Abstract
Introduction
Bariatric surgery has seen a sharp rise in India in the last decade. India is one of the 10 most obese nations of the world, ranking second in number of type 2 diabetics.
Aims
To evaluate clinical outcomes of bariatric surgery after 3 years of follow-up in terms of weight loss, co-morbidity resolution, complaints of gastroesophageal reflux disease and weight regain.
Methodology
All patients who underwent bariatric surgery from January to December 2013 with a minimum follow-up of 3 years were included in the study. Their demographic, preoperative, and postoperative data were prospectively maintained on Microsoft Office Excel and analyzed statistically.
Results
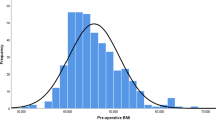
One hundred seventy-eight patients (157 lap. sleeve gastrectomy and 21 patients lap. RYGB) completed 3 years of follow-up. In the LSG group, patients had a pre-operative BMI 44.8 ± 8.33 kg/sq. m (mean ± S.D.) and excess body weight 52.3 ± 23.0 kg. In the RYGB group, pre-operative BMI was 42.7 ± 8.82 kg/sq. m and excess body weight 45 ± 18.7 kg. In the LSG group, % excess weight loss (EWL) at 1 year was 87.6 ± 24.4% and 3 years was 71.8 ± 26.7%. In the RYGB group, % EWL at 1 year was 97.2 ± 27.3% and at 3 years was 85.8 ± 25.3%. Diabetes resolution was seen in 32 (80%) in LSG group and 11 (91.7%) in RYGB group (Figs. 1, 2, 3, and 4).
Conclusion
Our study reflects that there is no statistically significant difference between outcomes of sleeve gastrectomy and Roux-en-Y gastric bypass surgery in terms of weight loss and diabetes resolution at 3 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery numbers have seen a sharp rise in India in the last decade. A country known for its undernourished population, India has witnessed recent economic growth coupled with greater influence of western culture and foods. The obesity epidemic is on the rise and now India is one of the 10 most obese nations of the world besides being second only to China in the number of type 2 diabetics (1). Of the numerous bariatric procedures performed worldwide, the popularity of laparoscopic sleeve gastrectomy (LSG) has increased from 4.5% in 2008 to 37% in 2013, and it became the most popular operation in the USA in 2015 (2). It is technically easier to perform, achieves good weight loss and resolution of comorbidities comparable to Roux-en-Y gastric bypass (RYGB) and is the most appropriate option in extremely obese patients (3). However, long-term weight maintenance and comorbidity resolution are better with RYGB compared to LSG (4). In some studies, comparable results have been seen in terms of weight loss and comorbidity resolution in both procedures (5). This can be explained with patient selection, multidisciplinary approach and follow-up protocol variations between different programs. We present our experience from a single surgeon from our center, which is a Center of Excellence in Bariatric and Metabolic surgery, on LSG and RYGB as primary procedures for treatment of morbid obesity in terms of weight loss and comorbidity resolution 3 years after surgery.
Aims and Objective
The aim of this study was to evaluate clinical outcomes of laparoscopic bariatric surgery after 3 years of follow-up in terms of weight loss, comorbidity resolution, gastroesophageal reflux disease (GERD) symptoms and weight regain.
Materials and Methods
This study was performed at a Centre of Excellence (COE) in Bariatric and Metabolic Surgery. The study was approved by the local Ethics Committee and the need for patients’ informed consent was waived due to the retrospective nature of the study. All patients who underwent bariatric surgery from the same surgeon from January 2013 to December 2013 and had a minimum follow-up of 3 years after surgery were included in the study. For each patient, demographic, preoperative, and postoperative data were prospectively maintained on Microsoft Office Excel. Two hundred thirty-four patients underwent surgery in this period, of which 178 (76%) patients reported for 3 years follow-up whose data was retrospectively reviewed. Fifty-six patients who did not report for 3 years follow-up were excluded from the study.
Statistical Analysis
Categorical data were expressed as percentage (%). Continuous data were presented as mean and standard deviation. Statistical analysis was performed using SPSS version 24.0 (IBM Corp. N.Y.). Continuous data was analyzed using Mann-Whitney test. Categorical data was analyzed using Pearson’s chi-square test or Fisher’s exact test. A p value of less than 0.05 was considered statistically significant.
