Abstract
Background
The use of fully covered self-expandable metallic stents (FCSEMS) has opened the door to treat stenosis in the post-bariatric stomach. We hypothesized that endoscopically securing a FCSEMS would be technically feasible, effective, and safe for > 30-day dwell time.
Objectives
To assess the technical feasibility, clinical efficacy, and safety of endoscopically secured FCSEMS in the stomach for > 30 days.
Methods
A retrospective review (September 2016 to April 2018) of consecutive patients who underwent FCSEMS suturing in the stomach at a single academic institution was reviewed. Technical success, stent dwell time, symptoms, and adverse events were recorded.
Results
Fifteen patients (median age of 49 (31–70)) were included. Stents were inserted for gastrojejunal (GJ) stricture or gastric stenosis in 9/15 and 6/15 of patients, respectively. All procedures were technically successful (100%). Immediate and short-term clinical success (prior to stent removal) was 100% in patients who did not have stent migration. Stent migration was seen in 3 cases (20%) after a median dwell time of 211 days. However, 2/3 (66.6%) had not attended their scheduled removal. Recurrence of symptoms after stent removal was seen in 53.3% of patients with 40% undergoing repeat stenting. Median stent dwell was 117 (30–342) days. Sixty percent and 33% of patients had stent dwell of at least 90 and 180 days, respectively.
Conclusions
A FCSEMS, if secured, may be safe and effective for even > 90-day dwell time in the post-bariatric stomach and may result in long-term clinical success for GJ stricture after stent removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing population of patients undergoing bariatric surgery will invariably lead to a growing number of patients who present with late complications requiring revision. Revision surgeries accounted for 13.9% of all bariatric surgeries performed in 2016, a rise from 6% in 2011. The increased need for revision of post-bariatric anatomy has resulted in the development of minimally invasive endoscopic interventions to treat post-bariatric complications [1].
In the post-bariatric surgery population, obstruction can result from various anatomical changes including gastric sleeve stenosis after laparoscopic sleeve gastrectomy (LSG) and gastrojejunal (GJ) anastomotic stricture after Roux-en-Y gastric bypass (RYGB). Gastric stenosis occurs in 0.7 to 4% of patients after LSG and results in obstructive symptoms including nausea, vomiting, and abdominal pain [2,3,4]. GJ anastomotic stricture is the most common early complication associated with RYGB, with reported rates between 3.4 and 7.1% [5, 6]. One method in the arsenal of endoscopic therapies is endoscopic stent insertion, which has proven success in the treatment of benign upper gastrointestinal obstruction [7,8,9].
Fully covered metal stents are advantageous due to their removability, which allows them to be useful in the treatment of benign upper GI conditions, including post-surgical complications. Stent migration remains a significant problem, however, occurring in 58% of patients in a study using fully covered stents to treat post-bariatric anastomotic complications [8] and 35.5% of patients in a study for benign upper gastrointestinal indications [10]. Risk factors for stent migration include benign conditions and the use of a FCSEMS [10], which are both present in post-bariatric cases. Stent migration may cause adverse events and require reintervention or surgery [8].
One solution for stent migration is the endoscopic fixation of stents. Stent fixation through endoscopic scope clips and over the scope clip and suturing are some solutions [11,12,13]. However, an ex vivo study has shown sutured stents to be superior to clips or non-sutured stents in strength of the force required to dislodge the stent and ability to reduce stent migration [13]. Successful endoscopic suturing of stents for benign upper gastrointestinal conditions was first reported by Kantsevoy and Bitner [14]. Studies since then have shown a variable success rate. Ngamruengphong et al. (2016) performed the first large study to assess the outcomes of sutured versus non-sutured FCSEMS for benign upper gastrointestinal conditions. Patients with a sutured stent had significantly less stent migration and longer time to migration, with a similar rate of adverse events [15]. However, there are no studies thus far that have looked at the use of sutured FCSEMS in the post-bariatric stomach. Additionally, there have been no studies looking at the use of FCSEMS for long-term dwell in this subpopulation. The ability to secure FCSEMS in the stomach by endoscopic suturing has opened the door to treat post-surgical anatomical complications refractory to more conservative management. This study describes a novel approach for the management of this pool of patients that is refractory to other therapies. We aimed to assess the technical feasibility, clinical efficacy, and safety of endoscopically secured FCSEMS (S-FCSEMS) in the stomach for greater than 30 days. Our primary objective was to assess technical feasibility of suturing a FCSEMS in the post-bariatric stomach. Our secondary objectives included assessing the adverse event rate, with a focus on stent migration rate, in addition to the short- and long-term clinical success of a S-FCSEMS. We hypothesized that a S-FCSEMS would be technically feasible, safe, and effective for at least 30-day dwell time in the post-bariatric stomach.
