Abstract
Background and Aims
Partially covered self-expandable metallic stents (PCSEMS), although an effective treatment for anastomotic/staple line leaks and strictures, can be difficult to remove. This study examines the effectiveness of the inversion technique for the removal of PCSEMS in the treatment of leaks and strictures that occurred post-sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB).
Methods
Consecutive patients who underwent PCSEMS removal for a leak and/or stricture post-SG or RYGB between July 2013 and December 2016 at the Johns Hopkins Medical Institutions were reviewed. All PCSEMS removals were first attempted via the inversion technique, which involves grasping the distal end of the stent and inverting it through itself.
Results
Fourteen patients (four males) underwent PCSEMS removal via the inversion technique for an anastomotic/staple line leak (50%), stricture (29%) or both (21%) post-SG (79%) or RYGB (21%). Technical success (successful removal of the stent) was achieved in one endoscopic session for 13 of the 14 PCSEMS (93%). One PCSEMS required the use of the stent-in-stent technique for removal. The median dwell time was 47 days (range 5–72). A distal partial occlusion developed in five patients (35%) due to tissue overgrowth and one PCSEMS (7%) migrated, necessitating premature removal. Eight patients (57%) experienced clinical success at follow-up, and six patients (43%) required subsequent treatment due to persistence or recurrence of the pathology.
Conclusions
The inversion technique is a safe, effective, and efficient method of removing PCSEMS placed to correct anastomotic/staple line leaks and strictures post-SG and RYGB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to a recently published systematic review, adverse events develop after 4.6% of sleeve gastrectomy (SG) and 10.6% of Roux-en-Y gastric bypass (RYGB) operations [1]. Common adverse events include anastomotic/staple line leakage, stricture, small bowel obstruction, and hemorrhage. The most worrisome adverse event, an anastomotic/staple line leak, is reported to occur in 0–7% of SG and 0.4–5.2% RYGB [2]. Fortunately, many post-surgical adverse events, including leaks and strictures, can be safely and effectively treated using self-expandable metallic stents (SEMS) [2,3,4,5].
Stenting is also associated with potential adverse events, including migration, ulceration, and fistulization [6]. Fully covered self-expandable metallic stents (FCSEMS) often fail to seal against the esophageal wall, which can result in the interposition of food and/or liquid between the stent and the digestive wall. FCSEMS are also prone to migration [7]. Unlike FCSEMS, partially covered self-expandable metallic stents (PCSEMS) promote the formation of a water-tight seal and have a significantly lower migration rate. This is due to tissue hyperplasia, both proximal and distal to the leak, that projects through the strut material of the stent wall [6,7,8]. However, this tissue ingrowth makes the stent difficult to remove, with some reporting the need for surgical removal [6,7,8].
Due to the limitations of FCSEMS, clinicians have been searching for more effective methods for PCSEMS removal. A recent example of this, referred to as the “stent-in-stent technique” involves inserting a FCSEMS inside of the previously placed PCSEMS to decrease vascular granulation [6, 8]. The disadvantage of this method is that it necessitates an additional procedure, as well as an additional stent, which increases costs. Furthermore, this technique carries the risk of both stents migrating.
Bariatric endoscopists at Johns Hopkins University have performed a technique to remove PCSEMS, called the “inversion technique.” This technique, which has previously been used in the removal of stents placed to treat issues of the esophagus, such as esophageal disease, strictures, and ruptures, has the advantage of conferring removal in a single session using standard endoscopic instruments [9, 10]. The objective of this study is to examine the effectiveness and safety of this technique in patients with anastomotic/staple line leaks and strictures that arose as adverse events of SG or RYGB.
Subjects and Methods
This retrospective study was approved by our Institutional Review Board for Human Research and complied with Health Insurance Portability and Accountability Act (HIPAA) regulations. All patients who underwent an upper endoscopy with foreign body removal at Johns Hopkins Hospital were identified from an institutional claims database. The electronic medical record of patients who underwent procedures with a CPT code of 43,247 (esophagogastroduodenoscopy, flexible, transoral; with removal of foreign body(s)) by two endoscopists (PIO and VK) between July 2013 and December 2016 were reviewed. Analysis is limited to all patients who had a PCSEMS removed as part of the treatment of an anastomotic/staple line leak or stricture that occurred as an adverse event of SG or RYGB.
