Abstract
Background
Sleeve gastrectomy plus side-to-side jejunoileal anastomosis (JI-SG), a relatively new approach to bariatric surgeries, has shown promising results for treating obesity and metabolic comorbidities. This study investigated the feasibility and safety of JI-SG in weight loss and diabetes remission compared with sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB).
Methods
Forty 10-week-old male Zucker diabetic fatty rats were randomly assigned to four groups: control, SG, JI-SG, and RYGB. Their body weights, food intake, and levels of gut hormones (ghrelin, insulin, and glucagon-like peptide-1 (GLP-1)) and lipids were measured.
Results
Rats in the SG, JI-SG, and RYGB groups demonstrated lower food intake and more weight loss 2 weeks postoperatively compared with control rats. Furthermore, rats in the JI-SG group achieved more weight loss (mean 242.7 ± 11.2 g) compared with those in the SG and RYGB groups (SG, 401.4 ± 15.1 g and RYGB, 298 ± 12 g, both P < 0.01). All surgery groups demonstrated a decreased fasting insulin, serum glucose, lipid levels, and increased GLP-1 postoperatively. The JI-SG group had lower fasting ghrelin levels than the RYGB group (168 ± 19.8 ng/L vs. 182 ± 16.7 ng/L, P < 0.01) and higher fasting GLP-1 levels than the SG group (1.99 ± 0.11 pmol/L vs. 1.71 ± 0.12 pmol/L, P < 0.01) at 12 weeks postoperatively. Over the experimental period, the ghrelin levels slowly increased in all surgical groups but remained lower than the preoperative and control levels.
Conclusions
JI-SG induced higher ghrelin and GLP-1 levels and improved glycemic control in Zucker diabetic fatty rats. Compared with SG and RYGB, JI-SG appeared to be a simple, relatively safe, and more effective procedure for treating type 2 diabetes and obesity in this animal model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery, including sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB), has been shown to improve control of type 2 diabetes and provide long-term benefits [1, 2]. Recently, SG has been used increasingly for its simplicity, safety, and lower rate of postoperative nutritional deficiencies. However, SG is generally considered less effective for weight loss, diabetes control, and metabolic improvements than RYGB [3, 4]. To improve its effectiveness, an additional type of intestinal bypass surgery combined with traditional SG, sleeve gastrectomy plus side-to-side jejunoileal (JI-SG), has recently been investigated and has shown promising results in weight loss and improvement of the type 2 diabetes remission rate [5, 6].
The two combined surgeries in JI-SG involve two physiological changes. The first is the removal of the gastric body and fundus of the stomach, which decreases stomach volume. Studies have confirmed that SG effectively leads to weight loss and type 2 diabetes remission [7, 8]. The other is side-to-side anastomosis of the ileum and jejunum, which moves food more quickly into the terminal ileum, without excluding or resecting any segments of the digestive tract. According to the hindgut theory, this process of expediting nutrient delivery to the hindgut yields better glycemic control [9]. Based on these findings, combined surgery with JI-SG has been established as a relatively new bariatric procedure. Melissas et al. performed laparoscopic JI-SG in 27 patients and observed more weight loss and a higher rate of diabetes resolution with JI-SG compared to SG [10]. Long-term follow-up and animal studies are needed to ensure the feasibility and safety of this new procedure.
The Zucker diabetic fatty (ZDF) rat has been studied extensively as a model for type 2 diabetes and presents similar features of the metabolic symptoms of type 2 diabetes [11, 12], including progressive insulin resistance, hyperglycemia, hyperinsulinemia, and hyperlipidemia [13, 14], that are analogous to the progression of obesity and diabetes in humans [15, 16]. The aim of this study was to further assess the feasibility and safety of JI-SG to induce weight loss and diabetes remission using an obese ZDF rat model. JI-SG was compared with SG and RYGB in terms of body weight, food intake, glycemic control outcomes, and levels of gut hormones, including ghrelin, insulin, glucagon-like peptide 1 (GLP-1), and lipids for 12 weeks postoperatively.
Materials and Methods
Animals and Diets
Forty male 10-week-old ZDF rats were purchased form Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All animals were housed under standard conditions (constant temperature and humidity with a 12-h light-dark cycle) in the animal center at Tongji University with free access to water and food. All animals were fed with a 6.5 % fat rat chow diet (Purina 5008, Vital River Laboratory Animal Technology) for 3 weeks preoperatively. The Animal Care and Utilization Committee of Tongji University approved all animal experiments in this study.