Methods
Patients
We performed a retrospective review of a prospectively collected database between September 2016 and April 2018 of consecutive patients who had a received an S-FCSEMS for a benign upper gastrointestinal stenosis or stricture subsequent to bariatric surgery. This included patients with gastric stenosis after LSG and patients with GJ stricture after RYGB.
GJ stricture was defined as a GJ diameter less than 8 mm and an inability in traversing the stomach to the jejunum using a standard endoscope. Gastric stenosis was diagnosed either radiographically by barium swallow or endoscopically in symptomatic patients. In particular, rotation of the staple line, inability to visualize lumen distal to the presumed stenotic area, and a dilated upstream stomach were used to make the diagnosis. Patients with gastric stenosis were included if they were refractory to at least one balloon dilation. At our center, an algorithm for the endoscopic management of gastric stenosis has been developed and put into practice. A patient with gastric stenosis initially undergoes 30-mm achalasia balloon dilation, then 35-mm achalasia balloon dilation up to 4 times if persistent. In cases that remain symptomatic after four dilations or in cases where there is no or minimal improvement with earlier dilations, a FCSEMS is placed across the stenosis and endoscopically fixed. This algorithm has been proven successful with 88.2% of patients reporting symptom improvement [16]. As the safety and efficacy of S-FCSEMS became more apparent during clinical practice, we were more likely to introduce S-FCSEMS earlier in patients, especially those who had no response to balloon dilations, who were within 4 weeks of their surgery or had sleeve lumens too small to comply with the > 30-mm dilation balloons.
All patients were offered surgical revision including seromyotomy or conversion/revision to Roux-en-Y gastric bypass in the case of stenosis/stricture. Cases were discussed as part of a multidisciplinary team including a clinical bariatric nurse, bariatric surgeon, and bariatric endoscopist. All patients gave fully informed consent to undergo the procedure.
Procedure
All procedures were performed by a single endoscopist with experience in endoscopic suturing (VK). Stent insertions were performed under general anesthesia. A standard endoscope was first advanced into the stomach to assess the stenosis or stricture with an attempt to reach the pyloric area in patients post-LSG or jejunal limb in patients post-RYGB. If gastric stenosis or GJ stricture was found, a guidewire was advanced and a FCSEMS was deployed over the guidewire. Prior experience had revealed that the WallFlex™ esophageal FCSEMS (Boston Scientific, Natick, MA) was found to cause mucosal erosions and tissue invagination into the distal end of the stent. Thus, most patients in this study received a Niti-S (Taewoong, Seoul, South Korea) FCSEMS, which is softer and less likely to cause injury to the gastric wall [17].
The stent was endoscopically secured with up to four 2-0 Prolene sutures with the use of a full-thickness endoscopic suturing system, OverStitch (Apollo Endosurgery, Austin, TX, USA). The suture pattern was as follows: bite using the tissue helix through the gastric wall, bite through the stent, and then bite using the tissue helix through the gastric wall (Video 1). Sutures were placed such that there was minimal tension on the gastric wall. To facilitate this, sutures were not cinched too tightly and were simply snug, and the distance between the gastric wall bites and the bite through the stent was minimized.
