Abstract
Objective:
To evaluate early changes in glycemia, insulin physiology and gut hormone responses to an easily tolerated and slowly ingested solid, low-carbohydrate mixed meal test (MMT) following laparoscopic adjustable gastric banding (LAGB) or Roux-en-Y gastric bypass (RYGB) surgery.
Subjects/Methods:
This was a prospective non-randomized study. Plasma glucose, insulin and c-peptide (to estimate hepatic insulin extraction; %HIE), incretins (GIP, aGLP-1) and pancreatic polypeptide (PP) responses to the MMT were measured at 4–8 weeks before and after surgery in obese, metabolically healthy patients (RYGB=10F or LAGB =7F/1M). Supplementary clamp data on basal endogenous glucose production (EGP) and peripheral insulin action (Rd=rate of glucose disposal) and metabolic clearance rates of insulin (MCR-INS) were available in five of the RYGB patients. Repeated measures were appropriately accounted for in the analyses.
Results:
Following LAGB surgery, C-peptide and insulin MMT profiles (P=0.004 and P=0.0005, respectively) were lower with no change in %HIE (P=0.98). In contrast, in RYGB subjects, both fasting glucose and insulin (Δ=−0.66 mmol l–1, P⩽0.05 and Δ=−44.4 pmol l–1, P⩽0.05, respectively) decreased, and MMT glucose (P<0.0001) and insulin (P=0.001) but not c-peptide (P= 0.69) decreased. Estimated %HIE increased at fasting (Δ=8.4%, P⩽0.05) and during MMT (P=0.0005). Early (0–20 min) prandial glucose (0.27±0.26 versus 0.006±0.21 mmol l–1, P⩽0.05) and insulin (63(48, 66) versus 18(12, 24) pmol l–1, P⩽0.05) responses increased after RYGB. RYGB altered the trajectory of prandial aGLP-1 responses (treatment × trajectory P=0.02), and PP was lower (P<0.0001). Clamp data in a subset of RYGB patients showed early improvement in basal EGP (P=0.001), and MCR-INS (P=0.015).
Conclusion:
RYGB results in distinctly different changes in plasma glucose, insulin and gut hormone response patterns to a solid, slowly ingested low-carbohydrate MMT versus LAGB. Altered nutrient delivery, along with indirect evidence for changes in hepatic and peripheral insulin physiology, are consistent with the greater early improvement in glycemia observed after RYGB versus LAGB surgery.
Similar content being viewed by others
Introduction
Although patients are in significant caloric restriction during the first months after bariatric surgery, improvement in glycemic control occurs much sooner after Roux-en-Y gastric bypass (RYGB) than after the laparoscopic adjustable gastric band (LAGB) procedure.1 This earlier improvement is hypothesized to be due to, in addition to caloric restriction,2 anatomical alterations of the RYGB procedure including a diversion of nutrients away from the duodenum3, 4 and rapid delivery of incompletely digested nutrients to a more distal portion of the small intestine.5 Animal models indicate that nutrient delivery and subsequent altered vagal nutrient sensing in the gut is important for regulation of endogenous glucose production (EGP) and hepatic insulin sensitivity via neural and hormonal communication within a gut–brain–liver axis.6, 7, 8
Previous studies in humans report greater acute plasma glucose, insulin and GLP-1 responses to nutrient challenges following RYGB than LAGB surgery.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 These early post-operative differences are significant given that a robust early or acute insulin response is more important than overall plasma insulin levels for optimizing postprandial glycemia.20, 21 While perhaps useful in ‘unmasking’ differences in hormonal responses, many studies use oral glucose or carbohydrate (CHO)-based liquid meal challenges which induce nausea and vomiting in some patients, eliciting additional neural input and exaggerated glucose and hormonal responses. Indeed, these types of nutritional challenges are clinically contraindicated in patients after bariatric surgery.22 Many studies include subjects with type 2 diabetes mellitus (T2DM), making it challenging to disentangle the effects of the pre-operative T2DM severity and abrupt changes in glycemia that occur post-operatively, making it difficult to isolate the effect of the surgical methodology in these subjects. Finally, it is not always clear to what degree changes in peripheral plasma insulin concentrations are due to changes in pancreatic insulin secretion or changes in hepatic insulin clearance.23, 24, 25
Given these issues, our aim was to compare early (within 4–8 weeks) post-operative changes in fasting, prandial and post-prandial plasma concentrations of glucose, insulin, c-peptide and circulating hormones which may affect insulin secretion—including gastric inhibitory peptide (GIP), active glucagon-like peptide 1 (aGLP-1), and pancreatic polypeptide (PP)—in healthy, obese subjects without diabetes undergoing RYGB versus LAGB surgery. Rather than using an oral glucose tolerance test or high-CHO liquid meal challenge, we used an easily tolerated post-operative solid meal administered over 20 min in the exact same manner under strictly observed conditions before and after surgery. A subset of data available from five of the RYGB patients who underwent euglycemic–hyperinsulinemic clamps before and after surgery are provided as supplementary evidence for post-operative changes in basal EGP, insulin action (Rd=rate of glucose disposal) and metabolic clearance rates of insulin (MCR-INS) after the procedure.
