Abstract
Introduction
The use of self-expandable stents to treat postoperative leaks and fistula in the upper gastrointestinal (GI) tract is an established treatment for leaks of the upper GI tract. However, lumen-to-stent size discrepancies (i.e., after sleeve gastrectomy or esophageal resection) may lead to insufficient sealing of the leaks requiring further surgical intervention. This is mainly due to the relatively small diameter (≤30 mm) of commonly used commercial stents. To overcome this problem, we developed a novel partially covered stent with a shaft diameter of 36 mm and a flare diameter of 40 mm.
Methods
From September 2008 to September 2010, 11 consecutive patients with postoperative leaks were treated with the novel large diameter stent (gastrectomy, n = 5; sleeve gastrectomy, n = 2; fundoplication after esophageal perforation, n = 2; Roux-en-Y gastric bypass, n = 1; esophageal resection, n = 1). Treatment with commercially available stents (shaft/flare: 23/28 mm and 24/30 mm) had been unsuccessful in three patients before treatment with the large diameter stent. Due to dislocation, the large diameter stent was anchored in four patients (2× intraoperatively with transmural sutures, 2× endoscopically with transnasally externalized threads).
Results
Treatment was successful in 11 of 11 patients. Stent placement and removal was easy and safe. The median residence time of the stent was 24 (range, 18–41) days. Stent dislocation occurred in four cases (36 %). It was treated by anchoring the stent. Mean follow-up was 25 (range, 14–40) months. No severe complication occurred during or after intervention and no patient was dysphagic.
Conclusions
Using the novel large diameter, partially covered stent to seal leaks in the upper GI tract is safe and effective. The large diameter of the stent does not seem to injure the wall of the upper GI tract. However, stent dislocation sometimes requires anchoring of the stent with sutures or transnasally externalized threads.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
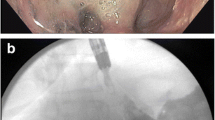
The use of self-expanding plastic (SEPS) or metal stents (SEMS) is an established treatment for leaks of the upper gastrointestinal (GI) tract. Available stents are either partially (PSEMS) or fully (FSEMS) covered SEMS or SEPS. However, it is currently unclear which type of stent is ideal for the treatment of leaks in the upper GI tract. The use of SEMS sometimes leads to insufficient sealing of leaks that may ultimately cause progressive sepsis and death (Fig. 1A) [1, 2]. We therefore preferentially use PSEMS, which are thought to promote mucosal ingrowth into the stent flares, a prerequisite for watertight sealing of leaks [2, 3]. Moreover, the mucosal ingrowth anchors the stent and reduces the risk of stent dislocation.
A Use of a commercial Leufen stent (diameter: 24/30 mm) in patient 11 at the gastroesophageal junction after a laparoscopic fundoplication was attempted to treat an esophageal perforation during balloon dilatation of achalasia. Orally applied contrast agent is found between the stent and the esophageal wall (→) and forms a fluid collection (►). B After changing to the novel stent (diameter: 36/40 mm) tight apposition to the esophageal wall can be observed without extraintestinal contrast agent
However, commercially available PSEMS often fail to seal leaks in the upper GI tract (i.e., after sleeve gastrectomy, esophagojejunostomy, esophagogastrostomy) due to the discrepancy between the relatively small stent diameter (≤30 mm) and the larger diameter of the upper GI tract. Commercially available stents (usual diameter: ≤30 mm) often are too small to cause apposition and thus watertight sealing. To circumvent this problem, we developed a novel PSEMS with a larger diameter (Leufen Medical GmbH, Aachen, Germany) and used it to treat leaks in the upper GI tract in 11 patients.
Patients and methods
From September 2008 to September 2010, 11 patients with postoperative suture or staple line leaks (gastrectomy, n = 5; sleeve gastrectomy, n = 2; fundoplication after esophageal perforation, n = 2; Roux-en-Y gastric bypass, n = 1; esophageal resection, n = 1) were treated with the novel large diameter PSEMS at our institution. Mean follow-up was 25 (range, 14–40) months. Treatment with a commercially available stents (2× Ultraflex Stent, partially covered, 23/28 mm, Boston Scientific, Natick, MA, United States; 1× Leufen Axistent®, partially covered, 24/30 mm, Leufen Medical GmbH, Aachen, Germany) had been unsuccessful in three patients before treatment with the novel stent (Table 1).