Patient Selection
BMI above 32.5 kg/m2 with significant comorbidities or BMI above 37.5 kg/m2 without comorbidity were considered for bariatric surgery according to Consensus statement for Asians (6).
Preoperative Preparation
Prior to surgery, every patient underwent multidisciplinary evaluation by a bariatric surgeon, physician, anesthetist, counselor, and dietician. If required, the patients were also sent to an endocrinologist or psychiatrist for further evaluation.
Patients with history of alcohol intake or smoking were explained the enhanced risk related to healing, weight regain, and accelerated liver damage. They were refused surgery till they demonstrated adequate cessation.
Every patient underwent upper gastrointestinal endoscopy to rule out significant upper gastrointestinal tract pathology. For patients with symptoms of obstructive sleep apnea, polysomnography was performed.
The patients were counseled in detail about the surgical choices of sleeve gastrectomy (LSG) and Roux-en-Y gastric bypass (RYGB), various postoperative complications, possible nutritional deficiency, and redo surgery if need arises. In our center, patients with hiatus hernia or diabetes of more than 5 years duration were preferred for RYGB over LSG. However, in LSG group, no exclusion criteria were applied based on pre-operative glycemic control, C peptide levels or medication history.
The patients with fatty liver were put on low calorie liquid diet for at least 2 weeks and admitted 1 day before the procedure. Deep vein thrombosis prophylaxis was started 12 h before surgery with low molecular weight heparin subcutaneous injections and continued for 1 week after surgery.
Operative technique: All surgeries were performed by the same primary surgeon and assistants.
-
1.
Laparoscopic sleeve gastrectomy: The patients were operated under general anesthesia in steep reverse Trendelenberg position with compression stockings and sequential compression devices attached. The surgeon stood on the right, the camera assistant and the second assistant on the left side of the patient. Pneumoperitoneum was created using a long Veress needle which was inserted at left hypochondrium in accordance with size of left lobe of liver on ultrasound of abdomen. A 10-mm 30-degree telescope was used for insertion of first trocar and five trocars were inserted in upper abdomen (1 × 12 mm, 1 × 10 mm and 3 × 5 mm trocars). Subsequently, the 10-mm 30-degree scope was used. Intra-abdominal pressure was kept at 14 mm of Hg. The procedure began by dividing the greater omentum using ultrasonic shears opposite to Crow’s feet. This was continued cephalad dividing short gastric vessels up to the angle of His. Fundus of stomach was dissected off the left crus to ensure adequate posterior mobilization. The division of the greater omentum was then continued distally up to the pylorus. The posterior surface of the stomach was dissected free of any adhesion with the pancreas. A 36 French gastric calibration tube (GCT) was inserted per orally into the duodenum through the pylorus. Stomach sleeve was constructed by laparoscopic 60-mm staplers across the bougie, the first firing starting 5 cm proximal to the pylorus. The staple heights were decided according the tissue thickness. As a routine, we re-inforced proximal 2 cm of staple line with PDS 3–0 sutures and omental patch in all patients. At the end of the procedure, leak test was performed by flooding abdomen with saline and injecting air through the stomach tube while occluding pylorus distally. Specimen was removed from 12 mm port. No drains or naso-gastric tubes were kept. Ports larger than 5 mm were closed under vision using PDS no. 1 suture.
-
2.