Experimental Design
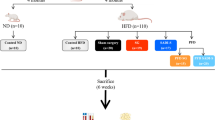
Forty ZDF rats were divided equally into four groups: sham-operated (control), SG, JI-SG, and RYGB. The rats’ body weight, food intake, and blood glucose levels were monitored weekly postoperatively. Blood glucose levels were analyzed with a hand-held glucometer (Accu-Chek; Roche Diagnostics, Shanghai, China) after an overnight fast. Ghrelin, insulin, GLP-1, and lipids were measured preoperatively and on the second, fourth, sixth, eighth, tenth, and twelfth postoperative week. The operation times (from the midline abdominal incision to complete skin closure) of all rats were recorded.
Biochemical Tests
Blood samples were collected from the tail vein into tubes that contained chilled ethylenediaminetetraacetic acid and dipeptidyl peptidase 4 inhibitor. Following centrifugation (1000×g) at 4 °C for 15 min, serum was immediately separated and stored at −80 °C until analysis. Lipids and gastrointestinal hormones (ghrelin, GLP-1, and insulin) were measured using enzyme-linked immunosorbent assay kits (USCN Life Science Inc., Wuhan, China).
Surgery
Preoperative Care
Rats were fed a nonresidue diet for 2 days and fasted for 12 h preoperatively. Rats were also given prophylactic antibiotics 30 min preoperatively and immediately after closure (gentamicin, 10 mg/kg; North China Pharmaceutical Group Corp., Hebei, China). Rats were anesthetized with ketamine (75 mg/kg body weight, Hengrui Medicine Co., Ltd., Jiangsu, China) during surgery.
SG
SG was performed by dissection of the greater curvature, including the lower part of the stomach and the forestomach with ligation of the short gastric vessels, according to a previously described technique [17]. The operative specimen, including most of the forestomach, was removed. The gastric wound was closed with a continuous invaginating extramucosal suture with a 5–0 silk thread (Ningbo Medical Needle Co., Ltd., Ningbo, China).
JI-SG
SG was performed first and then side-to-side anastomosis between the jejunum (20 cm distal to the ligament of Treitz) and the distal ileum (20 cm proximal to the ileocecal valve) was performed (Fig. 1). Stabilizing continuous bowel-to-bowel suturing with 5–0 silk on each side of the anastomosis for a distance of approximately 10 cm was used to prevent twisting of the bowel and reduce the possibility of mechanical obstruction.
RYGB
Gastric bypass was completed using a standard rat protocol [18]. The midportion of the stomach of animals allocated to gastric bypass was freed from adhesions on the greater and lesser curvature. Horizontal section of the stomach was performed, leaving only a small rim of glandular stomach on the proximal part. The distal and proximal stomachs were closed using 5–0 sutures (Ningbo Medical Needle). Jejunal section was performed 15 cm from the ligament of Treitz; gastrojejunal anastomosis and jejunoileal anastomosis were then performed. Finally, the abdominal wound was closed.
Control Group
Rats in the control group underwent sham operation in which the same abdominal incisions and gastrointestinal incision were performed, but removal was not performed. For consistency with the more complex surgical techniques, an interval of 20 min was observed before closing the gastric incision with a continuous extramucosal technique using a 5–0 suture (Ningbo Medical Needle).
Postoperative Care
All animals were given 5 mL prewarmed saline and placed in warm conditions for revival. Rats were allowed water for 12 h and a nonresidue diet 48 h postoperatively. On the third postoperative day, a high-fat diet (preoperatively) and water were not limited. Weight and food intake were measured weekly (at 5:00 p.m).
Statistical Analysis
All statistical analyses were performed according to standard methods using the Statistical Package for Social Sciences software (version 13.0; SPSS Inc., Chicago, IL, USA). Results were expressed as mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance with Bonferroni test for multiple comparisons and Student’s t test for single comparisons. P < 0.05 was considered statistically significant.
