Abstract
We aim to review the available literature on obese patients treated with robotic or laparoscopic sleeve gastrectomy, in order to compare the clinical outcomes and intraoperative parameters of the two methods. A systematic literature search was performed in PubMed, Cochrane Library and EBSCOhost databases, in accordance with the PRISMA guidelines. Sixteen studies met the inclusion criteria incorporating 29,787 patients. Robotic sleeve gastrectomy (RSG) technique showed significantly higher mean operative time and increased length of hospital stay. Post-operative incidence of leakage, wound infection and bleeding, along with weight reduction, were comparable. The majority of the studies assessing charges found increased cost in RSG population. Well-designed, randomized controlled studies, comparing RSG to laparoscopic sleeve gastrectomy (LSG), are necessary to assess further their clinical outcomes and cost-effectiveness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obesity is a rising morbid condition, while bariatric surgery continues to be the main therapeutic mode for a high rate of sustainable weigh loss [1]. A stand-alone bariatric procedure that is increasingly performed is the laparoscopic sleeve gastrectomy (LSG) [2]. In fact, LSG was the most frequent bariatric procedure in the USA in 2013 [3]. However, the need for fewer complication rate, smaller hospital stay and better cosmetic results has led to the introduction in use of robotic technology in bariatric surgery.
Robotic sleeve gastrectomy (RSG), using the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA), has been proposed as an alternative approach to conventional LSG. RSG offers to operators certain advantages, such as improved control, through enhanced wrist movement and articulation, and visualization [4]. As the number of studies assessing the feasibility of RSG increases, it is necessary to examine whether the results between the two techniques are at least equivalent. The purpose of this study is to summarize the existing evidence comparing the surgical outcomes of LSG and RSG.
Materials and Methods
Search Strategy and Articles Selection
The present study was conducted in accordance with the protocol agreed by all authors and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [5]. PubMed database, Cochrane Library and EBSCOhost were searched (last search: May 30, 2016) using the following terms in every possible combination: “robotic,” “robot-assisted,” “robotically,” “da vinci,” “robotic-assisted,” “laparoscopic,” and “sleeve gastrectomy.” Inclusion criteria were (1) original reports with ≥10 patients, (2) written in the English language, (3) published from 2010 to 2016, (4) conducted on human subjects and (5) reporting outcomes of LSG or RSG on obese patients. Two independent reviewers (VST, DEM) extracted the data from the included studies. Any discrepancies between the investigators about the inclusion or exclusion of studies were discussed with the guarantor author (DZ) in order to include articles that best matched the criteria, until consensus was reached. Moreover, the reference lists of all included articles were assessed for additional potentially eligible studies.
Data Extraction
For each eligible study, data were extracted relative to demographics (number of patients, mean age, sex, comorbidities, preoperative body mass index (BMI)) and to the intraoperative parameters and outcomes (mean operative time; length of hospital stay; bougie diameter; conversion rate; intraoperative and post-operative complications; charges; BMI after 1, 6 and 12 months and % excess weight loss (%EWL) after 1, 6 and 12 months). Moreover, categorical outcomes were 2 × 2 tabulated, referring patients presenting the outcome and patients free of the outcome, separately for the LSG and RSG groups. Regarding continuous outcomes, we extracted the mean, the standard deviation and the number of patients. In cases that standard deviation was not available, it was calculated using the available data.
Statistical Analysis
Regarding the categorical outcomes, the odds ratio (OR) and 95% confidence interval (CI) were calculated, based on the extracted data, by means of fixed-effects model (Mantel-Haenszel statistical method), where the number of studies providing data was sufficient. OR < 1 denoted that outcome was more frequent in the RSG group. Continuous outcomes were evaluated by means of weighted mean difference (WMD) with its 95% CI, using fixed-effects and random-effects (inverse variance statistical method) models, appropriately to calculate pooled effect estimates. In cases where WMD < 0, values in the RSG group were higher. Between-study heterogeneity was assessed through Cochran’s Q statistic and by estimating [6].