If the stent covered the gastroesophageal transition, acid suppression therapy was administered to prevent reflux disease. Stents were placed under sedation and removed under general anesthesia to prevent aspiration. Twenty-four hours after placement, leak sealing was assessed by oral application of methylene blue (Akorn, Inc., Lake Forest, IL, USA) or by a CT scan with an oral contrast agent. All patients were started on fluids followed by solids as tolerated after a negative leak test. Indwelling drains were removed 7–10 days after the negative leak test if secretion and signs of ongoing infection were absent.
Institutional review board approval was granted for the evaluation of patients with postoperative suture and staple line leaks in a retrospective manner from a prospective database. All patients were informed that the novel stent was a specially manufactured device and that no data was available regarding its use. Consent was obtained before each procedure.
Stent design
The novel stent is a partially covered metal stent with a shaft diameter of 36 mm and a flare diameter of 40 mm. The noncovered flare area is only 5-mm long, in contrast to the 10–15 mm used in commercial stents (Fig. 2). The short, noncovered area still permits mucosal ingrowth to obtain water tightness (Fig. 3) but facilitates atraumatic extraction. Retrieval sutures are attached at both ends of the stent for removal or repositioning.
Stent anchoring
Due to dislocation, the novel large diameter stent needed anchoring in four patients (2× intraoperatively, 2× endoscopically; Table 1). Despite fixation, all anchored stents could be easily removed endoscopically. Only a slight laceration of the mucosa occurred in the area of the noncovered stent flares.
Endoluminal anchoring
In patients 3 and 10, the stent had dislocated 24 h after its placement. After endoscopic repositioning, two sutures were threaded through the noncovered flare on two opposing sides of the stent with a grasper (FG-6L-1, Olympus, Tokyo, Japan). Their respective ends were then lead out transnasally, covered up to the stent flare with nasogastric tubes to prevent mucosal injury, and anchored to the nose under slight tension with adhesive tape (Fig. 4). After mucosal ingrowth into the stent flares, usually 4 days later, the threads were removed. No stent dislocation was observed thereafter.
Intraoperative transmural anchoring
After a leak of an esophagojejunostomy, patient 8 developed a refractory anastomotic stenosis, which was treated with an esophageal resection. However, the esophagocolostomy also developed a leak that was treated with the novel large diameter stent. The neck wound was covered with a vacuum dressing. Due to dislocation, the stent was anchored with a transmural PDS 5-0 suture under endoscopic control while the vacuum dressing was changed. No stent dislocation was observed thereafter.
Patient 11 was treated for a distal esophageal rupture with a commercial stent (Leufen; diameter: 24/30 mm). The patient was transferred to our institution after persistent leakage did not subside 4 days after a fundoplication had been performed.
During surgical revision, transhiatal drainage was performed and the novel stent was placed. Because the stent dislocated during intraoperative placement due to its angulated positioning, it was anchored with two transmural PDS 5-0 sutures to the esophagus (Ethicon PDS-II, Jonson & Johnson). No further stent dislocation occurred thereafter.
Results
Treatment was successful in 11 of 11 patients in whom the novel large-diameter stent was used. All leaks healed and no long-term problems, such as strictures or fistulas, were observed during follow-up. Moreover, the novel stent was used successfully if prior treatment with a commercial stent had failed, such as in patients 1, 10, and 11 (2× Ultraflex, diameter: 23/28 mm; 1× Leufen, diameter: 24/30 mm) and thus seems superior to other commercial stents (Fig. 1B).
The most common cause of treatment failure using commercial PSEMS in our clinical practice was persistent leakage between the stent and the GI wall due to the insufficient stent diameter relative to the large lumen of the UGI tract (i.e., sleeve gastrectomy, esophagojejunostomy, esophagogastrostomy; Fig. 1A). Figure 5A shows an extracted commercial Leufen stent (diameter: 24/30 mm) 3 weeks after placement to treat a leak in an esophagojejunostomy in a patient who was not part of the collective treated with the novel large diameter stent. The proximal stent flare clearly shows mucosal ingrowth but its distal flare had insufficient contact to the jejunal mucosa to promote mucosal ingrowth. In contrast, the extracted novel stent (diameter: 36/40 mm), which was used to treat another patient with a leak in an esophagojejunostomy clearly shows mucosal ingrowth at both of its flares (Fig. 5B), demonstrating how large-size discrepancies between the upper GI tract and the stent may lead to insufficient sealing of leaks.