Materials and methods
Study design
This was a prospective, non-randomized study of 18 patients approved for either RYGB(10female) or LAGB (8=7 female/1 male) recruited from a single-bariatric center. Inclusion criteria were: ⩾18 years of age and ⩽150 kg (weight limit of DXA scanner), no prior diagnosis of T2DM, and no history of taking medications for or evidence of hypertension, dyslipidemia or other metabolic or endocrine disorders. Within 2 months before their surgery date, patients reported to the clinical research unit in the morning after a minimum 10-h fast on two occasions for: (1) obtaining written, informed consent and medical history, physical examination and determination of glucose regulation status by oral glucose tolerance test, and (2) assessment of fasting blood and urine profiles to rule out kidney, liver, thyroid or hematological disorders and recent drug or medication use; administration of a mixed meal test (MMT); and assessment of body composition. Except for the oral glucose tolerance test, these studies were repeated in the same manner 4–8 weeks after surgery. Five RYGB patients were available for additional visits to complete pre- and post-operative euglycemic–hyperinsulinemic clamps with tracer. The study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Adiposity
Body composition was estimated by total body dual energy X-ray absorptiometry (DPX-L; GE/Lunar Radiation, Madison, WI) from which percent body fat, fat mass and fat-free mass (FFM) were calculated.
Oral glucose tolerance test
After a 10–12 h overnight fast, glucose tolerance was determined by 75-g oral glucose tolerance test with measurements of fasting, 30 min, 1-h and 2- h plasma glucose and insulin concentrations and classified according to the 2003 ADA diagnostic criteria.26
Mixed meal test
Patients were admitted the morning after a 10–12-h overnight fast. An intravenous catheter was placed in an antecubital vein and fasting blood samples were obtained at −15 and 0 min prior to the start of the MMT. Patients were given a 200 kcal breakfast with a macronutrient composition of 15% carbohydrate (7.5 g), 65% fat (14 g), and 20% protein (10 g) consisting of scrambled egg, peanut butter and sliced banana. Patients were instructed to chew thoroughly before swallowing. To assure that the MMT be well-tolerated at 1–2 months’ post-surgery, meals were consumed over 20 min with ~25% of the calories consumed over each 5- min period to maintain a consistent rate of consumption across meals and participants. Blood samples for measurement of plasma glucose, insulin, c-peptide, PP, GIP and aGLP-1 were drawn from the catheter before, mid-way and at completion of the meal (at 0, 10, 20 min) and then additionally at 15, 30, 60, 90, 120 and 180 min after completion of the meal.
Euglycemic–hyperinsulinemic clamp
The euglycemic–hyperinsulinemic clamp was performed to assess insulin action (Rd) at physiological hyperinsulinemia.27, 28 Briefly, after an overnight fast a catheter was placed in the antecubital vein and a primed continuous (3-3H) glucose infusion was started to determine EGP. A second catheter was inserted retrograde into a vein on the dorsum of the hand for blood sampling and the hand kept heated (60 °C) for the duration of the study. At least 2 h after starting the (3-3H) glucose infusion, a primed continuous intravenous insulin infusion was administered for 100 min at a constant rate of 40 mU m−2 min−1. Arterialized blood samples were collected every 5 min for plasma glucose determination and a 20% glucose infusion was adjusted to maintain the plasma glucose concentration at about 5.6 mmol l–1. Blood samples for measurement of (3-3H) glucose specific activity and plasma glucose and insulin concentrations were collected at the end of the basal period (22, 15, 8 and 1 min prior to insulin infusion) and every 10 min during the final 40 min of insulin infusion.
Clamp measures
Under fasting conditions, basal EGP was calculated as the (3-3H) glucose infusion rate divided by the steady-state plasma (3-3H) glucose specific activity. Whole-body insulin action (Rd) was determined during the last 40 min of the insulin infusion. EGP and Rd were normalized to estimated metabolic body size (EMBS), which is directly derived from FFM but takes into account the intercept of the relation between metabolic rate and FFM (−17.7 kg in our laboratory (that is, EMBS=FFM+17.7 kg).29 Rd was further corrected for individual variation in average plasma glucose concentrations during the last 40 min of the clamp30 by normalizing to a reference value 5.55 mmol l–1. The metabolic clearance rate of insulin (MCR-INS) was defined as insulin infusion rate divided by incremental (clamp minus basal) plasma insulin concentration during the last 40 min of the insulin infusion. Because of significant differences in plasma insulin levels during insulin infusion for pre- versus post-operative clamps, changes in peripheral insulin action were assessed from differences in the slope of Δglucose/Δinsulin where Δglucose= (Rd–Basal EGP) and Δinsulin= (steady-state plasma insulin during clamp-fasting plasma insulin).