Stent Removal
All stents were intended to remain in situ for > 30 days. The rationale behind long-term dwell was that stent insertion confers proven symptomatic improvement when in situ. However, this symptomatic improvement may not persist post-stent removal. Our goal was to prolong symptom relief by prolonging stent dwell times safely. Patients were scheduled for a follow-up esophagogastroduodenoscopy (EGD) for stent removal at different time intervals depending on the clinical indication, pathology encountered, and number of sutures successfully placed. Stents were removed earlier than was intended if patients presented with symptoms of intolerance or in case of stent migration.
During stent removal, retained sutures connected to the FCSEMS were cut with Ensizor Flexible Endoscopic Scissors (Slater Endoscopy, Miami, FL, USA), and stent removal was performed using stent grasping forceps. In patients where we had noted partially uncovering of the stents with tissue ingrowth, stent removal was performed using the inversion technique [18]. In this technique, the endoscope was passed forward through the stent, grasping its distal end with the forceps. Then, endoscope and forceps were pulled backwards together, peeling off the stent and removing it in an inverted manner.
Outcomes
Patient demographics including age and sex were collected. Prior surgery, indication for procedure, and prior therapies were collected. Procedural data such as stent deployment location, stent size, and number of sutures used was also collected. The primary endpoint of technical success was defined as successful stent deployment and securing of the stent with sutures. Immediate clinical success was defined as improvement or complete resolution of symptoms 1 week after stent insertion. Short-term clinical success was defined as continued resolution or improvement of symptoms at the time of stent removal. Long-term clinical success was defined as continued resolution or improvement of symptoms after stent removal or migration for the entire duration of the follow-up period. Stent migration was defined as displacement of stent from its initial placement site and was evaluated if patients had symptom recurrence or development of new symptoms.
Results
Baseline Characteristics
Fifteen patients received S-FCSEMS between September 2016 and April 2018. Patients’ age ranged between 31 and 70 years with a median age of 49 (80% female). Nine out of 15 patients (60%) were post-RYGB with a GJ stricture, and 6 out of 15 patients (40%) were post-sleeve gastrectomy with gastric body stenosis. Presenting symptoms included nausea, vomiting, regurgitation, heartburn, dysphagia, and food intolerance. Patients frequently had a constellation of symptoms resulting from additional anatomical or physiological abnormalities that resulted in a complicated clinical presentation not directly related to the stenosis itself. Three out of 15 (20%) patients had concurrent complications due to post-surgical anatomy. One patient had a staple line leak, one patient had a gastrogastric fistula, and a third patient had a gastrogastric fistula and marginal ulcer.
Prior Therapies
Most patients were initially referred from bariatric surgeons directly to our center or to another endoscopist who had attempted other interventions before referring to our center. Prior therapies are shown in Table 1. In patients with gastric stenosis, all patients had prior pneumatic balloon dilations. Patient GS2 had a concurrent staple line leak which was treated prior by stenting. In this patient, overlapping stents were placed from the distal esophagus to the gastric antrum such that adequate diversion of the leak would be achieved and the stenosis would be traversed. No other prior interventions were performed in this patient. In patients with GJ stricture, 2 out of 9 (22.2%) had prior steroid injections and 2 (22.2%) patients had prior four-quadrant knife incisions. Two patients (22.2%) had previous stents placed at an outside institution that were not secured and had migrated.
Procedural Characteristics
Average room time (anesthesia preparation and recovery as well as endoscopy time) was 71.9 ± 24.5 min. Only 2 patients with gastric stenosis had WallFlex™ (Boston Scientific, Natick, MA) FCSEMS placed, of size 23 × 125 mm. The remaining gastric stenosis patients and all GJ stricture patients had Niti-S™ (Taewoong, Seoul, South Korea) FCSEMS placement. The diameter of the Niti-S FCSEMS was 18 mm in all patients. The 60-mm-length stent was used in 6/13 of patients, and the 80-mm-length stent was used in 7/13 patients.
