Abstract
The composite bread was prepared from cassava flour (CF) and wheat flour (WF) in various proportion of 100:0 (100% CF), 50:50 (50% CF: 50% WF), 20:80 (20% CF: 80% WF), 10:90 (10% CF: 90% WF) and 0:100 (100% WF) and flavored with 2 g of cocoa powder (FL). Forty male Wistar rats, weighing 170–200 g were divided into eight groups of five animals each. They were subsequently placed on different formulated bread including control (100% WF Bread without cocoa powder) and the commercial bread for two weeks. The rats were sacrificed by cervical dislocation, blood was rapidly collected by direct heart puncture and the liver was excised. The results of the lipid profile revealed that there were significant (p < 0.05) decrease in plasma total cholesterol, triglycerides, LDL of the normal rats fed with composite bread as compared to the 100% WF bread control. However, a significant (p < 0.05) increase was revealed in HDL-C level in the plasma of the rats fed with composite bread with 100% CF bread + FL as compared with the control. In addition, significant increases (p < 0.05) by superoxide dismutase and catalase activities in the liver of the rats fed with composite bread of 100% CF bread + FL showed the highest antioxidant properties. Moreso, no significant (p > 0.05) difference in the activities of plasma ALP, AST, ALT was observed in rats fed with composite bread as compared to that of the control rats. The results suggest that cassava flour bread blends could serve as cheap functional food recipe with high antioxidant qualities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary Heart Disease is among of the largest contributors to morbidity and mortality worldwide. Globally, Coronary Heart Disease accounts for 17.5 million deaths in 2012, with over 75% of the deaths occurring in developing countries [1,2,3]. By 2015, 16% of all deaths were caused by Coronary Heart Disease [2, 4]. Broadly speaking, the cause of this disease is multifactorial in which some of them can be modified [5]. One of the risk factors is dyslipidemia. Dyslipidemia is therefore defined as a metabolic disorder of lipid characterized by an elevation and reduction in lipid fraction in plasma [6]. Low-density lipoprotein cholesterol (LDL- Cholesterol), triglyceride, and low level of high-density of lipoprotein cholesterol (HDL- Cholesterol) are the major risk factors of atherosclerosis affecting large and medium side arteries and consequently causing ischemia in the heart [7]. Dyslipidemia is thought to be a primary risk factor considered for Coronary Heart Disease which may play a role before other risk factors appear [8, 9]. Cholesterol is a risk factor that can be changed from hypertension, so the higher the total cholesterol level, the higher the likelihood of hypertension [10].
Free radicals or oxidative stress have been implicated in all the incidences and progressions of several health conditions such as neurodegenerative disorder, diabetes, cancer, atherosclerosis, cardiovascular disorders and other chronic pathologies [11]. Oxidative stress is a cellular disorder which normally occur as a result of the imbalances in physiology between the level of antioxidants and oxidants [11, 12]. Fortunately, organisms with antioxidant enzymes can salvage or slow down the deleterious effects of Reactive Oxygen Species (ROS) which results to cells injury [13]. Under normal physiological conditions, cell protect themselves against ROS damage through antioxidants that scavenge free intermediate radicals and inhibit oxidases [12, 13]
Antioxidants play a very crucial role in the body against ROS [14, 15]. Antioxidants are the first line of defence mechanism against free radical damage, and are important for maintenance of optimum health and overall well-being [14, 15]. Regular consumption of anti-oxidative vegetables and fruits has been recognised as reducing the risk of chronic diseases [16]. Studies have demonstrated that diets rich in antioxidant have positive health impact in the long run [17, 18]. Antioxidant are potent inhibitors of the oxidative process even at relatively small concentration and thus have several physiological functions the body [19, 20]. The dietary antioxidant such as polyphenols, vitamin-A, ascorbic acid, glutathione, beta-carotenoids, alpha-tocopherols, coenzyme Q, as well as antioxidant enzymes include superoxide dismutase, catalase, glutathione-peroxidases, glutathione-s-transferases and glutathione reductases have been investigated for the prevention and treatment of pathologies resulting from oxidative damages [21,22,23]. The use of plant extracts as natural antioxidants has developed a fast interest as a result of concerns of synthetic antioxidants that have developed negative health effect [24, 25].
Cassava being major source of carbohydrate in local diets, and when biofortified with increased levels of carotenoids, provides many other nutritional health benefits, such as improvement of visual ability and immune system [26]. The new variety of vitamin-A cassava was introduced by the Federal government of Nigeria under Harvest plus which is 25% richer in β-carotene (a pro-vitamin A) than those earlier released in 2011 [27, 28]. The characteristic yellow colour associated with vitamin-A cassava variety is due to its higher vitamin A content [29]. Vitamin A deficiency is a major public health challenges in sub-Saharan countries [30].