The diagnosis of a leak, stricture, or a combination of both was made via imaging and esophagogastroduodenoscopy (EGD) (Fig. 1). In the case of leakage, abdominal collections were drained percutaneously prior to stent placement.
The PCSEMS were deployed under fluoroscopic and endoscopic guidance. Stents were positioned so as to cover the pathology both proximally and distally (Fig. 2). Three PCSEMS were used: Wallflex esophageal partially covered (Boston Scientific, Natick, MA), Niti-S Taewoong esophageal partially covered (Taewoong Medical, Seoul, South Korea), and Ultraflex esophageal stent (Boston Scientific, Natick, MA), with lengths varying from 6 to 15 cm as necessary. Patients were started on oral nutritional intake after a negative leak test as determined by an upper GI series at days 3 to 5 post-procedure.
The duration of stent dwell was variable with no clear pre-determined algorithm used. An oral contrast computed tomography (CT) scan or upper GI series was performed in the weeks post-PCSEMS insertion to confirm no ongoing leak and resolution of the abdominal collection. If the leak had not resolved, the patient was re-referred back to PIO or VK for further endoscopic manipulation.
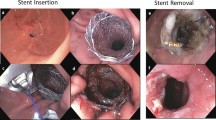
The specific technique of removal used for PCSEMS is called the “inversion technique” (Video 1). The inversion technique involves passing the endoscope through the lumen of the PCSEMS to its distal end and grasping it with stent grasping forceps. The forceps (now attached to the stent) are pulled back to the scope back while simultaneously keeping traction on the forceps. This allows the stent to invert through itself (Fig. 3) [9, 10]. If the inversion technique proved to be an unsuccessful removal method, the endoscopist resorted to implementing the stent-in-stent technique, as described by Vasilikostas et al. [11]. All PCSEMS removals were performed with general anesthesia under both endoscopic and fluoroscopic supports.
Immediately after the stent was removed, 60–120 ml contrast was injected under pressure through the working channel of the endoscope to assess for resolution of pathology (leak, stricture, or both) and to confirm no full-thickness disruption of the gastrointestinal wall as a result of PCSEMS removal. If there was persistent leak or stricture, it was up to the discretion of the endoscopist whether re-intervention (irrespective of technique) was necessary.
Outcomes considered include patient demographics (age, gender), bariatric surgery type (SG or RYGB), days to adverse event post-surgery, underlying pathology (leak and/or stricture), PCSEMS dwell time, technical success, clinical success, and adverse events. Technical success was defined as successful removal of the PCSEMS via the inversion technique in one session. Clinical success was defined as resolution of underlying pathology at follow-up. Adverse events and incidents were defined according to Cotton et al. [11].
Results
Application of exclusion criteria resulted in a sample of n = 14 patients. This sample consists of ten females (71%) and four males (29%), with a median age of 50 (range 24–67). Of these 14 patients that previously underwent bariatric surgery, 11 (79%) had a SG and 3 (21%) had a RYGB. In terms of post-surgical adverse events, seven (50%) presented with only a leak, four (29%) presented with only a stricture, and three (21%) presented with both a leak and a stricture. Of the ten patients that suffered a leak, the median number of days between surgery and leak identification was 15 (range 1–29). Of the three patients that suffered solely a stricture, the median number of days between surgery and stricture identification was 52.5 (range 8–63). Generally, the patients presented with symptoms of pain, nausea, and vomiting prior to pathology identification.
The PCSEMS employed in this cohort ranged from 6 to 15 cm in length, with a median length of 15 cm and an average length of 13 cm (Table 1). Stent length did not appear to impact the ease of removal. The median PCSEMS dwell time was 47 days (range 5–72) (Table 2) (Figs. 4 5, and 6). All PCSEMS used in this study crossed the gastroesophageal junction or gastrojejunal anastomosis, in patients that had RYGB.
