Abstract
Background
Laparoscopic sleeve gastrectomy (LSG) has become an increasingly popular metabolic surgical procedure. Alteration in motor gastric function is a fundamental feature following LSG but still remains controversial.
Purpose
To determine the gastric emptying time 3 month after LSG, correlations between gastric emptying time and body weight, weight loss, and glycemia levels, alongside determining predictive factors of weight loss at the 3-month follow-up.
Materials and Methods
Twenty-one patients were recruited in this study. Gastric emptying time was measured using a standard solid-phase gastric emptying scan at both baseline and 3 months after LSG. Paired sample t tests and a general linear model with repeated measures were applied to investigate the alterations in major parameters after surgery. Univariate analyses were performed to evaluate the factors predicting weight loss at the 3-month follow-up.
Results
Compared with baseline levels, gastric emptying time, body weight, and HbA1c levels decreased significantly at 3 months after LSG (P < 0.001). Significant positive correlations were detected between HbA1c levels and gastric emptying time at baseline (P = 0.03). Significant positive correlations were detected between HbA1c levels at baseline and change in gastric emptying time (P = 0.03). Univariate logistic regression revealed a lower baseline BMI level to be independently associated with %EWL (P < 0.001).
Conclusions
The rate of gastric emptying increased following LSG. Patients with a higher risk of type 2 diabetes at baseline had longer gastric emptying times prior to treatment and significantly shortened emptying times following surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic surgery, as a recommended treatment option for patients with clinically diagnosed morbid obesity, is gaining increasing popularity. In addition to substantial weight loss, an increasing number of studies have highlighted that metabolic surgery can substantially ameliorate obesity-related metabolic diseases, including, but not limited to, type 2 diabetes mellitus (T2DM) [1,2,3], hypertension, dyslipidemia, obstructive sleep apnea–hypopnea syndrome (OSAHS) [4], and polycystic ovary syndrome (PCOS) [5] in patients with severe obesity.
According to the 2016 International Federation for the Surgery of Obesity and Metabolic Disorder (IFSO) survey [6], sleeve gastrectomy is currently the most frequently performed surgical procedure among metabolic surgeries worldwide, followed by Roux-en-Y gastric bypass surgery. Sequential randomized clinical trials (RCTs) indicated a significant reduction in body weight at 1 year, 3 years, and 5 years of follow-up after Roux-en-Y gastric bypass surgery compared with that of sleeve gastrectomy. However, according to evidence from the Swiss Multicenter Bypass Or Sleeve Study (SM-BOSS) RCTs [7, 8], both laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass (LRYGB) were equally efficient in causing loss of excess BMI at 3 years and 5 years of follow-up after surgery, respectively.
Compared with LRYGB, less alteration of the gastrointestinal anatomy makes LSG a more attractive surgical option. However, an alteration in motor gastric functional has been proven to occur after LSG, which may be associated with major gastric resection. A limited number of studies, which focused on the alteration of gastric functional features following LSG, using a gastric emptying time assessment have been reported. Furthermore, the results in previous studies were not comparable as they used different assessment methods, liquid or solid test meals, and there was variation in the control groups used. Time-resolved MRI following ingestion of liquids was reported to reveal motility changes after LSG. Based on dynamic steady-state free precession sequences, liquid test meals appeared to be the most serious limitation to previous studies [9]. A radiological upper gastrointestinal series with a water-soluble contrast medium was applied in another study [10]. As well as using liquid test meals, this test was not a measure of gastric function or motility, although the prolonged retention of barium in the stomach (> 6 h) observed in this study is suggestive of gastroparesis [11]. The solid-phase gastric emptying scan, measured using the radionuclide scintigraphic technique, is considered to be the best test currently available evaluate gastric emptying feature for gastric motor function assessment [11]. Thus, it would be a promising method to assess the gastric emptying time both objectively and quantitatively. Given the consideration of homogeneity in subjects, a self-controlled study is a superior design for this type of assessment.