Surgical techniques
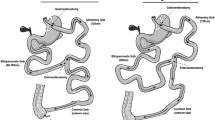
All laparoscopic RYGB and LAGB procedures were performed at a single-bariatric surgery center. RYGB was performed using a surgical technique similar to that reported by Wittgrove and Clark.31 In all cases, the anterior and posterior branches of the vagus nerve were identified and preserved. The anterior vagus was divided when the pouch was created but the proximal stomach pouch continued to enjoy innervation by both the anterior and posterior vagus. The innervation for the posterior vagus was preserved in the distal gastric remnant as well by limiting dissection directly onto the lesser curve of the stomach. LAGB was placed through a pars flaccida approach, using 1–3 permanent sutures to secure the fundus to the pouch.32 Both the anterior and posterior vagi were encircled within the band. A small partial fill of the band was done 3–4 weeks after surgery.
Analytic procedures
Plasma glucose concentrations were determined by the glucose oxidase method (Beckman Instruments, Fullerton, CA) and plasma insulin concentrations by an automated analyzer (Tosoh AIA System Analyzer, Tokyo). Plasma samples for measurement of PP, GIP and aGLP-1 samples were collected by specified methods and additives necessary for determination using multiplex (Luminex, Austin, TX, USA) technology (LINCO Diagnostics of Millipore, St. Charles, MO; Human Gut Hormone Panel 5plex). Plasma c-peptide was measured with ELISA techniques. Specifically, samples were collected in chilled EDTA tubes with immediate addition of DPPIV inhibitor (Millipore, 10 μl ml–1 blood for aGLP-1) or aprotinin (for c-peptide) and centrifuged at 4 °C within 30 min. Samples were stored at <−70 °C until assays were performed at the core NIDDK laboratory (Bethesda, MD). Samples were run in duplicate using a single assay for each hormone; intra-assay coefficient of variations were ⩽10% for all assays.
Hepatic insulin extraction (HIE)
The fraction of secreted insulin extracted from the liver before entering peripheral circulation was estimated from plasma samples during the MMT using the equation:
Statistical analysis
Statistical analyses were performed using the programs of the SAS Institute Inc. (Cary, NC). Based on previous reports,13, 33 we had 80% power at alpha of <0.05 to detect a difference in MMT 20 min’ insulin. Values are presented either as means (±s.d.) or, for variables with unequal distribution (that is, insulin, aGLP-1, GIP, PP), log transformed prior to analyses and presented as medians (25, 75 quartiles). Differences in physical and metabolic characteristics between the RYGB and LAGB groups were assessed using Student’s t-test or the Wilcoxon rank sum nonparametric test. Paired t-tests were used to compare pre- and post-operative values within individuals for plasma fasting concentrations and acute meal completion plasma responses (concentration at min 20 minus fasting concentration) of glucose, c-peptide, insulin, PP, GIP, aGLP-1 and measurements from the clamp (EGP, MCR-INS, Rd). Within each surgery group, comparisons of the trajectories of plasma responses for all measured factors during and for 3 h after meal ingestion between the pre- and post-op visits (TREATMENT) were assessed with analysis of variance using mixed models accounting for repeated measures. Both linear and quadratic terms to describe the trajectories over time were assessed in each model. Pre- and post-op differences were assessed by including an interaction term between TREATMENT and all relevant polynomial terms (time; time2) describing the response trajectories. Only the significant level of the greatest polynomial term describing the relationship with time is reported in the figures (referred to as trajectory). The effects of surgery and associated changes in body weight on changes in fasting (FPG) and meal test plasma glucose response curves were assessed in generalized linear regression and mixed model analyses, respectively. The significance level was set at <0.05.
Results
Subject pre- and post-operative characteristics and fasting profiles
Physical and fasting metabolic characteristics at pre- and post-op visits are presented and compared between the RYGB and LAGB groups (Table 1). Except for greater BMI (P=0.008) and lower fasting plasma GIP (P=0.03) in RYGB compared with LAGB, groups were similar before surgery. There were no differences in assessment time between the two groups (prior; RYGB: 39.6±6.6 versus LAGB: 28.8±4.3 days; P=0.21; after: RYGB: 38.1±4.2 versus LAGB: 43.5±4.1 days; P=0.42).