To secure the stent, the intention was to place 4 sutures per patient; however, this was not always technically feasible. In patients with GJ stricture, two sutures were applied in 1/9 of the patients, three sutures in 4/9 of the patients, and four sutures in 3/9 of the patients. In patients with gastric stenosis, two sutures were applied in 2/6 of the patients, three sutures in 2/6 of the patients, and four sutures in 2/6 of the patients. The technical success rate for the S-FCSEMS placement was 100%.
Post-Procedural Outcomes
The stents were well tolerated with only up to 24 h of mild post-procedural discomfort, with the exception of one patient who experienced significant post-procedural pain managed conservatively. Imaging was performed on this patient and the stent was confirmed in place. The pain was managed medically and the patient was discharged the next day pain-free.
Immediate clinical success (immediately after stent insertion) was 100%. Short-term clinical success (prior to stent removal) was 80% and was 100% in those who did not experience stent migration. Patients were followed up a median time of 17 months (range 7–24) from stent insertion. During the follow-up period, long-term clinical success was 46.7%, with 8/15 (53.3%) patients experiencing recurrence of their symptoms. Of those, 4 out of 6 (66.7%) were gastric stenosis patients and 4 out of 9 (44.4%) were GJ stricture patients. One patient was lost to follow-up after symptom recurrence, and all others underwent further management. One patient underwent EGD with dilation using a 30-mm achalasia balloon. Six patients underwent repeat stenting. Of those, patient GJ1 experienced stent migration necessitating surgical removal of the stent. Patient GJ9 failed repeat stenting and required surgical revision.
Two of the five patients who underwent repeat stenting received multiple consecutive S-FCSEMS. Patient GS1 received a S-FCSEMS for 246 days (8 months) for gastric stenosis and had an uncomplicated course. Due to symptom recurrence after removal, this patient received a total of 4 consecutive stents and experienced stent migration of the last two stents. Unfortunately, this patient developed recurrence of symptoms requiring surgical revision of her bypass with partial gastric resection. Patient GJ6 also experienced a recurrence of symptoms after removal, so a second S-FCSEMS was applied, which migrated at 180 days and was removed endoscopically without complication. A third stent was then applied, and a follow-up endoscopy after 180 days showed a wide open GJ with complete resolution of symptoms.
S-FCSEMS were intended to be removed after a preestablished time period which was determined by the provider taking clinical and procedural aspects into consideration. In most cases (11/15, 66.7%), stents were removed as scheduled. All patients with removal as scheduled were asymptomatic except for one patient who reported vomiting. Two patients did not require removal as the stent had migrated and passed in the stool, and one patient required surgical removal due to migration and intestinal obstruction. Overall, 9 (60%) and 5 (33%) out of 15 of the patients had stent dwell times > 90 and > 180 days, respectively.
Adverse events were seen in 3 cases (20%) in this series and all were stent migrations. However, worthy of note is that most of these patients (2/3, 66.6%) were non-compliant and had not attended their intended visit for stent removal. These patients had a median stent dwell time of 211 days, and only one patient had stent migration at less than 180 days (at 30 days). Only one patient required an emergency department visit with readmission.
Of the patients treated for gastric stenosis, patients GS2 and GJ9 had stent migration at 30 and 211 days, respectively. Patient GS2 required medical consultation and an abdominal X-ray to confirm that the stent had passed in the stool, a mild adverse event according to American Society for Gastrointestinal Endoscopy (ASGE) lexicon. In patient GS3, the passing of the stent was confirmed visually in the stool without complication.
Of the patients with GJ stricture, one patient experienced stent migration and had a complicated course. After the procedure, she refused to return for follow-up due to complete symptom resolution and fear of recrudescence of symptoms if the stent was removed. She missed her planned procedure for stent removal at 120 days (4 months). She presented at 276 days with a migrated stent causing intestinal obstruction, which required surgical management. This was graded as a severe adverse event according to ASGE lexicon.