In this study, solid-phase gastric emptying time was measured using a radionuclide scintigraphic technique and was performed in our hospital at baseline and the 3-month follow-up point, after LSG, for each patient. Additionally, changes in body weight and metabolic parameters were also recorded. The aims of this study were to prove our hypotheses that (1) gastric emptying time at 3-month follow-up after LSG would be different among patients; (2) there were possible correlations between gastric emptying time and body weight, weight loss, and glycaemia levels; and (3) gastric emptying time might be a predictive factor of weight loss at 3-month follow-up. To the best of our knowledge, this is the first study of gastric motor function based on a solid-phase gastric emptying scan in Chinese patients with obesity following LSG. Our study would increase the understanding of gastric emptying features after LSG and its underlying mechanisms for metabolic surgery.
Materials and Methods
Study Design and Patients
This was a retrospective, self-controlled, observational study to analyze gastric emptying features following LSG for patients with obesity in China. Eligible patients in this study were 18–65 years of age, with a diagnosis of morbid obesity according to the World Health Organization (WHO) criteria for obesity in Asian populations [12], who were receiving LSG for the first time in our hospital, and who had completed the solid-phase gastric emptying scan both before and after surgery. Preoperative (baseline) parameters, including gender, age, body weight, height, BMI, gastric emptying time, HbA1c, 3-h oral glucose tolerance test (OGTT), C-peptide and insulin levels to accompany the OGTT, were recorded. All related clinical data originated from our database, named “Beijing Friendship Hospital Metabolic Surgery Database (BFH-MSD)” (ClinicalTrials.gov ID: NCT03520699), which was approved by the Ethics Committees of Beijing Friendship Hospital where the study was conducted (NO.2017-P2-131-02). Informed consent was obtained from all individual participants included in this database.
Operative Procedure
The Bariatric and Metabolic Surgery Center in our hospital is one of the highest-volume centers in China, with at least 300 surgical procedures performed per year. All procedures of LSG in this study were conducted by our metabolic surgical teams, who have personal experience of at least 200 bariatric interventions. This surgical procedure was performed under laparoscopy according to international operation standards. After placement of trocars, a pneumoperitoneum was created by carbon dioxide gas with a pressure of 12 mmHg. Then, under a four-port laparoscopy, the greater curvature of the stomach was dissociated by dissecting the large omentum. The resection of the stomach started from 6 cm proximal to the pylorus towards the angle of His, using a linear stapler with the guidance of an intragastric bougie. Finally, a continuous suture was performed at the edge of the incision (Fig. 1).
Solid-Phase Gastric Emptying Scan
This test uses an externally positioned gamma scintillation camera to measure emptying of 1 mCi 99mTc-DTPA sulfur colloid from the stomach. The sulfur colloid is usually added to scrambled eggs in measured to ensure similarity in size, weight, and content. After fasting 12 h, patients ate the solid test meal in 3 min. Gastric emptying time was measured, and the anteroposterior position static phases were collected at the time points of 5 min, 30 min, 1 h, 2 h, 3 h, and 4 h. The gastric emptying curve was obtained by delineating the region of interest (ROI) area in the stomach. Finally, the time required for 50% of radioactivity to be excreted from the stomach was calculated using the ROI technique.
Follow-Up at 3 Months
According to guidelines for the follow-up of patients undergoing metabolic surgery [13], all of the patients were recommended for follow up at 3 months following LSG. Several previous studies also recommended the 3-month postoperative period as a visiting point for studying changes in gastric emptying in association with other measures [14, 15]. Moreover, patients were more compliant with a follow up at 3 months. Solid test meals would only be available after LSG at 3 months, allowing the solid-phase gastric emptying scan to be performed for patients enrolled in this study. Recorded clinical parameters were same as those in the baseline. Statistical analysis was performed following data collection.
Statistical Analysis
SPSS 21.0 software (SPSS, Inc., Chicago, IL) was used for all statistical analysis. Data were presented as mean (SD) for quantitative data and median (maximum, minimum) for qualitative data. For normally distributed quantitative data, paired samples t test and independent-samples t test were used to test the changes in body weight, BMI, gastric emptying time, HbA1c, plasma glucose levels, and other parameters. For non-parametric data, the Wilcoxon signed rank test was applied. Qualitative data were analyzed using chi-square (Fisher’s exact test). Pearson bivariate correlations were performed for correlation analysis of normally distributed quantitative data, while spearman bivariate correlations were used for non-normal distributions.