Both groups had significant (P⩽0.01) decreases in body weight, BMI, fat and FFM after surgery. The RYGB group had greater absolute and %weight loss (%WL), BMI and FFM (and subsequently a higher percent body fat) than the LAGB group after surgery.
Fasting plasma c-peptide significantly decreased after LAGB surgery. After RYGB, FPG and insulin (and therefore, HOMA-IR) significantly decreased and calculated % hepatic insulin extraction significantly increased.
Acute plasma responses during meal ingestion
Changes and differences in acute plasma responses during meal ingestion (defined as change from meal initiation to meal termination or minutes 0–20) were analyzed within and between surgery groups, respectively, at pre- and post-op visits (Table 2). RYGB and LAGB surgery groups were similar in meal responses prior to surgery and there were no significant differences in meal responses before and after LAGB. For RYGB, despite lower fasting concentrations after surgery, acute plasma responses of both glucose and insulin were greater (in absolute and relative terms) during this early phase. In contrast, the acute plasma PP MMT response was lower after RYGB.
Meal test plasma response curves before and after surgery
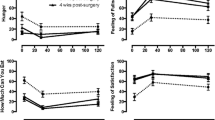
Prior to surgery, the RYGB and LAGB groups had similar plasma profiles and MMT responses for glucose, insulin, c-peptide, PP, GIP, aGLP-1 and estimated % hepatic insulin extraction during the 20-minute meal plus 3-h post-prandial period. Except for the aGLP-1 response in LAGB subjects, all measured factors demonstrated a significant response to the slowly ingested 200 kcal solid, low-carbohydrate meal (trajectory in Figures 1 and 2).
Plasma glucose (a and b), c-peptide (c and d) and insulin (e and f), and calculated % hepatic insulin extraction (HIE; g and h) responses (means; s.e.m.) during (20 min) and following (3 h) ingestion of a solid MMT before and 4–8 weeks after RYGB surgery or LAGB. RYGB: pre-operative=open squares; dashed lines; post-operative=closed squares; solid lines. LAGB: pre-operative=open circles; dashed lines; post-operative closed circles, solid lines. Results from mixed model analyses are provided for changes over time (trajectory), surgery effect (treatment) and interaction of trajectory × treatment.
Plasma PP (a and b), GIP (c and d) and aGLP-1 (e and f) responses (means; s.e.m.) during (20 min) and following (3 h) ingestion of a solid MMT before and 4–8 weeks after RYGB surgery or LAGB. RYGB: pre-operative=open squares; dashed lines; post-operative= closed squares; solid lines. LAGB: pre-operative=open circles; dashed lines; post-operative closed circles, solid lines. Results from mixed model analyses are provided for changes over time (trajectory), surgery effect (treatment) and interaction of trajectory × treatment.
When comparing main effect differences between pre- to post-op visits within each surgery group (TREATMENT), only the RYGB group had a significant shift downward in the overall plasma glucose MMT response after surgery although both groups had significant post-operative decreases in the overall insulin MMT response (Figure 1). In addition, the shape of the trajectory of the insulin response to the MMT was different post-RYGB (indicated by the trajectory × treatment interaction term), with an earlier peak response. A significant downward shift in the plasma MMT profiles of c-peptide was observed after LAGB, but not RYGB. Consequently, estimated %hepatic insulin extraction did not change after LAGB surgery but increased significantly after RYGB surgery (Figure 1). The magnitude and patterns of plasma PP, GIP and aGLP-1 MMT responses did not change significantly after LAGB. In contrast, the plasma aGLP-1 response to the MMT demonstrated an increased peak and prolonged response (trajectory × treatment, P=0.05) and overall plasma MMT responses of GIP and PP were lower after RYGB (Figure 2).
Associations between changes in body composition and glycemia
With both groups combined, weight change (WTΔ) was associated with FPGΔ (r=0.51, P=0.03). When including WTΔ in mixed models, RYGB surgery had an independent effect on lowering overall plasma glucose MMT profiles (fixed effects: trajectory, P=0.02; WTΔ and TREATMENT, both P<0.0001).
Measurements from the clamp
(Figures 3a–c) In the subset of five RYGB patients who had pre- and post-op euglycemic–hyperinsulinemic clamps, both basal EGP and steady-state plasma insulin concentrations during insulin infusion were lower (P=0.001 and 0.015, respectively; Figures 3a and b) with a subsequently higher calculated whole-body MCR-INS after RYGB surgery (pre: 0.52±0.12 l min–1, post: 0.64±0.09 l min–1, P=0.003). Improvement in peripheral insulin action was evident from a greater Δglucose/Δinsulin before and during the clamp after RYGB surgery (pre=0.014 ±0.006, post=0.023 ±0.006, P=0.03; Figure 3c). Because EGP was completely suppressed during clamps both before and after surgery, we were unable to directly assess hepatic insulin action.