Of the 14 PCSEMS placed in this study, 13 (93%) were successfully removed using the inversion technique. One patient required the use of alternative therapy for stent removal. The stent-in-stent technique was used in this case. In this patient, a 15-cm Wallflex PCSEMS was placed to treat a 5-mm leak at the angle of His that was not responsive to therapy with a percutaneous drain. The PCSEMS remained in situ for 56 days. At the time of removal, the endoscopist attempted the inversion technique, and although the distal end of the stent dislodged easily, the proximal end was not responsive to the endoscopist’s efforts. Upon determining that the inversion technique would not be a feasible removal method in this case, the endoscopist pushed the stent down but was unable to completely unravel it; therefore, the stent remained partially inverted. The endoscopist then inserted a 15-cm Wallflex FCSEMS through the original PCSEMS (Fig. 7). The patient was brought back 1 week later, at which time the endoscopist removed the FCSEMS followed by the PCSEMS without a problem.
Of the 13 cases in which technical success was achieved, one patient presented difficulty with PCSEMS removal using the inversion technique (dwell time 72 days). In this patient, the stent was not removed in one smooth motion, as it was caught in the inverted position while the proximal end of the stent was still attached. However, after several further pulls, the stent was dislodged and subsequently removed. While no perforation occurred, a significant portion of tissue did come off with the stent, and an iatrogenic stricture subsequently resulted. This was treated with a simple single through-the-scope balloon dilation to 20 mm.
The median duration of follow-up care post-PCSEMS removal was 3 months (range 0–18) (Table 2). Eight patients (57%) experienced clinical success. Of the six patients that did not, three were being treated for solely a stricture, two were being treated for solely a leak, and one was being treated for both a leak and stricture.
The median days to leak identification post-surgery in the cohort of patients that were treated for a leak and experienced clinical success was 14 (range 1–22). In patients that were treated for a leak but did not experience clinical success at follow-up, the median days to leak identification post-surgery was 15 (range 7–23). As for the patients treated for a stricture (with or without the presence of a leak), the median days to identification post-surgery in those that did experience clinical success was 16 (range 14–63), and in those that did not experience clinical success, the median days to identification post-surgery was 36 (range 8–72).
An adverse event of stent treatment was reported in one patient (7%) [12]. In this case, a PCSEMS had migrated, requiring premature removal. Additionally, upon retrieval, in five patients (36%), distal partial occlusion developed due to a combination of tissue ingrowth and stent-related ulceration (Fig. 8) [12]. No stent was fractured during removal. Of the six patients that experienced migration or distal partial occlusion, all were being treated for an anastomotic/staple line leak.
Discussion
Endoscopic treatment of adverse events arising post-bariatric surgery has been the topic of a number of recent publications, as there is significant morbidity associated with surgical re-intervention. A myriad of inventive techniques have been documented, but while balloon dilation is the standard method of treating strictures, there is no universally favored method for the treatment of anastomotic/staple line leaks [6, 13,14,15,16]. Diversion therapy, via the insertion of a SEMS, boasts advantages including early oral alimentation and early discharge, reducing the likelihood of suffering adverse events and costs associated with prolonged hospital stays [14, 16,17,18,19,20,21,22,23,24]. Stenting has also been successfully used to treat strictures that do not respond to balloon dilation [13, 17, 25]. However, drawbacks to using SEMS are well documented. FCSEMS are disposed to migration and suboptimal diversion, while PCSEMS solve these problems but are prone to difficult removal [14, 15, 21].
Recently, clinicians have been seeking mechanisms to overcome these obstacles, and significant advancements have been made. Endoscopic suturing of FCSEMS, for one, reduces migration, but the problem of suboptimal diversion remains, in addition to the increased cost associated with suturing [7, 8, 13, 19, 20, 26, 27]. Similarly, using a second stent to remove a PCSEMS has proven effective, but warrants an additional procedure and thus higher costs [19, 20]. Thus, the field of bariatric endoscopy lacks a proven method that eliminates additional procedures and appliances. This study addresses that deficiency by presenting the inversion technique for the removal of PCSEMS placed to treat leaks and strictures post-bariatric surgery. The inversion technique, as demonstrated in this analysis, is safe and effective.