To evaluate the differences in predictive factors based on weight loss control, we divided the samples into two groups according to the %EWL (percent of excess weight loss). The cutoff point of %EWL was determined by clinical observation. Predictive factors of %EWL were than analyzed by univariate logistic regression analyses.
To analyze the variations of plasma glucose, C-peptide, and insulin levels with time in the OGTT, a general linear model with repeated measures was performed. All tests were two-tailed, and the level of significance was set at 0.05 (P < 0.05).
Results
Patients
From April 2016 to September 2017, 21 patients were recruited to participate in this study. In the baseline demographic data, 62% of the patients were women. The mean (SD) age was 32 (7.26) years old, and the mean BMI was 38.89 (7.55) kg/m2. Before surgery, the mean gastric emptying time was 67.10 (33.43) min. Additionally, the baseline characteristics for metabolic parameters (the levels of glucose, insulin, and C-peptide in OGTT) are shown below (Table 1).
Gastric Emptying Time
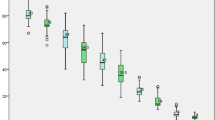
Twenty-one patients (100%) completed the self-comparison in terms of solid-phase gastric emptying scan. Compared with baseline levels, gastric emptying time was significantly decreased at 3 months following LSG (Table 1; Fig. 2a; P < 0.01). Gastric emptying time at baseline and change in gastric emptying time were not significantly correlated with baseline body weight, %EWL, %TWL (percent of total weight loss), or well as weight loss (Figure S1 in the Supplementary Appendix; P > 0.05) but were significantly correlated with HbA1c at baseline level (Fig. 3; P = 0.03, r = 0.474; P = 0.03, r = 0.470, respectively). However, in terms of fasting glucose, fasting insulin, and fasting C-peptide levels, there were no significant correlations shown with gastric emptying time at baseline (Figure S2 a–c in the Supplementary Appendix; P > 0.05). In terms of peak and time to peak levels of C-peptide and insulin, there were no significant correlations with gastric emptying time at 3 months (Figure S2 d–g in the Supplementary Appendix; P > 0.05).
Gastric emptying time, body weight, BMI, and obesity degree from baseline to 3 months. a The difference in gastric emptying time between baseline and 3 months was statistically significant (P < 0.01). b The difference in body weight between baseline and 3 months was statistically significant (P < 0.01). c The difference in BMI between baseline and 3 months was statistically significant (P < 0.01). d The difference in the degree of obesity between baseline and 3 months was statistically significant (P < 0.01)
Correlation analyses between gastric emptying time at baseline, change in gastric emptying time and HbA1c at baseline. a The correlation between gastric emptying time at baseline and HbA1c at baseline was statistically significant (P = 0.03, r = 0.474). b The correlation between change in gastric emptying time and HbA1c at baseline was statistically significant (P = 0.03, r = 0.470)
Weight Loss
At 3 months, reductions in body weight, BMI, and degree of obesity were statistically significant (Table 1; Fig. 2b–d; P < 0.01 for all self-comparisons). To evaluate predictive factors of body weight loss, a logistic regression analysis was conducted. Instead of gastric emptying time at baseline and change in gastric emptying time, BMI at baseline could be used as a predictive factor of %EWL within a 3-month follow-up. According to the univariate analysis, body weight and BMI at baseline were significant predictive factors for postoperative weight loss (Table 2; both P < 0.01).
Glycemic Control
In self-comparison, a significant change from baseline to 3 months after LSG showed that HbA1c was decreased by 1.06% (Table 1; P < 0.05), with 11 patients represented in the data after LSG. As for OGTT, reductions were observed in fasting plasma glucose levels, 2- and 3-h postprandial plasma glucose levels, fasting and 3-h postprandial C peptide levels, C peptide-time to peak, and fasting and 3-h postprandial insulin levels (Table 1; P < 0.05). In addition, the result of HOMA-IR in self-comparison showed a significant decrease at 3 months after LSG (Table 1; P < 0.05).