Clamp measurements before and 4–8 weeks after RYGB surgery (n=5). Individual changes within 4–8 weeks after RYGB surgery in (a) basal EGP, (b) steady-state plasma insulin concentrations and (c) the mean relationships of glucose disposal rate (Rd) to plasma insulin concentrations from fasting state to euglycemic–hyperinsulinemia before (open triangles, dashed line) and after (closed triangle, solid line) RYGB surgery (insulin infusion=40 mU m–2 min–1; with 3-3H-glucose tracer). Fasting Rd was assumed to be equal to basal EGP (Ra, steady-state assumption). P value is for pre- versus post-operative differences in Δglucose/Δinsulin where Δglucose= (Rd–basal EGP) and Δinsulin= (steady-state plasma insulin during clamp-fasting plasma insulin).
Discussion
In obese but otherwise healthy patients, RYGB and LAGB procedures resulted in distinctly different early changes in body composition, and fasting, prandial and post-prandial response patterns of plasma glucose, insulin, and the gut hormones GIP, aGLP-1 and PP by 4–8 weeks after surgery. Specifically, RYGB patients had a greater loss of FFM, significant decreases in fasting and post-prandial plasma glucose and insulin concentrations, enhanced acute plasma glucose and insulin responses during meal ingestion, increased estimated hepatic insulin clearance rates, alterations in plasma aGLP-1 MMT response patterns, and attenuation of MMT plasma GIP and PP profiles. Clamp data from a subset of RYGB subjects, pre- and post-surgery, confirmed early improvement in basal EGP and peripheral insulin action and clearance after gastric bypass surgery. Collectively, these differences are consistent with the greater improvement in glycemia observed after RYGB than LAGB surgery.
Improvement in glycemia after any bariatric surgery procedure is attributed to caloric restriction and subsequent weight loss,2 and, specifically to RYGB procedure, anatomical alterations3, 4 and rapid delivery of incompletely digested nutrients to a more distal portion of the small intestine.5 In our study, mean weights before and after surgery were similar between RYGB and LAGB groups and all patients had lost a significant amount of weight at the time of the post-op measurements. However, by 8 weeks, there was greater weight loss after RYGB than LAGB due to a greater FFM loss. Early accelerated loss of FFM particularly after RYGB has been previously reported.34, 35
Weight loss was associated with an overall decrease in FPG in our combined cohort but RYGB still had an independent effect on lowering overall plasma glucose MMT profiles. Studies with equal early weight losses between RYGB and gastric restrictive surgeries9, 10, 14, 36, 37 or between RYGB patients and matched dietary caloric restriction controls18, 38 have reported greater improvements in fasting and/or post-prandial plasma glucose regulation after RYGB. Similar decreases in FPG after either LAGB or RYGB surgery has been reported,13 but this was after a longer post-operative interval (⩾4–6 months).
Early improvement in glycemia after RYGB is attributed to anatomical alterations that result in direct jejunal nutrient delivery.3, 4 Although absolute plasma concentrations of glucose and insulin were lower after both RYGB and LAGB surgery, the RYGB procedure resulted in absolute and percent increases in early meal test (0–20 min) responses, as well as post-operative changes in overall plasma insulin and aGLP-1 meal test response patterns. Enhanced early glucose and insulin responses to nutrients concur with studies that have looked at RYGB alone,17, 22, 39 or in comparison to LAGB surgery9, 10, 11, 12, 13, 14, 15, 16 using high-CHO liquid challenges. Because the pattern of the early insulin response to a nutrient challenge is at least as, if not more, important than absolute plasma insulin levels for improving post-prandial glycemia,20, 21 these differences in acute meal plasma glucose and insulin responses may at least partially account for the greater improvement in overall glycemia that was observed after RYGB than LAGB surgery.
A small (~0.28 mmol l–1) acute increase in glucose lead to 150% increase in the acute insulin response indicating that modest changes in plasma glucose responses robustly affect insulin secretion. In contrast to earlier studies, the greatest proportion of the rise in plasma aGLP-1 in the RYGB group occurred later and not concurrently with or before the acute rise in insulin10, 15, 16 nor was there a greater MMT response in c-peptide profiles.13, 14, 22 These differences could be due to measuring active versus total GLP-1, exclusion of T2DM, and/or use of a slowly ingested lower-CHO, higher fat, solid meal test compared to other studies.