The mechanism underlying the inversion technique, which consists of grasping the stent at the distal end and inverting it, is effective because grasping the stent from the distal end results in a concentrated force at the site of traction. In contrast, the proximal end of the stent is easier to grasp and allows for more maneuverability of the scope, but results in more a more expanded force, more extension of the stent, higher risk of breakage of the stent, and higher risk of rupturing the tissue.
The success rate of PCSEMS removal using the inversion technique was 93% in this sample. The ease of removal did not appear to be affected by the length of the stent, nor the days to adverse event post-surgery. Furthermore, the use of this technique resulted in successful removal of PCSEMS that had been in place for over 2 months. This finding is particularly relevant because, due to the tissue ingrowth that occurs with PCSEMS, increased residence time generally correlates with increased potential for difficult removal [11, 14, 20, 28, 29]. Although there is currently no consensus as to the ideal timing of stent removal, we believe that 4 weeks is an optimal duration, as it is generally suitable for healing and allows for fairly easy stent removal [6, 8, 13, 30]. It is important to note that in the case in which the inversion technique was not successful, the PCSEMS had been in place well beyond this optimal time frame. However, due to the success of the inversion technique in a number of patients with PCSEMS in place beyond the optimal range, we recommend that this technique be considered in cases in which stents have remained in situ beyond the optimal window.
As previously described, the stent-in-stent technique requires two procedures, as well as two stents, in order to remove a PCSEMS [13, 30]. Therefore, the inversion technique is more time and cost efficient than the stent-in-stent technique because it eliminates the cost of a second stent, the cost of the procedure to insert the second stent, the cost of the lengthened hospital stay, and the additional time requirement from both the patient and clinician. Furthermore, the stent-in-stent technique may carry a unique risk when compared to the inversion technique. The second stent, specifically a FCSEMS, is inserted inside of the previously placed PCSEMS to assert radial pressure against ingrown tissue. If the second stent works correctly, this process will dislodge the PCSEMS from the esophageal wall and thus will allow for easier removal [6, 11, 28, 29]. However, this mechanism could have the potential to put both stents at a heightened risk of migration. Moreover, the stent-in-stent technique may not always be a viable method, as it requires a second stent with a diameter that is larger than the original stent for the mechanism to work [11]. Thus, in cases in which the PCSEMS needed to treat the pathology is the largest available, there may not be a FCSEMS large enough to use in conjunction. For these reasons, we recommend that the stent-in-stent technique be considered as a removal method only when the inversion technique is unsuccessful, or does not appear to be feasible as determined by the performing endoscopist.
When performing the inversion technique to remove PCSEMS, two noteworthy procedural novelties must be considered. First, despite the aggressive pull that is necessary to dislodge the PCSEMS from the tissue, there have been no reported perforations. If, however, a large portion of tissue does secede from the esophageal wall with the stent during the pull, a stricture can result. This may require the implementation of a single through-the-scope balloon, as was the case with patient 12 in this study. Fortunately, these iatrogenic strictures often respond to single balloon dilation. Secondly, the procedure reported in this study involved a contrast injection to confirm leak closure and stricture resolution, as well as to check that the esophageal wall was not perforated during PCSEMS removal. This step is indispensable because dislodging the PCSEMS results in substantial bleeding, so the ability to visually detect a persistent leak or perforation is not reliable.
Before performing the inversion technique, it is important to be able to recognize technical challenges and manage them. The traction necessary to remove the stent should be constant and moderate, with no dramatic pulls. Excessive force could hypothetically result in injury to either the stent or the esophageal wall. If the stent is too well embedded to comply with removal via the inversion technique, cauterizing the tissue with argon plasma coagulation is a strategy that can be used to expose the end of the stents. Further, twisting or using rotation while having the stent grasped during removal may be helpful. Furthermore, using a double-channel scope to allow the utilization of two grasping forceps allows one to grasp the stent at two separate points before twisting or inverting, which may also serve useful.