Results
Operation Time and Postoperative Complications
All operations were successful. The operation times in the control, SG, JI-SG, and RYGB groups were 32.2 ± 8.9 min, 63.4 ± 7.6 min, 94.7 ± 9.8 min, and 119.2 ± 8.6 min, respectively. The operation time in JI-SG rats was shorter than that in RYGB rats (P < 0.01) and was longer than that in SG rats (P < 0.01). One rat each in the JI-SG and RYGB groups died of anastomotic leaks on the second and fourth day postoperatively, respectively. One JI-SG rat was found dead at the tenth postoperative week due to malnutrition and low weight. No deaths or complications were observed in the control group.
Weight Loss and Food Intake
Postoperatively, SG, JI-SG, and RYGB rats achieved significant weight loss compared to the control group by the fourth postoperative week (Fig. 1). Thereafter, SG rats regained their body weight but at a lower rate than the control rats. JI-SG rats demonstrated the best weight control among the four groups, and RYGB rats had better weight control than SG rats each week (RYGB, 229.6 ± 12.3 g; JI-SG, 303 ± 18.2 g; and SG, 391.5 ± 12.1 g vs. control, 421.5 ± 19.1 g, P < 0.01). Compared with the control group, rats in all three surgery groups had a significantly decreased food intake from 4 weeks postoperatively (P < 0.01). Mean food intake of JI-SG rats was the lowest of the four groups (Fig. 2) and that of RYGB rats and JI-SG rats was lower than the SG group at all time points (P < 0.01).
Glucose
Compared with the control group, fasting glucose levels were significantly lower in the surgery groups (P < 0.01). Blood glucose levels were significantly lower in the JI-SG and RYGB rats compared to SG rats (data from the fifth week, 6.4 ± 2.4 μmol/L and 7.4 ± 1.9 μmol/L vs. 8.5 ± 1.3 μmol/L, P < 0.01) from 5 weeks postoperatively (Fig. 3). At 12 weeks postoperatively, the glucose levels of JI-SG rats were also lower than those of RYGB rats (data from the twelfth week, 6.2 ± 2.5 μmol/L vs. 7.4 ± 2.3 μmol/L, P < 0.05) and reflected good glucose control compared to preoperative measurements (data from the twelfth week, 6.2 ± 2.5 μmol/L vs. 12.4 ± 1.5 μmol/L, P < 0.01).
Fasting plasma glucose throughout 12 weeks postoperation. The fasting plasma glucose levels of rats in RYGB, SG, and JI-SG groups were lower than those in the control group since 2 weeks postoperatively. JI-SG obtained the lowest glucose level among surgery groups at 12 weeks postoperatively. *P < 0.01
Glucose Metabolism-Related Hormones
Insulin
At 2 weeks postoperatively, the insulin level exceeded preoperation values in the SG, JI-SG, and RYGB groups (Fig. 4). Subsequently, insulin decreased to a greater degree in the surgery groups than in the control. At postoperative week 12, JI-SG and RYGB rats demonstrated significantly lower levels of insulin than the preoperative levels. JI-SG rats had a lower level than RYGB rats at 12 weeks postoperatively (4.49 ± 1.3 mU/L vs. 5 ± 1.4 mU/L, P = 0.034).
Fasting insulin secretion of rats in all groups at 2, 4, 6, 8, 10, and 12 weeks postoperation. Comparing with SO group, all surgical groups had serum insulin levels higher at 2 weeks postoperation but lower at 4 weeks and remained lower throughout the rest of 12-week period. The insulin level of JI-SG group was the lowest among all groups at 12 weeks postoperation. *P < 0.01
Ghrelin
At 2 weeks postoperatively, SG, JI-SG, and RYGB rats achieved significantly lower fasting ghrelin levels than the controls (Fig. 5). Although the ghrelin level did not significantly differ between SG rats and JI-SG rats, both of these groups had lower ghrelin levels than RYGB rats. Over the experimental period, the ghrelin levels slowly increased in all surgical groups but remained lower than the preoperative and control levels.