Comparison of the novel stent (B) with a commercial stent (A) after both stents were used to seal a leak of an esophagojejunostomy. A Only the proximal stent flare of the commercial stent (diameter: 24/30 mm), which had contact to the esophageal wall, shows mucosal ingrowth, whereas its distal flare did not have sufficient contact to the jejunum to permit mucosal ingrowth. B The novel stent (diameter: 36/40 mm) also shows signs of mucosal ingrowth at its jejunally placed flare
Stent placement and extraction was safe and effective in all cases. The average residence time was 24 (range, 18–41) days. Even after a residence time of 41 days, stent extraction was easy due to the noncovered area of only 5 mm. All stents were removed at the planned time interval and no early removal was necessary. The stent removal procedure took less than 10 min in all patients. Stent inversion was necessary in four patients during the removal procedure.
No severe complications related to the large diameter of the stent were observed, and none of the patients experienced late strictures or leakage. However, stent dislocation occurred in 4 of 11 patients (36 %) but was preventable by endoscopic or open stent anchoring (Table 1). In particular, the large stent diameter did not cause pressure ulcerations or GI wall necrosis. Increased postinterventional pain, sometimes caused by stent expansion, or stent intolerance, presumably due to esophageal spasm, was not observed.
Discussion
The ideal stent for the treatment of suture or staple line leaks in the upper GI tract should seal leaks sufficiently, should not dislocate, prevent the formation of excessive granulation tissue, and be easily removable at any time after its placement. None of the currently available stents (SEPS, FSEMS, und PSEMS) fulfill all of the above criteria. Consequently, outcome did not depend on the type of stent used in a recent systematic review [4].
A commonly encountered problem is insufficient sealing of leaks if FSEMS and SEPS are used, in particular after esophageal resections, due to the insufficient diameter of commercial stents relative to the larger postoperative lumen found in the GI tract. Because commercial PSEMS are usually available with a flare diameter of up to 30 mm, lumen differences between the stent and GI tract may cause persistent liquid reflux from the distal end of the stent between the GI tract and the stent wall [2]. Due to the lack of water tightness, this may lead to clinically persistent leakage. To counter this problem, a special double-stent technique, called dumbbell technique, was developed [2]. This procedure was used successfully in 16 of 22 patients after esophageal resection where size discrepancies between the stent and GI tract are common. However, it was only needed to treat 2 of 15 patients with esophageal perforation, likely due to the smaller lumen that needed to be covered in this case [2]. Bridging of large GI lumina (i.e., after esophageal resection, sleeve gastrectomy) with a single commercial stent is associated with a failure rate of up to 22.4 % [2, 5, 6]. In contrast, we observed a 100 % success rate in our patient collective using the novel stent with a shaft diameter of 36 mm and a flare diameter of 40 mm (Fig. 2B). The noncovered area at the flares of our stent is only 5-mm long, which permits mucosal ingrowth to obtain water tightness while still facilitating stent extraction.
Compared with fully covered stents, mucosal ingrowth into the mesh at the stent flares in PSEMS may cause trauma during stent extraction or prevent it altogether [6, 7]. Due to the small noncovered area, tissue in- and/or overgrowth was less pronounced compared with commercially available stents, and stent extraction was always easy and safe, even after 41 days of residence. If extraction of the novel PSEMS by gentle pulling back with a rat-tooth forceps was not immediately successful due to mucosal hyperplasia, it was possible to invert the stent by pulling at its distal end before its extraction. SEPS insertion was never necessary to extract our stent [2].
Four of 11 patients (36 %) experienced dislocation of the novel stent, which is within the overall reported range of 6–43 % for PSEMS [2, 5, 6]. The relatively high rate of dislocation in our series may be due to the strong expansion force of the novel stent together with its small noncovered area that increases the risk of stent dislocation, in particular in the upper GI tract with its large lumen differences. Angular positioning of the stent may further increase this risk. Due to its tendency to straighten, the stent moves into an area where this is feasible, which leads to distal dislocation into the larger GI tract lumen. However, commercial PSEMS used to treat patients after bariatric surgery had a dislocation rate of up to 83 % [8–10], indicating that this may be a principal problem and not a particular characteristic of the novel stent. To remedy this problem and prevent future dislocation, endoscopic or surgical anchoring was used successfully in our series. Endoscopic anchoring with transnasally externalized threads was not performed preemptively, because it causes severe patient discomfort. This was not the case if the stent was placed intraoperatively and anchored with transmural sutures. We therefore recommend fixation in the latter case, in particular, because it does not seem to impair later stent extraction.