The rate of nutrient delivery to the gut is a key factor in regulating glycemia.40, 41 In humans, direct delivery of nutrients bypassing the duodenum and proximal jejunum via a nasogastric tube enhanced insulin sensitivity and clearance in both normal glucose tolerant and T2DM patients.42 Animal models indicate that nutrient sensing in the gut regulates EGP and hepatic insulin sensitivity via neural-hormonal communication within a gut–brain–liver axis.6, 7, 8 In humans, plasma PP can be used as a marker for vagal efferent signaling to the pancreas.43 Interestingly, the meal test plasma profile of PP was attenuated after RYGB, but not LAGB surgery consistent with other reports of diminished post-prandial PP response within 2 weeks after RYGB.22, 44
PP affects satiety and slows gastric emptying,45 but less is understood about other potential metabolic actions. Increased plasma PP levels can prolong the postprandial plasma glucose rise and delay meal-induced insulin responses45—actions which are compatible with the lower plasma PP levels, we observed after RYGB surgery. In patients with T2DM, lower plasma PP release after diet-induced weight loss was associated with improved ß-cell glucose sensitivity and function.46 These collective observations indicate that changes in plasma PP may explain the improved blood glucose regulation following RYGB.
Previous euglycemic–hyperinsulinemic clamp studies with tracers demonstrated that caloric restriction alone46 or RYGB surgery47, 48, 49, 50 improved hepatic insulin sensitivity and/or decreased EGP. Using a tracer during a liquid MMT, Bradley et al13 reported decrease in EGP 4–6 months after RYGB, but not LAGB surgery, despite similar weight loss and improvements in insulin action and B-cell activity. Our MMT results indicate that the decrease in fasting and post-prandial plasma insulin concentrations after RYGB were the result of increased hepatic insulin clearance rather than lower pancreatic insulin secretion.
In a limited number of subjects, our clamp data demonstrated decreased basal EGP with a lower fasting plasma insulin, increased whole-body MCR-INS, and improved peripheral insulin action. Other studies failed to find early improvement in whole-body glucose uptake rates after RYGB.50, 51, 52 We found a significant difference in the slope of Δglucose/Δinsulin from the basal to insulin-stimulated state after surgery. These results indicate that after RYGB, both insulin action and clearance were improved, however, because of the small sample size, we cannot draw definitive conclusions from our clamp data.
These data should be considered in the context of certain limitations. Although hepatic insulin clearance was estimated with indirect measures, our results concur with other human studies.39, 44, 50 Gastric emptying rates were not measured but meals were carefully delivered in the exact same manner so changes in plasma hormone response patterns most likely represent changes in nutrient delivery or absorption rates. We do not have data on plasma FFA levels which might have been informative as another potential mechanism for changes in liver metabolism.44, 50, 53 Finally, clamp data was only available from the RYGB group so we cannot speculate if similar changes in EGP or insulin action also occurred after LABG surgery. Although HOMA-IR—an estimate of fasting hepatic insulin sensitivity54—did decrease after LABG surgery, it was to a lesser degree than after RYGB surgery and was more so a function of a decrease in fasting plasma insulin than glucose.
A novel aspect and strength of our study was the use of a slowly ingested solid meal that was easily tolerated by all the patients and avoided any nausea or vomiting which might confound interpretation of plasma hormonal responses. The MMT consisted of foods preferred early after bariatric surgery and therefore was representative of their dietary intake. Without a glucose challenge, we demonstrated similar changes in the patterns of hormone responses as in other studies, but without exaggerated spikes in plasma glucose or insulin. Our results confirm that regardless of the nutrient challenge used, altered nutrient delivery—as reflected in changes in early hormone response patterns—is an important factor for regulation of glycemia. It also validates the current clinical guidelines for avoiding high-carbohydrate challenges early after surgery to prevent the large glucose spikes observed in other studies which may have potential adverse cardiovascular consequences.13
In obese but otherwise healthy patients, RYGB and LAGB procedures resulted in distinctly different early changes in body composition, and fasting, prandial and post-prandial responses of plasma glucose, insulin, GIP, aGLP-1 and PP by 4–8 weeks after surgery. The changes in acute glucose and insulin meal responses coupled with the evidence from the clamp data indicating early improvements in basal EGP, insulin clearance and insulin action observed after RYGB may explain the improved glycemia observed after RYGB compared to LAGB surgery.
References
Cummings DE . Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009; 33: S33–S40.
Camastra S, Gastaldelli A, Mari A, Bonuccelli S, Scartabelli G, Frascerra S et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 2011; 54: 2093–2102.
Pories WJ, Caro JF, Flickinger EG, Meelheim HD, Swanson MS . The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville Gastric Bypass. Ann Surg 1987; 206: 316–323.
Rubino F, Marescaux J . Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004; 239: 1–11.
Strader AD, Vahl TP, Jandacek RJ, Woods SC, D'Alessio DA, Seeley RJ . Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 2005; 288: E447–E453.
Wang PY, Caspi L, Lam CK, Chari M, Li X, Light PE et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature 2008; 452: 1012–1016.
Breen DM, Rasmussen BA, Kokorovic A, Wang R, Cheung GW, Lam TK . Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med 2012; 18: 950–955.