Fasting ghrelin in all groups at 2, 4, 6, 8, 10, and 12 weeks postoperation. The ghrelin levels in all groups (SG, JI-SG, and RYGB) were lower than those in the control group since 2 weeks postoperatively and continued to remain lower than control group and their preoperative levels after 12 weeks, *P < 0.01. In addition, the postoperative ghrelin levels in SG and JI-SG groups were lower than RYGB group, *P < 0.01
GLP-1
RYGB, SG, and JI-SG rats demonstrated higher GLP-1 levels compared with control rats 2 weeks postoperatively (Fig. 6). However, the GLP-1 level of SG rats did not significantly differ from that of control rats after 4 weeks postoperatively. By contrast, RYGB and JI-SG rats had significantly higher GLP-1 levels than control and SG rats throughout the 12-week period, as well as compared with preoperative levels.
Fasting serum GLP-1 secretion of rats in all groups at 2, 4, 6, 8, 10, and 12 weeks postoperation. The GLP-1 level of rats in RYGB, SG, and JI-SG groups was higher than that of the SO group at 2 weeks postoperation. Compared with control group, SG group showed no significant difference in GLP-1 level after 4 weeks postoperatively. *P < 0.01
Lipids
Preoperative lipid levels demonstrated no significant differences among the groups (Fig. 7). Postoperative lipid levels were significantly lower in the surgical groups (SG, RYGB, and JI-SG) than in the control. The JI-SG and RYGB rats achieved lower lipid levels compared with SG rats (93.5 ± 4.6 pg/mL vs. 116.2 ± 4.6 pg/mL, P = 0.012), and JI-SG rats demonstrated the lowest lipid level overall at 12 weeks postoperatively.
Discussion
Recently, many studies have demonstrated that bariatric surgery effectively improves type 2 diabetes and brings long-term advantages for patients [19–21]. A number of new bariatric surgeries have been developed to obtain an optimal procedure for those with type 2 diabetes [10, 22, 23]. It has been hypothesized that the stomach size and small bowel length are evolutionary remnants of a low-calorie, fiber-rich diet. Accordingly, Santoro et al. proposed partial biliopancreatic diversion of 3 m of the small bowel without excluding the duodenum [23]. Melissas et al. further exploited the relevant mechanisms of this proposal using JI-SG surgery [10]. However, many aspects of JI-SG were unknown until recently. In the present study, we performed JI-SG surgery on ZDF rats and evaluated its antidiabetic, weight control, and intestinal neuroendocrine effects.
Generally, compared with the control, all surgical groups (SG, JI-SG, and RYGB) in this study demonstrated greater weight loss, lower food intake, and lower fasting glucose, insulin, ghrelin, GLP-1, and lipid levels. Weight loss has been shown to play an important role in improving glucose homeostasis [24]. JI-SG achieved the highest weight loss and lowest food intake among these surgical procedures. The JI-SG and RYGB groups demonstrated consistently better weight loss results throughout the 12-week postoperative period and achieved a more sustained effect in the resolution of diabetes than the SG and control groups. In our study, all surgery groups exhibited a lower food intake after 4 weeks and significant weight loss 3 weeks postoperatively.
In the present study, the marked increase in GLP-1 secretion and significant improvement in insulin sensitivity and glucose homeostasis after JI-SG and RYGB supports the hindgut hypothesis. Plasma levels of fasting ghrelin, GLP-1, and lipids were significantly modified by surgical interventions. SG, RYGB, and JI-SG induced fasting ghrelin and increased the glucose-stimulated GLP-1 level from 2 weeks postoperatively. The JI-SG and SG groups demonstrated no difference in ghrelin levels at 2 weeks postoperatively. Although the role of ghrelin in diabetes remission remains controversial [25, 26], increasing evidence from recent studies supports the hypothesis that decreased ghrelin levels are linked to a lower food intake, weight loss, and improved insulin sensitivity [27]. Furthermore, the JI-SG and RYGB groups had similar levels of GLP-1 elevation at 12 weeks postoperatively. Both the JI-SG and RYGB groups had significantly higher GLP-1 levels than the SG group.
JI-SG is believed to be a simple, relatively safe, and effective method for enhancing the neuroendocrine response of the intestine to food consumption. The hindgut hypothesis states that food will stimulate L cells in the ileum to secrete GLP-1, which can improve glucose homeostasis [7]. The role of GLP-1 in improving insulin sensitivity has been confirmed [28], and GLP-1 analogs have been proposed for patients with type 2 diabetes [29]. Previous studies have also shown that SG and RYGB procedures increase GLP-1 secretion [30]. After JI-SG surgery, the undigested nutrients are more rapidly transferred to the distal bowel through side-to-side jejunoileal anastomosis. In addition, the JI-SG procedure removed approximately 70 % of the total stomach, which significantly promoted gastric emptying [31]. Also, the JI-SG and RYGB groups demonstrated higher glucose-stimulated GLP-1 levels than the SG group postoperatively. Therefore, we speculate that side-to-side jejunoileal anastomosis may enhance GLP-1 secretion. Further experiments are required to confirm this hypothesis.