Findings on Stent and Suture Removal
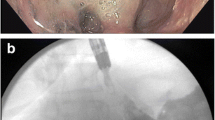
Twelve out of fifteen patients had endoscopic stent removal (Fig. 1). One patient had no attached sutures to the stent. In the other patients, sutures connected to the FCSEMS were removed with Ensizor Flexible Endoscopic Scissors (Slater Endoscopy, Miami, FL, USA). Stent removal was performed using Raptor Stent Grasping Device (US Endoscopy, Mentor, OH) in 11/12 patients (Video 2). Two patients had stents which became partially uncovered with tissue ingrowth at follow-up (Fig. 2), and stent removal was performed using the inversion technique [18]. After en bloc stent removal, contrast was injected and demonstrated excellent flow from the esophagus to the jejunum. The stricture had markedly improved.
a Endoscopic view of a gastrojejunal anastomotic stricture in a patient post-Roux-en-Y gastric bypass. b Insertion of an 18 × 60 Niti-S (Taewoong, Seoul, South Korea) fully covered self-expandable metal stent (FCSEMS). c Endoscopic suturing of the FCSEMS using the OverStitch (Apollo Endosurgery, Austin, TX, USA) endoscopic suturing device. d Endoscopic view of a patent FCSEMS after endoscopic placement of 4 sutures. e Endoscopic image of the FCSEMS in place and patent at 91 days with intact sutures. f Gastrojejunal anastomosis after stent removal using stent grasping forceps
– Video demonstration of the stent removal process using stent grasping forceps (MP4 52,440 kb)
Endoscopic evaluation at stent removal showed a patent stenosis in all evaluated patients. Findings at removal included a shallow ulceration at the distal end of the stent in one patient. One patient had their stent slightly dislodged proximally into the gastric pouch but still tethered to sutures. In one case, the proximal portion of the stent was frayed making it difficult to remove some sutures (Fig. 3). We did not consider the incidental EGD findings mentioned above to be adverse events because the patients were completely asymptomatic and these findings did not result in any further complications after stent removal.
Discussion
The development of stenosis in the post-bariatric surgery stomach is a common adverse event with limited endoscopic treatment options. Our case series is the first study to demonstrate that the use of an S-FCSEMS for the treatment of stenosis in the post-bariatric patient is technically feasible, safe, and efficacious. We have found that FCSEMS placed can remain in situ greater than 30 days and for up to 180 days (6 months) with a low rate of serious adverse event, indicating that long-term dwell is possible. Patients in this series experienced minimal pain post-procedure, demonstrating tolerability of this procedure.
Clinical outcomes showed excellent immediate clinical success (100%), with somewhat reduced short-term success (80%), due to stent migration from failure to remove the stent at the intended time. However, long-term clinical success was low, with 53.3% of patients developing symptom recurrence within the 17-month (range 7–24) follow-up period and requiring repeat treatment, medical intervention, or surgical intervention. Clinical success was thus seen mostly during the stent dwell time and was significantly decreased after stent removal, suggesting that long-term therapy may require multiple repeat treatments or revision surgery. In addition, patients with gastric stenosis had a higher rate of long-term clinical failure, with 66.7% of patients developing recurrence of symptoms, as compared with a 44.4% of patients who had GJ stricture. Even though the series was small and true comparisons cannot be made, we noted a trend towards better long-term outcomes in patients with GJ stricture. This may be attributable to the fact that the GJ stricture represents a true stenosis whereas the stenosis post-gastric sleeve is more functional in nature. Thus, it would not be surprising for the two groups to have similar short-term outcomes when the stent is in situ. However, this clinical success is short-lived in the gastric stenosis group. S-FCSEMS may be better suited for GJ stricture and would not be advised for gastric stenosis.