Furthermore, we analyzed the shape of the glucose, insulin, and C-peptide curves during OGTT. In terms of shape of glucose level, C-peptide and insulin levels, there were significant differences between levels at baseline and 3 months after surgery (Fig. 4; P < 0.001, respectively).
Glucose, C-peptide, and insulin curve during the OGTT (means shown). a The difference in the shape of glucose level between baseline and 3 months was statistically significant (P < 0.001). b The difference in the shape of C-peptide levels between baseline and 3 months was statistically significant (P < 0.001). c The difference in the shape of insulin level between baseline and 3 months was statistically significant (P < 0.001)
Discussion
Gastric Emptying Time Alteration Features After LSG
The results of this 3-month follow-up self-controlled observational study showed that the rate of gastric emptying was accelerated for solids following LSG. This result is in agreement with previous studies using scintigraphic evaluation of gastric emptying for solids after LSG [14, 16, 17]. However, in a previously published study evaluating gastric emptying with semi-solid meal, it was demonstrated that the rate of gastric emptying was not affected by LSG with antrum preservation [15]. The residual gastric volume would be relatively larger without removal of the gastric antrum, and this was probably the main reason for the difference in results.
The reasons for accelerated gastric emptying may be relevant to specific procedures. These include firstly entire fundus resection. The gastric fundus reflexively relaxes to comply and accommodate ingested food, which plays an important role in the physiology gastric emptying. The fundus has little phasic activity (with reference to the contractile activity of the smooth muscle); rather, it has elevated tone (with reference to resting pressure of the smooth muscle fibers), which contributes to the movement of ingested food motion from upper to lower stomach as a result of the pressure tone gradient [11]. After entire fundus resection, the function of the gastric reservoir is substantially reduced because initial receptive relaxation of the proximal stomach and its’ subsequent state of adaptive relaxation are eliminated by this procedure. Meanwhile, the tone of upper stomach might be further elevated, resulting in an increased pressure gradient, and which might account for the dysphagia and belching after a meal in postoperative patients. The second procedure this is relevant to is partial corpus resection. Pacemaker cells, also known as interstitial cells of Cajal (ICCs), are situated within the Auerbach plexus on the greater curvature of the stomach, where they generate contractions within the antrum and pylorus at a rate of three cycles per minute (cpm). The antrum can contract at a maximum frequency of 3 cpm, and this can be modulated by vagal splanchnic and hormonal input at the same time [18]. Following partial corpus resection, the pacesetter potential (also called gastric slow wave) activity is deeply modified leading to the alteration of maximum frequency. Meanwhile, the neural and hormonal stimulations remain. If appropriate stimulation is present (either neural or humoral), then the strength and amplitude of the gastric slow wave are increased and can be greatly influenced by these factors [11, 19]. The third procedure this is relevant to is partial antrum resection. The propulsion function of the antrum depends upon antral innervation through the Latarjet’s nerve, which remains almost intact during this surgical procedure. This ensures that antropyloric coordination remains intact, which plays a critical role in the timing of gastric emptying. Nevertheless, after partial antrum resection, the volume of lower stomach is visibly decreased. The process of trituration takes place in lower stomach, and this depends upon the size of the meal, the caloric content, the amount of liquid ingested, and the relative amounts of fat, fiber, protein, and carbohydrates [19]. Given the central role of antral pump and pylorus, tight dietary management provides a fundamental therapy for postoperative patients with obesity.