Quality and Publication Bias Assessment
The Newcastle-Ottawa Quality Assessment Scale (NOS) [7] was used as an assessment tool to evaluate non-randomized control trials (RCTs). The scale’s range varies from zero to nine stars, and studies with a score equal to or higher than five were considered to have adequate methodological quality to be included. There were no RCTs in the literature to be included. Two reviewers (VST, DEM) rated the studies independently, and final decision was reached by consensus.
The existence of publication bias could not be evaluated using the Egger’s formal statistical test [8], because the number of the included in the analysis studies was not adequate (less than ten), thus compromising substantially the power of the test.
Results
Article Selection and Patient Demographics
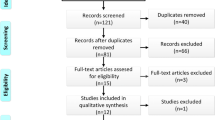
The flow diagram of the search of the literature is shown in Fig. 1. The characteristics of the included studies are summarized in Table 1. Sixteen studies were included in the qualitative synthesis [10–25]. The study design was retrospective in 9 [9–12, 15–20, 22, 23] and prospective in 4 studies [12, 14, 21, 22]. The included studies were conducted in the USA [11, 12, 16–21, 23], Greece [9, 10, 14, 24], Spain [22], India [13] and Brazil [21] and were published between 2011 and 2016. The RSG sample size ranged from 14 to 957 patients. The total sample size was 29,787; 27,535 patients treated with LSG and 2252 patients treated with RSG. Preoperative mean BMI was ≥30 kg/m2 in all included patients.
The most frequent comorbidities in patients undergoing LSG and RSG are presented in Table 2. The most prevalent comorbidity was hypertension (n = 327, 1.19% for the LSG group; n = 518, 23% for the RSG group). Other frequent comorbidities were diabetes mellitus (n = 181, 0.66% for the LSG group; n = 374, 16.61% for the RSG group), sleep apnea (n = 272, 0.98% for the LSG group; n = 480, 21.31% for the RSG group) and dyslipidemia (n = 93, 0.33% for the LSG group; n = 222, 9.86% for the RSG group). Further analysis of most frequent comorbidities is shown in Table 4. There were no significant differences.
Bougie Diameter
The use of bougie was assessed in this study (Table 3). Bougie was used in the following diameters: 32F [21, 22], 34F [18, 19], 36F [16, 24], 38F [13, 16, 17, 20] and 40F [12, 16]. In four studies [9, 10, 14, 15] (25%), we used endoscopes equivalent to 29F and 30F.
Mean Operative Time
Mean operative time ranged from 84.18 to 138 min for the LSG and from 95.5 to 148 min for the RSG (Table 3). Mean operative time was significantly greater in the RSG group (WMD −20.66 (−23.45, −17.88); p < 0.0001) as shown in Table 4 and Fig. 2.
Forest plot describing the differences in a incidence of leaks, b length of hospital stay and c mean operative time between robotic and standard laparoscopic sleeve gastrectomy. a Incidence of leaks was not significantly different between the robotic and the standard laparoscopic group. b Length of hospital stay was significantly greater in robotic than in standard laparoscopic sleeve gastrectomy. c Mean operative time was significantly greater in robotic than in standard laparoscopic sleeve gastrectomy
Length of Hospital Stay
The length of hospital stay ranged from 1.7 ± 1.8 to 4 ± 3 days for the RSG group, while it ranged from 1.2 ± 0.5 to 5.9 (4–13) days for the LSG group (Table 3). According to our analysis shown in Table 4 and Fig. 2, the length of hospital was greater in the RSG group (WMD −0.25 (−0.30, −0.20); p < 0.0001).
Conversions
Conversion rate in the RSG group was 0% in all studies measured, as shown in Table 3. In the LSG group, the number of conversions was zero in three studies [10, 19, 21], one in two studies [12, 17] and two in one study [24], with the conversion rate ranging from 0 to 2.5%.
Complications
The most prevalent post-operative complications are shown in Table 4. The risk of post-operative leakage was comparable between the two groups (OR 1.28 (CI 0.54, 3.03); p = 0.57). The frequency of wound infection was nearly similar (4.19 (0.20, 89.46); p = 0.36). Moreover, the risk of bleeding was similar for the LSG and RSG groups (1.76 (0.38, 8.09); p = 0.47). The rate of other complications reported was not significantly different (0.93 (0.51, 1.69); p = 0.81).