Typically, stenosis in the setting of post-surgical anatomy is managed conservatively first, and multiple interventions are performed before surgical intervention or gastric stenting is considered [16, 19]. In patients who are responsive to other therapies, the risk of potential stent migration and consequent adverse events outweighs the clinical benefit that is comparable to that achieved using balloon dilation, incision, or other methods. An additional concern with sutured stents is that leaving a stent in situ for > 4 weeks results in the potential for the development of tissue inflammation and/or ulceration. However, this case series shows success in preventing migration by suturing the stent without a significant increase in risk for ulceration or other adverse events. Particularly, we believe that the use of the softer, more pliable, Niti-S (Taewoong, Seoul, South Korea) FCSEMS in most cases prevented injury to the gastric wall [17]. Due to the success of this intervention in our small cohort, we believe further prospective studies should be performed evaluating this method. In particular, the safe use of this procedure would necessitate the elucidation of risk factors for migration and the development of an algorithm for determining the intended dwell time of each stent.
One advantage of placing S-FCSEMS is that it is repeatable. However, in our patient cohort, patients who received serial placement of S-FCSEMS were more likely to experience an adverse event, particularly stent migration. Eighty percent of patients who received serial stent placement experienced at least one stent migration. We hypothesize that repeated procedures that require sutures to be once again placed in the post-surgical stomach may not be as durable due to mild deformation, scarring, or inflammation of the mucosa and submucosa induced by the initial stent. Therefore, subsequent repeat suturing of a stent in the same location may result in gastric wall bites that do not adequately penetrate the full thickness of the gastric wall, compromising the long-term functionality of these sutures. Further study needs to be performed to ascertain if this is indeed true.
In post-bariatric surgery, the stomach has a small lumen, which makes maneuvering the endoscope difficult and endoscopic suturing technically challenging. However, our study had a 100% technical success rate, suggesting that this procedure is certainly technically feasible. Endoscopic suturing learning curve studies have shown efficiency attained between 7 and 38 cases, which is encouraging, but further studies may seek to look at the learning curve of stent suturing, particularly in the smaller gastric lumen of post-bariatric patients [20, 21]. The current OverStitch (Apollo Endosurgery, Austin, TX, USA) endoscopic suturing device requires the use of a double-channel therapeutic endoscope, limiting maneuverability and adoption. Future advances in the device model have the potential to make suturing technically easier and consequently make such procedures more accessible to patients.
Some important technical considerations in this procedure include size of stent, number of sutures, and suturing technique. Preference was to use a 60-mm-length stent in all patients. The rationale behind using a 60-mm-length stent was the concern for migration. Shorter stents, if they do migrate, are less likely to cause obstructive symptoms and serious adverse events. In some cases, when the distance between GJ and Roux limb was longer, an 80-mm-length stent was used instead to ensure the distal end did lie in the Roux limb. The goal in all patients was to place 4 equidistant sutures for each stent. However, this was not possible in all patients due to technical difficulty, specifically restricted luminal size. Another important goal was to use the tissue helix for each bite through the gastric wall. The belief was that full-thickness bites would minimize “cheese wiring” of the tissue through the stent. Furthermore, there was an emphasis on ensuring that we were not cinching too tightly to minimize tension on sutures. This is because we aimed to have some mobility in the stent, allowing for peristalsis to occur without compromising the attachment of the suture to the gastric wall. Previous reports have suggested that tighter sutures may go through the mucosa and remain only attached to the stent, rendering them non-functional [13]. Keeping sutures relatively loose also made it easier to cut the sutures during suture removal. Another method to minimize tension was to suture the stent where it was resting naturally, instead of forcing the stent to take a midline position in the lumen.