Correlations Between Glycaemia Levels and Gastric Emptying Time after LSG
In this study, HbA1c, fasting, and 2-h and 3-h postprandial glucose levels were significantly decreased. Meanwhile, C-peptide and insulin levels were substantially ameliorated at corresponding time points. Prior to the surgical procedure, the mean (± SD) HbA1c level was 5.91% (± 1.00), with postprandial glycemic excursions accounting for about 70% of variability when HbA1c is < 7.3% [20]. Furthermore, positive correlations between gastric emptying time/change in gastric emptying time and HbA1c at baseline were found in this study. A complex bidirectional relationship exists between gastric emptying and glycaemia [21]. Persuasive evidence exists that gastric emptying is a major determinant of postprandial glycaemia in health, as well as patients with diabetes [22,23,24]. The variability in glycaemia following intake of food is largely attributable to differences in the rate of gastric emptying [25]. This process determines the absolute and relative rates of release of the incretin hormones, glucagon-like peptide 1 (GLP-1), and gastric inhibitory polypeptide (GIP) from L cells (in the distal small intestine and colon) and K cells (locals in the duodenum and jejunum), respectively [19, 21, 26]. This process means that more rapid gastric emptying increases both GLP-1 and GIP secretion. GLP-1 and GIP stimulate beta cells in the pancreatic islets to secrete insulin and GLP-1 suppresses secretion of glucagon from alpha cells; as a result, postprandial glycaemia is decreased. Even relatively minor variations in gastric emptying can have a substantial impact on the postprandial glycaemia [22, 23, 27, 28]. This might account for the curative efficacy of metabolic surgery in control of glycaemia for diabetes. In addition, we found that there were no significant correlations between gastric emptying time and fasting C-peptide, fasting insulin, peak, and time to peak levels of C-peptide and insulin. Studies are needed to establish the mechanisms associated with islet cell function.
Predictive Factors of Weight Loss After LSG
%EWL allows for comparison of individuals with varying initial weights and varying excess weights, making it useful as a standard measure of commonly reported weight loss in metabolic surgery literature [29]. In this study, the %EWL cutoff is set at 50% at 3 months after LSG. In univariate logistic regression analysis, it seems that BMI (weight) is a significant, valuable predictor of weight loss. Furthermore, lower weight at baseline predicted increased effects of surgery on weight loss. However, such statistical differences were not found in terms of baseline gastric emptying time (change in gastric emptying time). The following reasons for this should be taken into consideration: firstly, although %EWL has been the standard metric for reporting weight loss in general, the disadvantage of using %EWL is that it may not reflect successful weight loss in very high BMI patients. In fact, patients with super-obesity often have lower %EWL than lower BMI groups despite achieving greater absolute weight loss [29]. Therefore, %EWL has limitations when used as the sole measure of success following metabolic surgery. Secondly, although the mean level of gastric emptying time (change in gastric emptying time) is visibly lower in %EWL ≥ 50% group, the standard deviation (SD) were slightly wider meaning that our sample size was not large.
Limitations
The following limitations to our study should be considered when interpreting the results. Firstly, the sample size was not large, albeit similar to or greater than that of previous studies [14, 16, 17]. Investigation with a larger patient cohort is definitely necessary, but the fact that we could already identify statistically significant changes in this study was encouraging. Secondly, some clinical data was missed in follow-ups. For postoperative patients, although intensive follow-ups were performed according to the guidelines [13], we missed some data in follow-ups due to the lack of compliance of some patients. Further investigations with a larger sample size and increased compliance are still needed to further clarify the correlation between gastric emptying time and metabolic surgery.
Conclusions
In conclusion, the rate of gastric emptying was accelerated following LSG in Chinese patients with obesity. Interestingly, patients with higher risk of type 2 diabetes at baseline were observed to have a longer gastric emptying time before treatment and a significantly shortened emptying time after surgery. Increased understanding of gastric emptying features fundamental to gastric motor function might provide opportunities for demonstrating the underlying mechanisms of metabolic surgery on diabetes remission. The alterations of gastric emptying features after LSG render it an attractive surgical therapy and research area for individuals with morbid obesity.
References
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–13.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641–51.
Arterburn D, Courcoulas A. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014;349:g3961.
Christ JP, Falcone T. Bariatric surgery improves hyperandrogenism, menstrual irregularities, and metabolic dysfunction among women with polycystic ovary syndrome (PCOS). Obes Surg. 2018;28:2171–7.
Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;
Peterli R, Wolnerhanssen BK, Vetter D, et al. Laparoscopic sleeve gastrectomy versus roux-Y-gastric bypass for morbid obesity-3-year outcomes of the prospective randomized Swiss Multicenter Bypass Or Sleeve Study (SM-BOSS). Ann Surg. 2017;265(3):466–73.
Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–65.
Baumann T, Kuesters S, Grueneberger J, et al. Time-resolved MRI after ingestion of liquids reveals motility changes after laparoscopic sleeve gastrectomy—preliminary results. Obes Surg. 2011;21(1):95–101.