The primary limitation of this study is the sample size, as it describes a series of only 14 patients. The heterogeneity of the sample is also limiting because patients treated for both a leak and/or stricture after either RYGB or SG are included, but statistical analysis between these groups is not valuable due to the small sample size. Additionally, comparisons of clinical success between this study and other studies are limited because, as this paper focuses on the implementation of the inversion technique, we do not report final clinical success rates after all additional follow-up treatment was completed. Finally, the retrospective nature of the study is unfavorable, and thus larger, prospective studies on this subject are necessary.
Use of the inversion technique has the potential to reduce the number of procedures, required materials, and associated costs to the patient, when compared to other removal modalities, such as the stent-in-stent technique for the removal of PCSEMS placed to treat leaks and/or strictures post-bariatric surgery. Furthermore, this technique resulted in a 93% success rate, even in stents that have remained in situ beyond the optimal timeframe and thus are likely to command a difficult removal. It is for these reasons that we recommend that the inversion technique be implemented by trained endoscopists for the removal of PCSEMS placed to treat anastomotic/staple line leaks and strictures that arise as adverse events of SG or RYGB.
References
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England). 2014;384(9945):766–81.
Guirat A, Guenzi M, Pereira P, et al. What is the role of the sleeve gastrectomy in the surgical treatment of morbid obesity? Eur Surg. 2014;46(5):181–8.
Griffith PS, Birch DW, Sharma AM, et al. Managing complications associated with laparoscopic Roux-en-Y gastric bypass for morbid obesity. Can J Surg J canadien de chirurgie. 2012;55(5):329–36.
Kumar N, Thompson CC. Endoscopic management of complications after gastrointestinal weight loss surgery. Clin Gastroenterol Hepatol Off Clin Prac J Am Gastroenterol Assoc. 2013;11(4):343–53.
Moon RC, Shah N, Teixeira AF, et al. Management of staple line leaks following sleeve gastrectomy. Surg Obes Relat Dis Off J Am Soc Bariatric Surg. 2015;11(1):54–9.
Murino A, Arvanitakis M, Le Moine O, et al. Effectiveness of endoscopic management using self-expandable metal stents in a large cohort of patients with post-bariatric leaks. Obes Surg. 2015;25(9):1569–76.
Seven G, Irani S, Ross AS, et al. Partially versus fully covered self-expanding metal stents for benign and malignant esophageal conditions: a single center experience. Surg Endosc. 2013;27(6):2185–92.
van Boeckel PG, Dua KS, Weusten BL, et al. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC Gastroenterol. 2012;12:19.
Weigt J, Barsic N, Malfertheiner P. A novel approach to esophageal stent removal in the setting of proximal stenosis and failure of the primary retrieval mechanism. Endoscopy. 2015;47:129–30.
van Heel NCM, Haringsma J, Wijnhoven BPL, et al. Endoscopic removal of self-expandable metal stents from the esophagus (with video). YMGE Gastrointest Endosc. 2011;74(1):44–50.
Vasilikostas G, Sanmugalingam N, Khan O, et al. ‘Stent in a stent’--an alternative technique for removing partially covered stents following sleeve gastrectomy complications. Obes Surg. 2014;24(3):430–2.
Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71(3):446–54.
Aryaie AH, Singer JL, Fayezizadeh M, et al. Efficacy of endoscopic management of leak after foregut surgery with endoscopic covered self-expanding metal stents (SEMS). Surgical endoscopy. 2016
Eisendrath P, Deviere J. Major complications of bariatric surgery: endoscopy as first-line treatment. Nat Rev Gastroenterol Hepatol. 2015;12(12):701–10.
Kumbhari V, Cai JX, Schweitzer MA. Endoscopic management of bariatric surgical complications. Curr Opin Gastroenterol. 2015;31(5):359–67.
Kumbhari V, Tieu AH, Cai JX, et al. Novel technique for the management of staple line leaks after sleeve gastrectomy. Gastrointest Endosc. 2015;82(4):748.
Kim J, Azagury D, Eisenberg D, et al. ASMBS position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and nonoperative management. Surg Obes Relat Dis Off J Am Soc Bariatric Surg. 2015;11(4):739–48.
Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis Off J Am Soc Bariatric Surg. 2016;12(7):1278–85.