Laparoscopic Roux-en-Y gastric bypass (RYGB): The patients were operated under general anesthesia in steep reverse Trendelenberg leg-split position with compression stockings and sequential compression devices. The surgeon stood on the right, the camera assistant in between the legs and the second assistant on the left side of the patient. Pneumoperitoneum was created using a long Veress needle which was inserted at left hypochondrium in accordance with size of left lobe of liver on ultrasound of abdomen. A 10-mm 0 degree telescope was used for insertion of first trocar and five trocars were inserted in upper abdomen (2 × 12 mm, 1 × 10 mm and 2 × 5 mm trocars). Subsequently 10 mm 30 degree scope was used. Intra-abdominal pressure was kept at 14 mmHg. Gastric pouch was created by dividing stomach horizontally 5 cm below gastroesophageal junction and vertically along a 36F bougie. In patients with hiatus hernia diagnosed preoperatively, hiatal dissection was done, both crura identified and cruroplasty was done with prolene 1–0 interrupted sutures. A biliopancreatic limb was created by stapled division at 75 cm from DJ flexure. Alimentary limb was measured at 150 cm and stapled jejuno-jejunostomy was done with biliopancreatic limb, enterotomy closed with PDS 3–0 in 2 layers. Stapled ante-colic gastro-jejunostomy was done with 25 mm circular stapler. Enterotomy was closed by linear stapler. Both mesenteric defects were closed with prolene 3–0. A 28F abdominal drain was placed. Air fluid leak test was performed by injecting 250 cm3 air through the gastric pouch. Ports larger than 5 mm were closed with PDS 1–0 under vision.
Postoperative Care
Patients were allowed clear liquids 6 h after surgery and gradually increased to full liquid diet over the next 2 days. The patients were then discharged usually by third postoperative day after drain removal. First follow-up was on the seventh day of the discharge when nutritional supplements were started. Liquid diet was continued for 2 weeks and then the patients were gradually shifted to pureed diet, soft diet over the next 2–4 weeks. Full diet was started after 6 weeks in LSG patients and after 4 weeks in RYGB patients. Subsequent follow-up was at the first, third, sixth, and 12th months postoperatively and then annually. Additionally, patients were encouraged to attend quarterly support group meetings.
On their first follow-up visit, patients were started on a multivitamin and calcium preparation with vitamin D3. All medicines were crushed before intake. For gall stone prophylaxis, tablet ursodiol 300 mg twice daily was prescribed for the initial 6 months to patients with intact gallbladder.
Laboratory estimation for protein, vitamin, and mineral deficiencies was performed at 6 months and then yearly, with relevant deficiencies corrected. Protein supplementation was generally given for 1 year in LSG group and continued life-long in RYGB group.
Weight charting was done on each follow-up and patients having gained weight more than 10 kg from nadir were classified as “weight regain” (7).
Results
In the year 2013, a total of 234 patients underwent bariatric surgery at our center, of which 178 (76%) completed 3 years of follow-up with our program, whose data was analyzed. One hundred fifty-seven underwent LSG and 21 underwent RYGB.
In the LSG group (n = 157), patients had a preoperative BMI 44.8 ± 8.33 kg/sq. m and excess body weight 52.3 ± 23.0 kg (mean ± S.D.). In the RYGB group (n = 21), preoperative BMI was 42.7 ± 8.82 kg/sq. m and excess body weight 45 ± 18.7 kg. The rest of the parameters are mentioned in the Table 1.
In the LSG group, 40 (25.5%) patients suffered from type 2 diabetes, of which 30 (75%) were on OHAs alone, 9 (22.5%) were on OHAs + insulin and 1 (2.5%) was on insulin alone. In the RYGB group, 12 (57.2%) patients suffered from type 2 Diabetes, of which 7 (58.3%) were on OHAs alone, 3 (25%) on OHAs + insulin and 2 (16.7%) were on insulin alone (Table 2).
In the LSG group, 62 (39.5%) suffered from hypertension, 88 (56%) suffered from sleep apnea, 10 (6.4%) had symptomatic or endoscopically proven GERD and 65 (41.4%) suffered from joint pain. In the RYGB group, 10 (47.6%) suffered from hypertension, 12 (57.2%) from sleep apnea, 15 (71.4%) from GERD, and 10 (47.6%) from joint pain (Table 2).
All procedures were successfully completed laparoscopically. No cases of postoperative bleeds, leaks, or deep vein thrombosis were seen in either group. Port site infection was seen in 5 (3.2%) in LSG group at specimen extraction port and 1 (4.7%) in RYGB group. Incisional hernia occurred in 1 (0.6%) in LSG group which required repair. Three (1.9%) patients required cholecystectomy subsequently in LSG group. No revisions or mortality occurred in either group at 3 years follow-up (Tables 3, 4, 5, and 6).