In the first years after the introduction of stent therapy for leaks in the upper GI tract, we immediately tested for water tightness after stent placement using an oral contrast agent swallow. However, this procedure is not sensitive for small contrast agent leaks [11], which led us to use CT scans with oral contrast agent swallows. Today, we only perform a CT scan to rule out additional intra-abdominal fluid collection. If the clinical course is uneventful, we orally administer methylene blue routinely 2–3 days after stent placement to determine water tightness. At this point, the stent is fully expanded and mucosal ingrowth is sufficient to cause water-tight sealing of the leak. In our series, 2 days after methylene blue administration, colored abdominal drain secretions were observed in patient 10. Due to a lack of additional symptoms, only the correct stent placement was verified and an additional methylene blue administration 2 days later did not cause a change of color in the abdominal drains.
Taken together, the novel large diameter PSEMS with a short, noncovered flare is ideal for the treatment of leaks in the upper GI tract if large diameter lumina require sealing and may be superior to current commercial stents. The diameter of 40 mm does not cause major complications or harm the GI wall. Moreover, no other known complication, such as tracheal compression, cardiac compression, perforation, bleeding, or lethal gastroaortic fistula was observed [2, 5, 12, 13]. However, the risk of stent dislocation is relatively high due to the strong expansion force and the short noncovered flares. If the stent is placed during surgery, it should be anchored with sutures. If necessary, transnasal anchoring can be performed endoscopically. We recommend the routine use of this stent for large diameter leaks in the upper GI tract. However, a larger series together with a randomized trial is necessary to evaluate its potential advantages fully.
References
Ott C, Ratiu N, Endlicher E, Rath HC, Gelbmann CM, Scholmerich J, Kullmann F (2007) Self-expanding Polyflex plastic stents in esophageal disease: various indications, complications, and outcomes. Surg Endosc 21:889–896
Swinnen J, Eisendrath P, Rigaux J, Kahegeshe L, Lemmers A, Le MO, Deviere J (2011) Self-expandable metal stents for the treatment of benign upper GI leaks and perforations. Gastrointest Endosc 73:890–899
Fischer A, Thomusch O, Benz S, von Dobschuetz E, Baier P, Hopt UT (2006) Nonoperative treatment of 15 benign esophageal perforations with self-expandable covered metal stents. Ann Thorac Surg 81:467–472
van Boeckel PG, Sijbring A, Vleggaar FP, Siersema PD (2011) Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther 33:1292–1301
Blackmon SH, Santora R, Schwarz P, Barroso A, Dunkin BJ (2010) Utility of removable esophageal covered self-expanding metal stents for leak and fistula management. Ann Thorac Surg 89:931–936
Tuebergen D, Rijcken E, Mennigen R, Hopkins AM, Senninger N, Bruewer M (2008) Treatment of thoracic esophageal anastomotic leaks and esophageal perforations with endoluminal stents: efficacy and current limitations. J Gastrointest Surg 12:1168–1176
Freeman RK, Ascioti AJ, Wozniak TC (2007) Postoperative esophageal leak management with the Polyflex esophageal stent. J Thorac Cardiovasc Surg 133:333–338
Edwards CA, Bui TP, Astudillo JA, de la Torre RA, Miedema BW, Ramaswamy A, Fearing NM, Ramshaw BJ, Thaler K, Scott JS (2008) Management of anastomotic leaks after Roux-en-Y bypass using self-expanding polyester stents. Surg Obes Relat Dis 4:594–599
Bege T, Emungania O, Vitton V, Ah-Soune P, Nocca D, Noel P, Bradjanian S, Berdah SV, Brunet C, Grimaud JC, Barthet M (2011) An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest Endosc 73:238–244
Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, de la Torre RA, Thaler KJ, Miedema BW, Scott JS (2008) Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg 206:935–938
Low DE (2011) Diagnosis and management of anastomotic leaks after esophagectomy. J Gastrointest Surg 15:1319–1322
D’Cunha J, Rueth NM, Groth SS, Maddaus MA, Andrade RS (2011) Esophageal stents for anastomotic leaks and perforations. J Thorac Cardiovasc Surg 142:39–46
Hirdes MM, Vleggaar FP, Van der LK, Willems M, Totte ER, Siersema PD (2011) Esophageal perforation due to removal of partially covered self-expanding metal stents placed for a benign perforation or leak. Endoscopy 43:156–159
Acknowledgments
This work was funded by departmental funds of the Department of Surgery, University of Freiburg, Freiburg, Germany.
Disclosures
Drs. Ficher, Bausch, and Richter-Schrag have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fischer, A., Bausch, D. & Richter-Schrag, HJ. Use of a specially designed partially covered self-expandable metal stent (PSEMS) with a 40-mm diameter for the treatment of upper gastrointestinal suture or staple line leaks in 11 cases. Surg Endosc 27, 642–647 (2013). https://doi.org/10.1007/s00464-012-2507-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2507-x