Concerns that arise when leaving stents in the gastric body for greater than 30 days include stent occlusion and mucosal ulceration. Based on experience from a previous study, we chose the Niti-S (Taewoong, Seoul, South Korea) stent in most cases because it results in less mucosal ulceration [16]. In this series, only one patient had ulceration after 342 days. Additionally, no patients had stent occlusion. These findings are promising and suggest that long-term dwell of these sutured stents may be safe. An interesting finding in our study was that in some patients, a part of the covered stent became effectively uncovered. The acidity of the stomach, movement of gastric contents through the stent, and the presence of sutures disrupting the stent covering are all factors which limit the time of dwell of these stent and necessitate stent exchange. In these cases, the inversion technique, used for partially covered stents would be the best method for stent retrieval [18]. In this study, 33.3% of patients had a stent dwell of 180 days (6 months) or greater. Stent migration occurred in 20% of our patients. Importantly, only 1 out of 15 patients experienced stent migration at less than 180 days (6 months). Our experience with this case series highlights the importance of close follow-up, both clinical and endoscopic, in patients with S-FCSEMS. Patients who were lost to follow-up and failed to present for stent removal cited symptom resolution/improvement and reluctance to remove the stent in the absence of any adverse effects as the reason for improper follow-up. Thus, stent migration could be eliminated with close follow-up and proper patient education about the importance of stent removal. As we accumulated clinical experience with S-FCSEMS, it also became apparent that an endoscopic follow-up is vital in patients with S-FCSEMS for > 60 days (2 months) due to concerns about stent migration. We suggest performing a relook endoscopy at 60 or 90 days post-insertion in every patient to determine whether stent removal is necessary or whether stent reinforcement with additional sutures is needed.
This study has all limitations inherent to a retrospective design. There is significant variability between patients in terms of case presentation, prior interventions, and intended time of dwell, which makes the results difficult to generalize. A future prospective study may consider standardizing the patients by performing treatment based on a predetermined algorithm taking into account patient history, failed interventions, and degree of stenosis. Another limitation is that all procedures were performed by an endoscopist with significant experience in endoscopic suturing. Endoscopists naïve to the use of endoscopic suturing may have a lower technical success rate, influencing outcomes. Finally, we cannot comment on the long-term clinical efficacy after stent removal due to the short term of follow-up.
Limitations of the procedure include the cost of the procedure and the additional procedural time required for endoscopic suturing, with a mean of 71.9-min room time in this study. Additionally, this procedure is technically difficult, and even when an endoscopist with significant suturing experience performed the procedure, stent migration remained an issue, particularly when patients received multiple consecutive stents. Additionally, long-term clinical success is not guaranteed. Since this therapy requires two procedures (stent insertion and removal) and often requires multiple consecutive treatments, serious discussions must be had with patients before moving forward with a treatment plan.
In conclusion, our case series is the first to demonstrate the technical feasibility of suturing FCSEMS in the post-surgical stomach. Using a standard suturing technique and preference for the Niti-S (Taewoong, Seoul, South Korea) FCSEMS, we achieved stent dwell of up to 90 days with a good safety profile and acceptable clinical success. However, clinical success seemed to be mostly limited to the duration of stent dwell and to patients with GJ stricture. With future advances in the endoscopic suturing system and stent size and design, S-FCSEMS have the potential to become a mainstay in the treatment of GJ stricture.
References
Kumbhari V, Cai JX, Schweitzer MA. Endoscopic management of bariatric surgical complications. Curr Opin Gastroenterol. 2015;31(5):359–67.
Burgos AM, Csendes A, Braghetto I. Gastric stenosis after laparoscopic sleeve gastrectomy in morbidly obese patients. Obes Surg. 2013;23(9):1481–6.
Parikh A, Alley JB, Peterson RM, et al. Management options for symptomatic stenosis after laparoscopic vertical sleeve gastrectomy in the morbidly obese. Surg Endosc. 2012;26(3):738–46.
Rebibo L, Hakim S, Dhahri A, et al. Gastric stenosis after laparoscopic sleeve gastrectomy: diagnosis and management. Obes Surg. 2016;26(5):995–1001.
Garcia-Garcia ML, Martin-Lorenzo JG, Liron-Ruiz R, et al. Gastrojejunal anastomotic stenosis after laparoscopic gastric bypass. Experience in 280 cases in 8 years. Cirugia espanola. 2014;92(10):665–9.
Ribeiro-Parenti L, Arapis K, Chosidow D, et al. Gastrojejunostomy stricture rate: comparison between antecolic and retrocolic laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2015;11(5):1076–84.