Pomerri F, Foletto M, Allegro G, et al. Laparoscopic sleeve gastrectomy—radiological assessment of fundus size and sleeve voiding. Obes Surg. 2011;21(7):858–63.
Lacy BE, Weiser K. Gastric motility, gastroparesis, and gastric stimulation. Surg Clin North Am. 2005;85(5):967–87. vi-vii
Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
O'Kane M, Parretti HM, Hughes CA, et al. Guidelines for the follow-up of patients undergoing bariatric surgery. Clin Obes. 2016;6(3):210–24.
Braghetto I, Davanzo C, Korn O, et al. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19(11):1515–21.
Bernstine H, Tzioni-Yehoshua R, Groshar D, et al. Gastric emptying is not affected by sleeve gastrectomy—scintigraphic evaluation of gastric emptying after sleeve gastrectomy without removal of the gastric antrum. Obes Surg. 2009;19(3):293–8.
Melissas J, Daskalakis M, Koukouraki S, et al. Sleeve gastrectomy—a “food limiting” operation. Obes Surg. 2008;18(10):1251–6.
Melissas J, Koukouraki S, Askoxylakis J, et al. Sleeve gastrectomy: a restrictive procedure? Obes Surg. 2007;17(1):57–62.
Stevens JE, Jones KL, Rayner CK, et al. Pathophysiology and pharmacotherapy of gastroparesis: current and future perspectives. Expert Opin Pharmacother. 2013;14(9):1171–86.
Phillips LK, Deane AM, Jones KL, et al. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. 2015;11(2):112–28.
Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881–5.
Marathe CS, Rayner CK, Jones KL, et al. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. 2013;36(5):1396–405.
Pilichiewicz AN, Chaikomin R, Brennan IM, et al. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab. 2007;293(3):E743–53.
Ma J, Pilichiewicz AN, Feinle-Bisset C, et al. Effects of variations in duodenal glucose load on glycaemic, insulin, and incretin responses in type 2 diabetes. Diabet Med. 2012;29(5):604–8.
Ishii M, Nakamura T, Kasai F, et al. Altered postprandial insulin requirement in IDDM patients with gastroparesis. Diabetes Care. 1994;17(8):901–3.
Rayner CK, Samsom M, Jones KL, et al. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24(2):371–81.
Trahair LG, Horowitz M, Rayner CK, et al. Comparative effects of variations in duodenal glucose load on glycemic, insulinemic, and incretin responses in healthy young and older subjects. J Clin Endocrinol Metab. 2012;97(3):844–51.
Gonlachanvit S, Hsu CW, Boden GH, et al. Effect of altering gastric emptying on postprandial plasma glucose concentrations following a physiologic meal in type-II diabetic patients. Dig Dis Sci. 2003;48(3):488–97.
O'Donovan DG, Doran S, Feinle-Bisset C, et al. Effect of variations in small intestinal glucose delivery on plasma glucose, insulin, and incretin hormones in healthy subjects and type 2 diabetes. J Clin Endocrinol Metab. 2004;89(7):3431–5.
Brethauer SA, Kim J, el Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11(3):489–506.
Acknowledgments
The authors would like to thank all of the involved study investigators, staffs, clinicians, nurses, and technicians for dedicating their time and skills to the completion of this study.
Funding
This study was supported by National Key Technologies R&D Program (Grant No. 2015BAI13B09); Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (Grant No. ZYLX201504); Research Foundation of Beijing Friendship Hospital, Capital Medical University (Grant No. yyqdkt 2017-31); and Wu Jieping Medical Foundation (Grant No. 320.2710.1813).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in this study.
Statement of Human and Animal Rights
This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committees of Beijing Friendship Hospital, Capital Medical University.
Electronic Supplementary Material
ESM 1
(DOCX 1604 kb)
Rights and permissions
About this article
Cite this article
Li, M., Liu, Y., Jin, L. et al. Alterations of Gastric Emptying Features Following Laparoscopic Sleeve Gastrectomy in Chinese Patients with Obesity: a Self-Controlled Observational Study. OBES SURG 29, 617–625 (2019). https://doi.org/10.1007/s11695-018-3571-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3571-7