Lam TK . Neuronal regulation of homeostasis by nutrient sensing. Nat Med 2010; 16: 392–395.
le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006; 243: 108–114.
Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ . Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 2007; 3: 597–601.
Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L . Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008; 16: 298–305.
Bose M, Machineni S, Olivan B, Teixeira J, McGinty JJ, Bawa B et al. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity (Silver Spring) 2010; 18: 1085–1091.
Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E et al. Gastric bypass and banding equally improve insulin sensitivity and beta cell function. J Clin Invest 2012; 122: 4667–4674.
Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010; 34: 462–471.
Salehi M, Prigeon RL, D'Alessio DA . Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011; 60: 2308–2314.
Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007; 30: 1709–1716.
Falken Y, Hellstrom PM, Holst JJ, Naslund E . Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab 2011; 96: 2227–2235.
Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008; 93: 2479–2485.
Magkos F, Bradley D, Eagon JC, Patterson BW, Klein S . Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on gastrointestinal metabolism of ingested glucose. Am J Clin Nutr 2016; 103: 61–65.
Groop PH, Melander A, Groop LC . The relationship between early insulin release and glucose tolerance in healthy subjects. Scand J Clin Lab Invest 1993; 53: 405–409.
Pratley RE, Weyer C . The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia 2001; 44: 929–945.
Jacobsen SH, Olesen SC, Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg 2012; 22: 1084–1096.
Polonsky KS, Rubenstein AH . C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes 1984; 33: 486–494.
Campioni M, Toffolo G, Basu R, Rizza RA, Cobelli C . Minimal model assessment of hepatic insulin extraction during an oral test from standard insulin kinetic parameters. Am J Physiol Endocrinol Metab 2009; 297: E941–E948.
Bojsen-Moller KN, Dirksen C, Jorgensen NB, Jacobsen SH, Serup AK, Albers PH et al. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes 2014; 63: 1725–1737.
Expert Committee on the D, Classification of Diabetes M. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003; 26: S5–S20.
Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Jarvinen H, Freymond D et al. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med 1988; 318: 1217–1225.
Steele R . Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci 1959; 82: 420–430.
Lillioja S, Bogardus C . Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes Metab Rev 1988; 4: 517–540.
Best JD, Taborsky GJ Jr., Halter JB, Porte D Jr . Glucose disposal is not proportional to plasma glucose level in man. Diabetes 1981; 30: 847–850.
Wittgrove AC, Clark GW . Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3-60 month follow-up. Obes Surg 2000; 10: 233–239.
Varela JE, Hinojosa MW, Nguyen NT . Perioperative outcomes of bariatric surgery in adolescents compared with adults at academic medical centers. Surg Obes Relat Dis 2007; 3: 537–540.
Hansen EN, Tamboli RA, Isbell JM, Saliba J, Dunn JP, Marks-Shulman PA et al. Role of the foregut in the early improvement in glucose tolerance and insulin sensitivity following Roux-en-Y gastric bypass surgery. Am J Physiol Gastrointest Liver Physiol 2011; 300: G795–G802.
Zalesin KC, Franklin BA, Lillystone MA, Shamoun T, Krause KR, Chengelis DL et al. Differential loss of fat and lean mass in the morbidly obese after bariatric surgery. Metab Syndr Relat Disord 2010; 8: 15–20.
Levitt DG, Beckman LM, Mager JR, Valentine B, Sibley SD, Beckman TR et al. Comparison of DXA and water measurements of body fat following gastric bypass surgery and a physiological model of body water, fat, and muscle composition. J Appl Physiol (1985) 2010; 109: 786–795.
Pattou F, Beraud G, Arnalsteen L, Seguy D, Pigny P, Fermont C et al. Restoration of beta cell function after bariatric surgery in type 2 diabetic patients: a prospective controlled study comparing gastric banding and gastric bypass. Obes Surg 2007; 17: 1041–1043.
Lee W, Lee Y-C, Chen J-C, Ser K-H, Chen S-C, Lin C-M . PL-03: A randomized trial comparing laparoscopic sleeve gastrectomy versus gastric bypass for the treatment of type 2 diabetes mellitus: Preliminary report. Surg Obes Relat Dis 2008; 4: 290.
Plum L, Ahmed L, Febres G, Bessler M, Inabnet W, Kunreuther E et al. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity (Silver Spring) 2011; 19: 2149–2157.
Bojsen-Moller KN, Dirksen C, Jorgensen NB, Jacobsen SH, Hansen DL, Worm D et al. Increased hepatic insulin clearance after Roux-en-Y gastric bypass. J Clin Endocrinol Metab 2013; 98: E1066–E1071.
Chandarana K, Batterham RL . Shedding pounds after going under the knife: metabolic insights from cutting the gut. Nat Med 2012; 18: 668–669.