Eloubeidi MA, Talreja JP, Lopes TL, et al. Success and complications associated with placement of fully covered removable self-expandable metal stents for benign esophageal diseases (with videos). Gastrointest Endosc. 2011;73(4):673–81.
Eubanks S, Edwards CA, Fearing NM, et al. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206(5):935–8. discussion 8-9
Thomas T, Abrams KR, Subramanian V, et al. Esophageal stents for benign refractory strictures: a meta-analysis. Endoscopy. 2011;43(5):386–93.
Sharaiha RZ, Kim KJ, Singh VK, et al. Endoscopic stenting for benign upper gastrointestinal strictures and leaks. Surg Endosc. 2014;28(1):178–84.
Diana M, Swanström LL, Halvax P, et al. Esophageal covered stent fixation using an endoscopic over-the-scope clip. Mechanical proof of the concept and first clinical experience. Surg Endosc. 2015;29(11):3367–72.
Vanbiervliet G, Filippi J, Karimdjee BS, et al. The role of clips in preventing migration of fully covered metallic esophageal stents: a pilot comparative study. Surg Endosc. 2012;26(1):53–9.
Rieder E, Dunst CM, Martinec DV, et al. Endoscopic suture fixation of gastrointestinal stents: proof of biomechanical principles and early clinical experience. Endoscopy. 2012;44(12):1121–6.
Kantsevoy SV, Bitner M. Esophageal stent fixation with endoscopic suturing device (with video). Gastrointest Endosc. 2012;76(6):1251–5.
Ngamruengphong S, Sharaiha RZ, Sethi A, et al. Endoscopic suturing for the prevention of stent migration in benign upper gastrointestinal conditions: a comparative multicenter study. Endoscopy. 2016;48(9):808.
Agnihotri A, Barola S, Hill C, et al. An algorithmic approach to the management of gastric stenosis following laparoscopic sleeve gastrectomy. Obes Surg. 2017;27(10):2628–36.
Khashab MA, Besharati S, Ngamruengphong S, et al. Refractory gastroparesis can be successfully managed with endoscopic transpyloric stent placement and fixation (with video). Gastrointest Endosc. 2015;82(6):1106–9.
Hill C, Khalil BK, Barola S, et al. Inversion technique for the removal of partially covered self-expandable metallic stents. Obes Surg. 2018;28(1):161–8.
Bhayani NH, Swanstrom LL. Endoscopic therapies for leaks and fistulas after bariatric surgery. Surg Innov. 2014;21(1):90–7.
Saumoy M, Schneider Y, Zhou XK, et al. A single-operator learning curve analysis for the endoscopic sleeve gastroplasty. Gastrointest Endosc. 2018;87(2):442–7.
Hill C, El Zein M, Agnihotri A, et al. Endoscopic sleeve gastroplasty: the learning curve. Endosc Int Open. 2017;5(9):E900–e4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval Statement
For this type of study, formal consent is not required.
Informed Consent Statement
Informed consent does not apply.
Conflict of Interest
Mouen A Khashab is on the medical advisory board for Boston Scientific and Olympus America and is a consultant for Boston Scientific, Olympus America, and Medtronic. Anthony N Kalloo is a founding member, equity holder, and consultant for Apollo Endosurgery. Vivek Kumbhari is a consultant for Medtronic, Reshape Lifesciences, Boston Scientific, and Apollo Endosurgery. He also receives research support from ERBE USA and Apollo Endosurgery. All the other authors have nothing to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Video 1
– Video demonstration of the stent suturing process (MP4 296,017 kb)
Rights and permissions
About this article
Cite this article
Fayad, L., Simsek, C., Oleas, R. et al. Safety and Efficacy of Endoscopically Secured Fully Covered Self-Expandable Metallic Stents (FCSEMS) for Post-Bariatric Complex Stenosis. OBES SURG 29, 3484–3492 (2019). https://doi.org/10.1007/s11695-019-04021-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04021-0