In the LSG group, BMI at 1 year was 28.6 ± 5.6 kg/sq. m and at 3 years was 31.3 ± 6.2 kg/sq.m. In the RYGB group, BMI at 1 year was 27.67 ± 6.0 kg/sq. m and at 3 years was 29.0 ± 5.9. There was no statistical difference in the BMI at 1 year (p value 0.44) or 3 years (p value 0.13) in the two groups.
In the LSG group, % excess weight loss (EWL) at 1 year was 87.6 ± 24.4% and 3 years was 71.8 ± 26.7%. In the RYGB group, % EWL at 1 year was 97.2 ± 27.3% and at 3 years was 85.8 ± 25.3%.There was no difference in %EWL in two groups at 1 year (p value 0.42) or 3 years (p value 0.13). Surgical failure (less than 50% EWL) were 5 (3.2%) in LSG group and 1 (4.7%) in RYGB group.
In the LSG group, weight regain (more than 10 kg from nadir) was seen in 28 (17.8%) patients after 3 years. In RYGB group, weight regain was seen in 2 (9.5%) patients after 3 years.
Diabetics were categorized on the basis of their insulin or OHA requirement. Diabetes resolution (off all medication) was seen in 32 (80%) in LSG group and 11 (91.7%) in RYGB group (p value 0.315). The statistical insignificance could be due to unequal groups in our study. Hypertension was considered as resolved when there was normalization of systolic blood pressure in the absence of medication. Resolution of hypertension was seen in 37 (55.2%) in LSG group and 8 (80%) in RYGB group (p value 0.13). Sleep apnea and joint pathology were assessed clinically. GERD was assessed clinically and confirmed endoscopically in symptomatic patients.
Discussion
The epidemic of obesity is on the rise, attributed to changes in behavioral patterns of various Indian communities in recent times. Obesity related comorbidities are also known to occur at a lower BMI in Asian-Indians (8). In recent years, LSG has emerged as a commonly used bariatric procedure worldwide and has been constantly compared with the standard procedure, the RYGB. This is due to the simplicity and efficacy of this procedure, with preference in adolescence and super-obese cases (9). It is also being performed with increasing frequency in India (8). In patients with comorbid conditions like type 2 Diabetes and hypertension, RYGB is preferred, possibly because RYGB has proven its ability to treat comorbidities, particularly type 2 diabetes (10). In our study, patients with type 2 diabetes of duration longer than 5 years, those with poor glycemic control or on insulin treatment were preferred for RYGB. Patients with symptomatic reflux or hiatus hernia diagnosed endoscopically were preferred for RYGB. Patients unwilling underwent LSG with hiatal dissection and cruroplasty. Jacobs et al. (11) reported that hiatus repair is easier at the time of LSG due of the absence of the bulky stomach occluding the view of the crura, making dissection easier after sleeve gastrectomy.
In our study, all procedures were successfully completed laparoscopically, without any significant intraoperative complications in either group. No postoperative bleeds, leaks or mortality were seen in either group. In a comparison between RYGB (n = 1345) and LSG (n = 686), Weiner et al. (15) reported a higher incidence of early major complications for the LSG group (4.9 vs. 7.14%; p = 0.039). In a study by De Maria et al. with 57,918 operated patients (12), the incidence of intraoperative complications was similar for both procedures (1.35% for LSG and 1.41% for RYGB). In another study by Gupta et al. (13) with 11,023 patients, the reported 30-day mortality was 0.2% in RYGB patients. A study by Rubin et al. (14) reported no staple line leaks, bleeds or postoperative mortality after LSG.
Our study showed similar % EWL in both groups at 1 year (LSG-87.6 ± 24.4%, RYGB 97.2 ± 27.3%) (p value 0.42)) and 3 years (LSG-71.8 ± 26.7%, RYGB 85.8 ± 25.3%) (p value 0.13). A study by Trelles and Gagner (16) showed a mean % EWL after sleeve gastrectomy ranging from 45 to 83% at 1 year, 47 to 83% at 2 years, and 66% at 3 years. A systematic review by Fischer et al. (17) showed that the % EWL at 12 months was significantly higher after RGBP than after LSG (68.3 vs. 56.1%; p < 0.01). Studies have shown weight loss after RYGB is significantly greater than LSG in all postoperative years of the study on the 12th–60th postoperative month (4, 18, 19). However, some studies report equal weight loss for RYGBP as compared to SG (5, 8) in the first 12 and up to 36 postoperative months.