Sarruf DA, Bonner-Weir S, Schwartz MW . New clues to bariatric surgery's benefits. Nat Med 2012; 18: 860–861.
Salinari S, Carr RD, Guidone C, Bertuzzi A, Cercone S, Riccioni ME et al. Nutrient infusion bypassing duodenum-jejunum improves insulin sensitivity in glucose-tolerant and diabetic obese subjects. Am J Physiol Endocrinol Metab 2013; 305: E59–E66.
Schwartz TW . Pancreatic polypeptide: a hormone under vagal control. Gastroenterology 1983; 85: 1411–1425.
Campos GM, Rabl C, Havel PJ, Rao M, Schwarz JM, Schambelan M et al. Changes in post-prandial glucose and pancreatic hormones, and steady-state insulin and free fatty acids after gastric bypass surgery. Surg Obes Relat Dis 2014; 10: 1–8.
Schmidt PT, Naslund E, Gryback P, Jacobsson H, Holst JJ, Hilsted L et al. A role for pancreatic polypeptide in the regulation of gastric emptying and short-term metabolic control. J Clin Endocrinol Metab 2005; 90: 5241–5246.
Kahleova H, Mari A, Nofrate V, Matoulek M, Kazdova L, Hill M et al. Improvement in beta-cell function after diet-induced weight loss is associated with decrease in pancreatic polypeptide in subjects with type 2 diabetes. J Diabetes Complications 2012; 26: 442–449.
Promintzer-Schifferl M, Prager G, Anderwald C, Mandl M, Esterbauer H, Shakeri-Leidenmuhler S et al. Effects of gastric bypass surgery on insulin resistance and insulin secretion in nondiabetic obese patients. Obesity (Silver Spring) 2011; 19: 1420–1426.
Dunn JP, Abumrad NN, Breitman I, Marks-Shulman PA, Flynn CR, Jabbour K et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care 2012; 35: 137–142.
Immonen H, Hannukainen JC, Iozzo P, Soinio M, Salminen P, Saunavaara V et al. Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non-diabetic patients. J Hepatol 2014; 60: 377–383.
de Weijer BA, Aarts E, Janssen IM, Berends FJ, van de Laar A, Kaasjager K et al. Hepatic and peripheral insulin sensitivity do not improve 2 weeks after bariatric surgery. Obesity (Silver Spring) 2013; 21: 1143–1147.
Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg 2010; 14: 15–23.
Lima MM, Pareja JC, Alegre SM, Geloneze SR, Kahn SE, Astiarraga BD et al. Acute effect of roux-en-y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 2010; 95: 3871–3875.
Viljanen AP, Iozzo P, Borra R, Kankaanpaa M, Karmi A, Lautamaki R et al. Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J Clin Endocrinol Metab 2009; 94: 50–55.
Wallace TM, Matthews DR . The assessment of insulin resistance in man. Diabetes Med 2002; 19: 527–534.
Acknowledgements
The authors acknowledge the contributions of the clinical and dietary staffs of the Scottsdale Bariatric Center and Phoenix NIDDK Obesity and Diabetes Clinical Research Unit. We are indebted to the bariatric surgery patients who participated as research volunteers. The study was funded by Intramural Research funding of NIDDK.
Author contributions
JB wrote the study design, collected metabolic data, did statistical analysis and wrote the manuscript. RB wrote the study design, performed the surgeries and wrote/edited the manuscript. MST assisted on statistical analysis, and reviewed/edited the manuscript. KLV wrote/edited the manuscript. SV designed and supervised meal administration, wrote/edited the manuscript. JK wrote the study design, assisted with statistical analysis and reviewed/edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bunt, J., Blackstone, R., Thearle, M. et al. Changes in glycemia, insulin and gut hormone responses to a slowly ingested solid low-carbohydrate mixed meal after laparoscopic gastric bypass or band surgery. Int J Obes 41, 706–713 (2017). https://doi.org/10.1038/ijo.2017.22
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.22
- Springer Nature Limited
This article is cited by
-
Laparoscopic adjustable gastric banding with liraglutide in adults with obesity and type 2 diabetes (GLIDE): a pilot randomised placebo controlled trial
International Journal of Obesity (2023)
-
Length of biliopancreatic limb in Roux-en-Y gastric bypass and its impact on post-operative outcomes in metabolic and obesity surgery—systematic review and meta-analysis
International Journal of Obesity (2022)
-
Impact of Vagotomy on Postoperative Weight Loss, Alimentary Intake, and Enterohormone Secretion After Bariatric Surgery in Experimental Translational Models
Obesity Surgery (2022)
-
Potential Hormone Mechanisms of Bariatric Surgery
Current Obesity Reports (2017)