%EWL and Post-Operative BMI
In our study, we examined the post-operative %EWL and BMI after 1, 6 and 12 months. %EWL ranged between 18 and 29 after 1 month, between 34 and 67 after 6 months and between 48 and 67.3 after 12 months for the LSG group. In addition, %EWL for the RSG group ranged between 15.5 and 29.49 after 1 month, 39 and 66 after 6 months and between 48.89 and 65.5 after 12 months. BMI was calculated from 43 to 49 after 1 month, from 31.04 to 46 after 6 months and from 29.21 to 43 after 12 months for the LSG group. On the other hand, BMI ranged between 40 and 48 after 1 month, 31.28 and 41.5 after 6 months and between 27.14 and 39 after 12 months for the RSG group. Further meta-analysis was not performed due to the lack of comparative data.
Cost
Charges were assessed in five studies (Table 3). Four (80%) [15, 19, 21, 23] of them demonstrated significantly higher charges for the RSG procedure. The mean charges ranged between 4730 € and 49,498 reals for the LSG and between 5130 € and 56,646 reals for the RSG group. In one study [15], which compared LSG with RSG, the mean charges had a mean difference of $364.69 (p < 0.01). Pepper et al. [19] showed higher median total (p = 0.037) and perioperative cost (p = 0.001) for the RSG group. Schraibman et al. [21], also, found significantly higher cost for the RSG group (p < 0.001). In addition, Villamere et al. [23] showed significantly higher charges for the RSG group (p < 0.05). Diamantis et al. [14] found a difference of 400 € (4730 € for LSG and 5130 € for RSG), but they did not include in their analysis original purchase and maintenance costs. Meta-analysis was not performed due to lack of data.
Publication Bias
Publication bias was not calculated for the outcomes because of the small number of the studies that were included.
Discussion
This meta-analysis identified 16 articles describing LSG and RSG as two alternative bariatric procedures, measuring the patients’ outcomes and published between 2011 and 2016. No similar systematic review was identified through literature search. The articles included in this study bring us closer to linking the implementation of either method with improved standards of safety, efficiency and cost-effectiveness.
The present study demonstrates that RSG and LSG are well-tolerated, feasible and effective surgical approaches. The rate of comorbidities was evaluated for the LSG group and was relatively low. It may be related with lower mean BMI and rate of metabolic syndrome in the LSG population. According to previous studies [25], robot-assisted procedures are associated with greater mean operative time, due to the increased setup time. This is in accordance with our outcomes. In fact, in our study, mean operative time was significantly greater in the RSG group. Mean length of hospital stay was, also, significantly greater in the RSG group. However, Kannan et al. [17] showed that the RSG group had shorter hospital stay (p = 0.01) but with limited clinical impact. Moreover, both techniques are associated with small and comparable rates of complications and conversions, being significantly safe. Since stapling phase, in both groups, is not robotic, it would be interesting to examine the technique of oversewing or buttressing. However, the available data were not sufficient to address this technical aspect.
Leaks and hemorrhage are the main risks of bariatric procedures, due to the long stapled lines and gastrointestinal anastomosis. According to our findings, the incidence of leaks and hemorrhage were comparable between the two groups. No significant differences were reported.
From the studies included, two [15, 18] referred %EWL after 1 month in the LSG group and three [13, 15, 18] in the RSG group, four [9, 15, 16, 21] referred %EWL after 6 months in the LSG and six [12, 13, 15, 16, 19, 21] in the RSG group, four [8, 9, 15, 16] referred %EWL after 12 months in the LSG and four [13, 15, 16, 19] in the RSG group. The %EWL was comparable between the two methods and also comparable to previous meta-analysis of sleeve gastrectomy as a primary procedure [26]. In fact, in that study, published in 2009, the %EWL ranged between 36 and 85 [25].