Ngamruengphong S, Sharaiha RZ, Sethi A, et al. Endoscopic suturing for the prevention of stent migration in benign upper gastrointestinal conditions: a comparative multicenter study. Endoscopy. 2016;48(9):808.
Kumbhari V, Abu Dayyeh BK. Keeping the fistula open: paradigm shift in the management of leaks after bariatric surgery? Endoscopy. 2016;48(9):789–91.
Kumbhari V, Tieu AH, Ngamruengphong S, et al. Endoscopic management of stomal stenosis after Roux-en-Y gastric bypass. Gastrointest Endosc. 2015;82(4):747.
Shehab HM, Hakky SM, Gawdat KA. An endoscopic strategy combining mega stents and over-the-scope clips for the management of post-bariatric surgery leaks and fistulas (with video). Obes Surg. 2016;26(5):941–8.
van Wezenbeek MR, de Milliano MM, et al. A specifically designed stent for anastomotic leaks after bariatric surgery: experiences in a tertiary referral hospital. OBES SURG Obes Surg J Metab Surg Allied Care. 2016;26(8):1875–80.
Bouchard S, Eisendrath P, Toussaint E, et al. Trans-fistulary endoscopic drainage for post-bariatric abdominal collections communicating with the upper gastrointestinal tract. Endoscopy. 2016;48(9):809–16.
Simon F, Siciliano I, Gillet A, et al. Gastric leak after laparoscopic sleeve gastrectomy: early covered self-expandable stent reduces healing time. Obes Surg. 2013;23(5):687–92.
Fischer A, Bausch D, Richter-Schrag HJ. Use of a specially designed partially covered self-expandable metal stent (PSEMS) with a 40-mm diameter for the treatment of upper gastrointestinal suture or staple line leaks in 11 cases. Surg Endosc. 2013;27(2):642–7.
Wei W, Ramaswamy A, de la Torre R, et al. Partially covered esophageal stents cause bowel injury when used to treat complications of bariatric surgery. Surg Endosc. 2013;27(1):56–60.
DaVee T, Irani S, Leggett CL, et al. Stent-in-stent technique for removal of embedded partially covered self-expanding metal stents. Surg Endosc. 2016;30(6):2332–41.
Hirdes MM, Siersema PD, Houben MH, et al. Stent-in-stent technique for removal of embedded esophageal self-expanding metal stents. Am J Gastroenterol. 2011;106(2):286–93.
Malli CP, Sioulas AD, Emmanouil T, et al. Endoscopy after bariatric surgery. Ann Gastroenterol Q Publ Hell Soc Gastroenterol. 2016;29(3):249–57.
Author information
Authors and Affiliations
Contributions
All authors have contributed to and agreed on the content of this manuscript. Christine Hill, Bassem Khalil M.D., Patrick I. Okolo, M.D., and Vivek Kumbhari, M.B., Ch.B., contributed to conception and design, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, and final approval of the article. Sindhu Barola, Abhishek Agnihotri M.B., B.S., Vikesh K. Singh, M.D., M.Sc., Michael Schweitzer, M.D., Thomas Magnuson, M.D., and Mouen A. Khashab, M.D., contributed to analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, and final approval of the article.
Corresponding author
Ethics declarations
Conflict of Interest
Mouen A. Khashab is a consultant for Boston Scientific.
Vivek Kumbhari is a consultant for Boston Scientific and Apollo Endosurgery.
Vikesh K. Singh is a consultant for Abbvie, Novo Nordisk, and Calcimedica. He has served on advisory boards for Akcea, Nordmark, Celltrion, and Salix.
All other authors have no disclosures.
Electronic Supplementary Material
Video 1
Partially covered self-expandable metallic stent deployment and subsequent removal using the inversion technique. (MP4 29,782 kb)
Rights and permissions
About this article
Cite this article
Hill, C., Khalil, B.K., Barola, S. et al. Inversion Technique for the Removal of Partially Covered Self-Expandable Metallic Stents. OBES SURG 28, 161–168 (2018). https://doi.org/10.1007/s11695-017-2811-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2811-6