In our study, diabetes resolution at 3 years was similar in both LSG and RYGB groups. According to Melissas et al. (4), comorbidity resolution was better for diabetes, dyslipidemia, and sleep apnea syndrome after RYGBP in the first postoperative year. In the following second, third, fourth, and fifth years of follow-up, both procedures proved to be equally capable to treat the above conditions. RYGBP showed better results in the treatment of hypertension on the first and the second postoperative year, while in the subsequent years of follow-up, there were no statistically significant differences in the ability of both procedures to treat this disease. The STAMPEDE (35) report showed that at 1 year, patients had better glycemic control after gastric bypass (42% of patients) or sleeve gastrectomy (37%) than after intensive medical therapy (12%) (p < 0.001). At 3 years, the target glycated hemoglobin level of 6.0% or less was achieved in 5% of the patients in the medical therapy group, as compared with 38% of those in the gastric-bypass group (p < 0.001) and 24% of those in the sleeve gastrectomy group (p = 0.01). Our results are in accordance with those published by Cutolo et al. (20) and Yang et al. (21) having reported similar results in diabetes remission at 24- and 36-month follow-up.
In our study, hypertension and sleep apnea were resolved in 37 (55.2%) and 77 (90.6%) in LSG group and 8 (80%) and 12 (100%) in the RYGB group, respectively. A study by Auclair A. Et al (33) showed hypertension and sleep apnea were resolved in 11 (44%) and 20 (77%) patients, respectively. A study by Neff et al. (36) showed remission of hypertension was greater after RYGB than LAGB at 1 year (32 vs. 16%, p = 0.008) and at 5 years postoperatively (23 vs. 11%, p = 0.02). In our study, joint pain was the only comorbidity which showed better results after LSG at 3-year follow-up, which is also seen in study by Melissas et al. (4), probably due to the fact that higher BMI patients underwent LSG in both studies. A study by Wendy C. Et al. (34) showed majority of participants with severe knee or hip pain or disability at baseline experienced joint-specific improvements (for knee pain, 77.1% [95% CI, 73.5–80.7%]; hip pain, 74.1% [95% CI, 69.7–78.4%]; hip physical function, 79.2% [95% CI, 75.3–83.1%]), and the majority of participants with a mobility deficit at baseline experienced remission by year 1 after a bariatric procedure.
It is believed that GERD may be aggravated by LSG. In our study, 12 (6.7%) patients developed de novo GERD and 4 (40%) patients had persistent GERD, however 6 (60%) had improvement in GERD symptoms. Himpens et al. (22) reported in their series of 53 patients who had undergone LSG, developed new symptoms of GERD in 21% of the patient within 6 years of follow-up. A study by Prasad et al. (3) showed 1.8% patients developed GERD who were asymptomatic preoperatively. However, a study by Dr. Aggarwal et al. (37) showed a significant improvement in GERD after 12 months in LSG patients (GERD Symptom Score reduced from 2.28 to 1.06, p < 0.05).
Weight regain is a worrisome problem seen long term after bariatric surgery. Several studies have defined weight regain as increase in weight by more than 10 kg from nadir (29,30,31,32). In our study, using this definition of weight regain, in the LSG group, weight regain was seen in 29 (17.8%) while in RYGB group it was seen in 2 (9.5%) after 3 years. Following RYGB, an initial rapid weight loss reaches a nadir after 1.5–2 years at around 90% excessive weight loss (EWL) (23, 24). In the long term, % EWL diminishes to 68% after 10 years (25). Significant weight regain after RYGB has been defined as ≥ 25% weight gain from nadir and occurs in 10–37% of patients, accompanied by a rebound of comorbidities such as hypertension, obstructive sleep apnea (OSA), and diabetes (25,26,27). Liu et al. (28) employed their definition of weight regain (an increase in % EWL of 25), with regain rates of 0, 1.0, 11.6, 19.2, and 29.5% at 1, 2, 3, 4, and 5 years postoperatively, respectively.