Cost was also assessed in this study but was not further analyzed due to lack of available data. Five studies [14, 15, 19, 21, 23] analyzed the charges in the LSG group and five studies [14, 15, 19, 21, 23] in the RSG group. Total charges were approximately ranged from $8795 to 49,498 for LSG and from $10,556 to 56,646 for RSG [19, 23] in the USA, 4730 € for LSG and 5130 € for RSG in Greece [14] and 30,748.5 reals for LSG and 47,858.0 reals for RSG in Brazil [21]. Significantly higher charges for the RSG group were shown in four out of five (80%) studies [15, 19, 21, 23]. In only one study [14] that the difference was not significant. However, in this study, the purchase and maintenance charges were not included in the analysis [14]. The greater charges associated with robot-assisted surgery are primarily due to the initial cost of acquiring the robotic system and the semidisposable instruments [27].
This meta-analysis demonstrates the need for additional studies comparing LSG with the RSG. Ideally, these would be randomized controlled studies, with prospective design and longer follow-up. For rare events, such as conversions and mortality, a large sample is needed. The studies included offer specific linkage to patient outcomes, complication and weight loss.
The limitations of this systematic review and meta-analysis reflect the limitations of the studies included. Five studies [12, 14, 21, 22, 24] (31.25%) were prospective. There were no randomized controlled studies between the comparative studies included, thus posing a certain limitation in this study. The majority of the studies included [10–13, 16–21, 23] were retrospective (68.75%).
On the other hand, the strengths of this study are (1) the clear data extraction protocol, (2) the well-specified inclusion-exclusion criteria, (3) the search in three different databases, (4) the quality assessment of the included studies and (5) the detailed presentation of the results of data extraction and analysis.
Conclusion
This meta-analysis identified 16 unique peer-reviewed studies of LSG and RSG procedures with patient outcome data. These studies suggest that LSG and RSG are associated with comparable clinical outcomes, complications and %EWL. Mean operative time and length of hospital stay were significantly greater in the RSG group. The majority of studies showed significantly higher charges for RSG. These results should be interpreted with caution due to the lack of randomized controlled studies. Future studies with greater clarity in significant outcomes, as the %EWL, complications and charges are necessary to demonstrate the differences in efficacy between LSG and RSG.
References
Colquit JL, Picot J, Loveman E, et al. Surgery for obesity. Cochrane Database Syst Rev. 2009;2:CD003641.
Kueper MA, Kramer KM, Kirschniak A, et al. Laparoscopic sleeve gastrectomy: standardized technique of a potential standalone bariatric procedure in morbidly obese patients. World J Surg. 2008;32(7):1462–5.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery worldwide 2013. Obes Surg. 2015;25:1822–32.
Moser F, Horgan S. Robotically assisted bariatric surgery. Am J Surg. 2004;188(Supp):38S–44.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011 Available from www.cochrane-handbook.org
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Alexandrou A, Athanasiou A, Michalinos A, et al. Laparoscopic sleeve gastrectomy for morbid obesity: 5-year results. Am J Surgy. 2015; 209(2).
Alexandrou A, Michalinos A, Athanasiou A, et al. Laparoscopic sleeve gastrectomy for morbid obesity with intra-operative endoscopy: lessons we learned after 100 consecutive patients. Obes Surg. 2015;25:1223–8. doi:10.1007/s11695-014-1524-3.
Altieri MS, Yang J, Telem DA, et al. Robotic approaches may offer benefit in colorectal procedures, more controversial in other areas: a review of 168,248 cases. Surg Endosc. 2016;30:925–33. doi:10.1007/s00464-015-4327-2.
Ayloo S, Buchs NC, Addeo P, et al. Robot-assisted sleeve gastrectomy for super-morbidly obese patients. J Laparoendosc Adv Surg Tech. 2011; 21(4). doi: 10.1089/lap.2010.0398.
Bhatia P, Bindal V, Singh R, et al. Robot-assisted sleeve gastrectomy in morbidly obese versus super obese patients. J Soc Laparoendosc Surg. 2014; 18(3). doi: 10.4293/JSLS.2014.00099.