Bread is universally accepted as a very convenient form of food that is important to all populations. It is a good source of nutrients, such as macronutrients (carbohydrates, protein, and fat) and micronutrients (minerals and vitamins) that are essential for human health [31]. Efforts have been made to promote the use of flour from high protein seeds and locally grown crops to replace wheat flour used in baking bread, thereby decreasing the demand for imported wheat leading to the production of protein enriched bread [32]. Most tropical cereal grains and some tubers have been used to make composite flour for bread making [33].
The nutritional importance arising from cassava/wheat flour bread’s blends will be harnessed to promote well-being and healthy living for the consumer with associated health challenges. The research may serve as an eye opener to possible solutions to ravaging effect of cardiovascular challenges, hyperlipidaemia, liver damage and other disease conditions that arise from daily consumed diets which pose threat to human wellness and life existence. Therefore, this research is designed to investigate the effect of the composite bread blend’s blend produced from cassava flour (CF)/wheat flour (WF) flavoured with cocoa powder on the Lipid profile (total cholesterol, HDL-Cholesterol, LDL-Cholesterol, triglyceride), liver function biomarkers (AST, ALP and ALT) in the plasma, endogenous antioxidant assay (SOD and Catalase), MDA level, total thiol, non-protein thiol and nitric oxide level in the liver homogenate of the normal rats.
Materials and methods
Materials
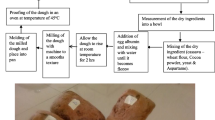
An improved variety cassava (Vitamin-A enriched Cassava) also known as Yellow Cassava was obtained from International Institute of Tropical Agriculture, IITA Ibadan, Oyo State, Nigeria. It was peeled, washed, cut into smaller pieces and oven dried in a cabinet dryer at 45 °C for 72 h before it was milled to flour while wheat flour (Golden penny), cocoa powder, yeast and eggs were purchased from Waso market, a major market in Ogbomoso city in Oyo State. Aspartame was purchased from a chemical supplier in Ilorin, Kwara State. All the chemicals used were analytical grade while the water was glass distilled.
Production of bread from vitamin-A cassava/wheat composite flour
The improved variety cassava was peeled, washed clean, cut into smaller pieces, then dried in the cabinet dryer at 45 °C for 72 h. The dried cassava was milled to CF. The composite mixture of an improved variety CF and WF in the ratios of 100:0 (100% CF and 0% WF), 50:50 (50% CF and 50% WF), 20:80 (20% CF and 80% WF), 10:90 (10% CF and 90% WF), and 0:100 (0% CF and 100% WF). Sweetening with Aspartame, Egg Albumin was used as emulsifier, cocoa powder as the flavouring agent (FL), also as additional phenolic source, yeast and water to taste in the production of composite breads. It was baked in a gas oven at 250 °C for 30 min or till it turned golden brown.
Effect of composite breads on biochemical indices in normal rats
Forty (40) rats weighing 170–200 g were randomly divided into eight groups of five albino rats each for two weeks of acclimatization. Group 1 to group 8 were placed on different formulated improved variety cassava/wheat flour composite bread including the control (100% WF Bread without cocoa powder) and the available commercial bread. The experiment lasted fourteen days after which the animals were subjected to night fasting prior the day of sacrifice. The rats were sacrificed using cervical dislocation and blood was collected rapidly by direct heart puncture. The liver was excised, rinsed in cold saline (0.9% Nacl) and homogenized in 0.1 M phosphate buffer (pH 7.4). The plasma was separated from the blood and used for assessing the liver function markers as well as the lipid profile using commercially available kits (Randox Laboratories Ltd, Crumlin, Dublin, Northern Ireland, UK). The liver homogenate was used for the assessment of antioxidant parameters.
Feed formulation and groups treatment
-
Group I: Normal rats fed with commercial bread.
-
Group II: Normal rats fed with 100% WF bread without cocoa powder.
-
Group III: Normal rats fed with 100% CF bread without cocoa powder.
-
Group IV: Normal rats fed with 100% CF bread + cocoa powder.
-
Group V: Normal rats fed with 50% CF + 50% WF bread + cocoa powder.
-
Group VI: Normal rats fed with 20% CF + 80% WF bread + cocoa powder.
-
Group VII: Normal rats fed with 10% CF + 90% WF bread + cocoa powder.
-
Group VIII: Normal rats fed with 100% WF bread + cocoa powder.
Methods
Preparation of plasma
At the expiration of the feeding trial, the rats were sacrificed and the whole blood was collected into EDTA bottle. The blood sample was centrifuged at 3000 rpm for 10 min to separate the plasma. The plasma was decanted into plain sample bottle and stored at 4 °C for further analysis.