It is understandable that the variation in results with published data may be related to robust follow-up protocols in a Center of Excellence. There are limitations to this study like it being a single center study with 3 years of follow-up and the inequality of the two groups making comparison difficult. Additional studies are required to consolidate our results and provide more clear answers on the long-term weight maintenance and resolution of comorbidities, following in these two procedures.
Conclusion
In our study, higher weight loss and diabetes remission was seen after 3 years in Roux-en-Y gastric bypass compared to sleeve gastrectomy, though no statistical significance can be established. A randomized control trial or a matched prospective controlled study is essential to establish superiority of a procedure. Also high diabetes resolution rates noticed in our study may be attributed to post bariatric protein diet in primarily cereal eating Indian population. These initial observations require a follow-up comparative study with racially different population groups.
References
Remedios C, Bhasker AG, Dhulla N, et al. Bariatric nutrition guidelines for the Indian population. Obes Surg. 2016;26:1057–68.
Khorgami Z, Shoar S, Andalib A, et al. Trends in utilization of bariatric surgery, 2010–2014: sleeve gastrectomy dominates. Surg Obes Relat Dis. 2017;13:774–8.
Prasad P, Tantia O, Patle N, et al. An analysis of 1–3-year follow-up results of laparoscopic sleeve gastrectomy: an Indian perspective. Obes Surg. 2012;22:507–14.
Melissas J., Stavroulakis, K., Tzikoulis, V. et al. Sleeve gastrectomy vs Roux-en-Y gastric bypass. Data from IFSO-European Chapter Center of Excellence Program. Obes Surg (2017) 27: 847.
Vidal P, Ramón JM, Goday A, et al. Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy as a definitive surgical procedure for morbid obesity. Mid-term results. Obes Surg. 2013;23:292–9.
Misra A, Chowbey P, Makkar BM, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. JAPI. 2009;57:163–70.
Lauti M, Kularatna M, Hill AG, et al. Weight regain following sleeve gastrectomy—a systematic review. Obes Surg. 2016;26:1326–34.
Chowbey PK, Dhawan K, Khullar R, et al. Laparoscopic sleeve gastrectomy: an Indian experience—surgical technique and early results. Obes Surg. 2010;20:1340–7.
Park JY, Kim YJ. Laparoscopic gastric bypass vs sleeve gastrectomy in obese Korean patients. World J Gastroenterol. 2015;21(44):12612–9.
Li P, Fu P, Chen J, et al. Laparoscopic Roux-en-Y gastric bypass vs. laparoscopic sleeve gastrectomy for morbid obesity and diabetes mellitus: a meta-analysis of sixteen recent studies. Hepato-Gastroenterology 2013;60(121):132–137.
Jacobs M, Bisland W, Gomez E, et al. Laparoscopic sleeve gastrectomy: a retrospective review of 1 and 2 years results. Surg Endosc. 2010;24:781–5.
DeMaria EJ, Pate V, Warthen M, et al. Baseline data from American Society for Metabolic and Bariatric Surgery-designated Bariatric Surgery Centers of Excellence using the bariatric outcomes longitudinal database. Surg Obes Relat Dis. 2010;6(4):347–55.
Gupta PK, Franck C, Miller WJ, et al. Development and validation of a bariatric surgery morbidity risk calculator using the prospective, multicenter NSQIP dataset. J Am Coll Surg. 2011;212(3):301–9.
Rubin M, Yehoshua RT, Stein M, et al. Laparoscopic sleeve gastrectomy with minimal morbidity. Early results in 120 morbidly obese patients. Obes Surg. 2008;18(12):1567–70.
Weiner RA, El-Sayes IA, Theodoridou S, et al. Early post-operative complications: incidence, management, and impact on length of hospital stay. A retrospective comparison between laparoscopic gastric bypass and sleeve gastrectomy. Obes Surg. 2013;22(12):2004–12.
Trelles N, Gagner M. Updated review of sleeve gastrectomy. The open Gastroenterol J. 2008;2:41–9.