Diamantis T, Alexandrou A, Nikiteas N, et al. Initial experience with robotic sleeve gastrectomy for morbid obesity. Obes Surg. 2011;21:1172–9. doi:10.1007/s11695-010-0242-8.
Ecker BL, Maduka R, Ramdon A, et al. Resident education in robotic-assisted vertical sleeve gastrectomy: outcomes and cost-analysis of 411 consecutive cases. Surg Obes Relat Dis. 2015; 00–00 doi: 10.1016/j.soard.2015.05.011.
Elli E, Gonzalez-Heredia R, Sarvepalli S, et al. Laparoscopic and robotic sleeve gastrectomy: short- and long-term results. Obes Surg. 2015;25:967–74. doi:10.1007/s11695-014-1499-0.
Kannan U, Ecker BL, Choudhury R, et al. Laparoscopic hand-assisted versus robotic-assisted laparoscopic sleeve gastrectomy: experience of 103 consecutive cases. Surg Obes Relat Dis. 2016;12:94–9. doi:10.1016/j.soard.2015.07.011.
Moon RC, Stephenson D, Royal NA, et al. Robot-assisted versus laparoscopic sleeve gastrectomy: learning curve, perioperative, and short-term outcomes. Obes Surg. 2016; doi:10.1007/s11695-016-2131-2.
Pepper VK, Rager TM, Diefenbach KA, et al. Robotic vs. laparoscopic sleeve gastrectomy in adolescents; reality or hype. Obes Surg. 2016; doi:10.1007/s11695-015-2029-4.
Romero RJ, Radomir K, Rabaza JR, et al. Robotic sleeve gastrectomy: experience of 134 cases and comparison with a systematic review of the laparoscopic approach. Obes Surg. 2013;23:1743–52. doi:10.1007/s11695-013-1004-1.
Schraibman V, Macedo ALV, Epstein MG, et al. Comparison of the morbidity, weight loss, and relative costs between robotic and laparoscopic sleeve gastrectomy for the treatment of obesity in Brazil. Obes Surg. 2014;24:1420–4. doi:10.1007/s11695-014-1239-5.
Vilallonga R, Fort JM, Caubet E, et al. Robotic sleeve gastrectomy versus laparoscopic sleeve gastrectomy: a comparative study with 200 patients. Obes Surg. 2013;23:1501–7. doi:10.1007/s11695-013-1039-3.
Villamere J, Gebhart A, Vu S, et al. Utilization and outcome of laparoscopic versus robotic general and bariatric surgical procedures at Academic Medical Centers. Surg Endosc. 2015;29:1729–36. doi:10.1007/s00464-014-3886-y.
Zacharoulis D, Sioka E, Papamargaritis D, et al. Influence of the learning curve on safety and efficiency of laparoscopic sleeve gastrectomy. Obes Surg. 2012;22:411–5. doi:10.1007/s11695-011-0436-8.
Markar SR, Karthikesalingam AP, Venkat-Ramen V, et al. Robotic vs. laparoscopic Roux-en-Y gastric bypass in morbidly obese patients: systematic review and pooled analysis. Int J Med Rob. 2011;7(4):393–400.
Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis. 2009;5(4):469–75.
Morino M, Pellegrino L, Giaccone C, et al. Randomized clinical trial of robot-assisted versus laparoscopic Nissen fundoplication. Br J Surg. 2006;93(5):553–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Does not apply.
Non-Blinded COI Statement
Dr. Magouliotis has nothing to disclose.
Ms. Tasiopoulou has nothing to disclose.
Dr. Sioka has nothing to disclose.
Dr. Zacharoulis has nothing to disclose.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Rights and permissions
About this article
Cite this article
Magouliotis, D.E., Tasiopoulou, V.S., Sioka, E. et al. Robotic versus Laparoscopic Sleeve Gastrectomy for Morbid Obesity: a Systematic Review and Meta-analysis. OBES SURG 27, 245–253 (2017). https://doi.org/10.1007/s11695-016-2444-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2444-1