As a bypass procedure, JI-SG has a long operative time. However, the JI-SG procedure was safe and feasible, with few postoperative complications, which is similar to RYGB. A recent clinical study has also reported that the JI-SG procedure is safe and feasible [10]. Furthermore, we observed better diabetic control induced by JI-SG than by SG in ZDF rats. To our knowledge, comparisons of the effects of JI-SG, SG, and RYGB surgeries in obese diabetic subjects have not been performed. Our present animal study suggests that the JI-SG procedure may be favorable in obese diabetic patients. Additionally, the JI-SG procedure may be an alternative surgery to RYGB in diabetic patients.
A limitation of our study was that all findings originated from an obese diabetic rat model, which does not necessarily reflect the situation in humans. However, the ZDF rat provides an effective and economical model to investigate the effects of JI-SG surgery. Our study showed that JI-SG surgery achieved similar amelioration of diabetes as RYGB and better results than SG, although the procedures differed in anatomical and some gut-hormone effects. Currently, the effects of JI-SG on many other hormones remain unclear. Therefore, further studies are needed to study the effects of JI-SG on glucose metabolism.
In conclusion, based on the present findings, JI-SG appears to be a simple, relatively safe, and effective procedure for treating obesity and metabolic comorbidities. JI-SG was very effective in achieving durable weight loss, reducing fasting ghrelin, and elevating glucose-stimulated GLP-1 associated with type 2 diabetes. JI-SG was more effective than SG and had similar metabolic effects as RYGB. Further clinical comparative studies and long-term follow-up in obese diabetic patients are necessary to confirm our findings and to evaluate the effectiveness of JI-SG surgery in humans.
References
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–13.
Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–25.
Rosenthal RJ. International Sleeve Gastrectomy Expert Panel, Diaz AA, et al. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8(1):8–19.
Li JF, Lai DD, Lin ZH, et al. Comparison of the long-term results of Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity: a systematic review and meta-analysis of randomized and nonrandomized trials. Surg Laparosc Endosc Percutan Tech. 2014;24(1):1–11.
DePaula AL, Macedo AL, Rassi N, et al. Laparoscopic treatment of metabolic syndrome in patients with type 2 diabetes mellitus. Surg Endosc. 2008;22(12):2670–8.
Raj PP, Kumaravel R, Chandramaliteeswaran C, et al. Laparoscopic duodenojejunal bypass with sleeve gastrectomy: preliminary results of a prospective series from India. Surg Endosc. 2012;26(3):688–92.
Li JF, Lai DD, Lin ZH, et al. Comparison of the long-term results of Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity: a systematic review and meta-analysis of randomized and nonrandomized trials. Surg Laparosc Endosc Percutan Tech. 2014;24(1):1–11.
Li P, Fu P, Chen J, et al. Laparoscopic Roux-en-Y gastric bypass vs. laparoscopic sleeve gastrectomy for morbid obesity and diabetes mellitus: a meta-analysis of sixteen recent studies. Hepato-Gastroenterology. 2013;60(121):132–7.
Patriti A, Aisa MC, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-Kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery. 2007;142(1):74–85.
Melissas J, Peppe A, Askoxilakis J, et al. Sleeve gastrectomy plus side-to-side jejunoileal anastomosis for the treatment of morbid obesity and metabolic diseases: a promising operation. Obes Surg. 2012;22(7):1104–9.
Lifante JC, Milone L, Korner J, et al. Sleeve gastrectomy improves glucose homeostasis in Zucker diabetic fatty rats. Obes Surg. 2012;22(7):1110–6.
Patel RT, Shukla AP, Ahn SM, et al. Surgical control of obesity and diabetes: the role of intestinal vs. gastric mechanisms in the regulation of body weight and glucose homeostasis. Obesity (Silver Spring). 2014;22(1):159–69.