Fischer L, Hildebrandt C, Bruckner T, et al. Excessive weight loss after sleeve gastrectomy: a systematic review. Obes Surg. 2012;22:721–31.
Zerrweck C, Sepúlveda EM, Maydón HG, et al. Laparoscopic gastric bypass vs. sleeve gastrectomy in the super obese patient: early outcomes of an observational study. Obes Surg. 2014;24(5):712–7.
Yaghoubian A, Tolan A, Stabile BE, et al. Laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy achieve comparable weight loss at 1 year. Am Surg. 2012;78(12):1325–8.
Cutolo PP, Nosso G, Vitolo G, et al. Clinical efficacy of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass in obese type 2 diabetic patients: a retrospective comparison. Obes Surg. 2012;22(10):1535–9.
Yang J, Wang C, Cao G, et al. Long-term effects of laparoscopic sleeve gastrectomy versus roux-en-Y gastric bypass for the treatment of Chinese type 2 diabetes mellitus patients with body mass index 28-35 kg/m2. BMC Surg. 2015;15:88.
Himpens J, Dobbeleir J, Peeters G. Long term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252(2):319–24.
Karmali S, Brar B, Shi X, et al. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013;23:1922–33.
Peterli R, Borbely Y, Kern B, et al. Early results of the Swiss multicentre bypass or sleeve study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg. 2013;258:690–5.
Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–40.
Cooper TC, Simmons EB, Webb K, et al. Trends in weight regain following Roux-en-Y gastric bypass (RYGB) bariatric surgery. Obes Surg. 2015;25:1474–81.
Nicoletti CF, de Oliveira BA, de Pinhel MA, et al. Influence of excess weight loss and weight regain on biochemical indicators during a 4-year follow-up after Roux-en-Y gastric bypass. Obes Surg. 2015;25:279–84.
Liu SYW, Wong SKH, Lam CCH, et al. Long-term results on weight loss and diabetes remission after laparoscopic sleeve gastrectomy for a morbidly obese Chinese population. Obes Surg. 2015;25(10):1901–8.
Abdallah E, El Nakeeb A, Yousef T, et al. Impact of extent of antral resection on surgical outcomes of sleeve gastrectomy for morbid obesity (a prospective randomized study). Obes Surg. 2014;24(10):1587–94.
Bohdjalian A, Langer FB, Shakeri-Leiden Muhler S, et al. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20(5):535–40.
Braghetto I, Csendes A, Lanzarini E, et al. Is laparoscopic sleeve gastrectomy an acceptable primary bariatric procedure in obese patients? Early and 5-year postoperative results. Surg Laparosc Endosc Percutan Tech. 2012;22(6):479–86.
Casella G, Soricelli E, Giannotti D, et al. Long-term results after laparoscopic sleeve gastrectomy in a large monocentric series. Surg Obes Relat Dis. 2016;12(4):757–62.
Auclair A, Biertho L, Marceau S, et al. Bariatric surgery-induced resolution of hypertension and obstructive sleep apnea: impact of modulation of body fat, ectopic fat, autonomic nervous activity, inflammatory and adipokine profiles. Obes Surg. 2017;27:3156.
King WC, Jia-Yuh Chen SH. Belle et al. change in pain and physical function following bariatric surgery for severe obesity. JAMA. 2016;315(13):1362–71.
Schauer P, Bhatt D, Kirwan J, et al. Et al. bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370:2002–13.
Neff KJ, Baud G, Raverdy V, et al. Renal function and remission of hypertension after bariatric surgery: a 5-year prospective cohort study. Obes Surg. 2017;27:613–9.
Sharma A, Aggarwal S, Ahuja V, et al. Evaluation of gastroesophageal reflux before and after sleeve gastrectomy using symptom scoring, scintigraphy, and endoscopy. Surg Obes Relat Dis. 2014;10(4):600–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Nasta, A.M., Goel, R., Dharia, S. et al. Weight Loss and Comorbidity Resolution 3 Years After Bariatric Surgery—an Indian Perspective. OBES SURG 28, 2712–2719 (2018). https://doi.org/10.1007/s11695-018-3218-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3218-8