Kasiske BL, O’Donnell MP, Keane WF. The Zucker rat model of obesity, insulin resistance, hyperlipidemia, and renal injury. Hypertension. 1992;19(1 Suppl):I110–115.
Lifante JC, Milone L, Korner J, et al. Sleeve gastrectomy improves glucose homeostasis in Zucker diabetic fatty rats. Obes Surg. 2012;22(7):1110–6.
Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19.
Jurgens CA, Toukatly MN, Fligner CL, et al. B-cell loss and B-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178:2632–40.
Trung VN, Yamamoto H, Yamaguchi T, et al. Effect of sleeve gastrectomy on body weight, food intake, glucose tolerance, and metabolic hormone level in two different rat models: Goto-Kakizaki and diet-induced obese rat. J Surg Res. 2013;185(1):159–65.
Peng Y, Murr MM. Roux-en-Y gastric bypass improves hepatic mitochondrial function in obese rats. Surg Obes Relat Dis. 2013;9(3):429–35.
Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304.
Brethauer SA, Aminian A, Romero-Talamás H, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258(4):628–37.
Nestvold TK, Nielsen EW, Lappegård KT. Bariatric surgery reduces risk factors for development of type 2 diabetes mellitus in morbidly obese, nondiabetic patients. Metab Syndr Relat Disord. 2013;11(6):441–6.
DePaula AL, Stival AR, Halpern A, et al. Surgical treatment of morbid obesity: mid-term outcomes of the laparoscopic ileal interposition associated to a sleeve gastrectomy in 120 patients. Obes Surg. 2011;21(5):668–75.
Santoro S, Malzoni CE, Velhote MCP, et al. Digestive adaptation with intestinal reserve: a neuroendocrine-based operation for morbid obesity. Obes Surg. 2006;16:1371–9.
Tvarijonaviciute A, Ceron JJ, Holden SL, et al. Effects of weight loss in obese cats on biochemical analytes related to inflammation and glucose homeostasis. Domest Anim Endocrinol. 2012;42(3):129–41.
Samat A, Malin SK, Huang H, et al. Ghrelin suppression is associated with weight loss and insulin action following gastric bypass surgery at 12 months in obese adults with type 2 diabetes. Diabetes Obes Metab. 2013;15(10):963–6.
Cruz-Domínguez MP, Cortés DH, Zarate A, et al. Relationship of ghrelin, acid uric and proinflammatory adipocytokines in different degrees of obesity or diabetes. Int J Clin Exp Med. 2014;7(5):1435–41.
Tamboli RA, Breitman I, Marks-Shulman PA, et al. Early weight regain after gastric bypass does not affect insulin sensitivity but is associated with elevated ghrelin. Obesity (Silver Spring). 2014;22(7):1617–22.
Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62(9):3044–52.
Aroor A, McKarns S, Nistala R, et al. DPP-4 inhibitors as therapeutic modulators of immune cell function and associated cardiovascular and renal insulin resistance in obesity and diabetes. Cardiorenal Med. 2013;3(1):48–56.
Svane MS, Madsbad S. Bariatric surgery-effects on obesity and related comorbidities. Curr Diabetes Rev. 2014;10(3):208–14.
Chambers AP, Smith EP, Begg DP, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014;306(4):424–32.
Disclosures
Authors’ contributions
KJW and XGZ were responsible for the study conception and design, analyses and interpretation of the data, and drafting of the manuscript. GQ, JJL, WG, and AAX generated the experimental data. KJW and JFZ provided advice on the study concept, conducted specific analyses, and critically revised the manuscript. JFZ is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was funded by the Health Bureau of Pudong New Area (Shanghai, pw2013A-3).
Conflict of Interest
The authors declare that they have no competing interests.
Statement of Animal Rights
The Animal Care and Utilization Committee of Tongji University approved all animal experiments in this study. All animals were housed under standard conditions with free access to water and food.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kaijing Wang and Xiaogang Zhou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, K., Zhou, X., Quach, G. et al. Effect of Sleeve Gastrectomy Plus Side-to-Side Jejunoileal Anastomosis for Type 2 Diabetes Control in an Obese Rat Model. OBES SURG 26, 797–804 (2016). https://doi.org/10.1007/s11695-015-1811-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1811-7