Preparation of liver homogenate
The rats were sacrificed, their livers were rapidly isolated placed on ice and weighed. These tissues were subsequently rinsed in cold 0.9% normal saline and homogenized in 0.1 M phosphate buffer (pH 7.4). The homogenates were centrifuged at 3000 rpm for 10 min, and a low-speed supernatant (SI) were kept for lipid peroxidation assay [34] and total protein determination.
Determination of plasma total cholesterol
Plasma total cholesterol was determined according to the method of Allain et al., [35]. About 1.0 ml of the mixture containing 4-aminoantipyrine, cholesterol esterase, cholesterol oxidase, phenol, peroxidase and 80 mM pipes buffer pH 6.8 was incubated with 10 µl of plasma for 5 min at 37 °C. The absorbance was then taken against the reagent blank within 60 min at 546 nm. The concentration of cholesterol in sample was subsequently calculated against a standard.
Determination plasma triacylglycerides
Plasma triglyceride was determined by the method of Tietz, [36]. About 10 µl of the sample was mixed with 1.0 ml of pipes reagent (40 mM phosphate buffer, 5.5 mM 4-chlorophenol and 1.75 mM Mg2+) and enzyme reagent (phosphate oxidase and peroxidase). Thereafter, the reaction mixture was incubated at 37 °C for 5 min and the absorbance was taken against blank within 60 min at 546 nm. The triglyceride concentration was subsequently calculated.
Determination of LDL-cholesterol
The LDL-cholesterol concentrated of the plasma samples was determined according to the equation of Friedewald et al., [37], expressed as.
LDL = TC-HDL-TG/2.2 mmol/L where
LDL = Low density lipoprotein
TC = Total Cholesterol
HDL = High density lipoprotein, and
TG = Triglycerides.
Determination of HDL-cholesterol
The precipitation was carried out according to the method of Lopes-Virella et al., [38] as described in kit’s manufacturer (Randox Laboratories Ltd) manual. Briefly, 200 µl of plasma was mixed with 500 µl of the precipitant (0.55 mM phosphotungstic chloride) and allowed to sit for 10 min at room temperature. Then, the mixture was centrifuged for 10 min at 800 × g. Thereafter, the clear supernatant was separated off and subjected to the same procedure for the determination of cholesterol described above. The HDL-cholesterol was thus calculated using a standard.
Determination of aspartate aminotransferase (AST) activity in plasma
The quantity of AST in plasma was determined as described in the manufacturer manual (Randox Laboratories Ltd). Briefly, 0.1 ml of the sample (plasma) was mixed with 0.5 ml of Buffer R1 comprising of 100 mmol/l, pH 7.4 phosphate buffer, 100 mmol/Ll l-aspartate and 2 mmol/l α-oxoglutarate. Then, the mixture was incubated for 30 min at 37 °C. Thereafter, 0.5 ml of reagent 2 (2 mmol/l 2, 4-dinitrophenylhydrazine) was added and allowed to stand for 20 min at 25 °C, after which 5 ml of 0.4 mol/l sodium hydroxide was added and thoroughly mixed. The absorbance of sample (Asample) was read against the reagent blank after 5 min at 546 nm using spectrophotometer. The activity of AST in the plasma was subsequently calculated.
Determination of alanine aminotransferase (ALT) activity in plasma
The quantity of ALT in plasma was determined as described in the manufacturer manual (Randox Laboratories Ltd). Briefly, 0.1 ml of the sample (plasma) was mixed with 0.5 ml of Buffer R1 comprising of 100 mmol/l, pH 7.4 phosphate buffer, 200 mmol/l l-alanine and 2.0 mmol/l α-oxoglutarate. Then, the mixture was incubated for 30 min at 37 °C. Thereafter, 0.5 ml of solution R2 (2.0 mmol/ 2, 4-dinitrophenylhydrazine) was added and allowed to stand for 20 min at 25 °C, after which 5 ml of 0.4 mol/l sodium hydroxide was added and thoroughly mixed. The absorbance of sample (Asample) was read against the reagent blank after 5 min at 546 nm using spectrophotometer. The activity of ALT in the plasma was subsequently calculated.
Determination of alkaline phosphatase (ALP) activity in plasma
The quantity of ALP in plasma was determined using colourimetry method according to the recommendations of Deutsche Gessellschaft fur Klinische Chemie (1972). Briefly, 0.02 ml of the sample (plasma) was mixed with 1 ml of reagent R1 comprising 1 mol/l, pH 9.8 Diethanolamine buffer, 0.5 mmol/l MgCl2 and 10 mmol/l p-nitrophenylphosphate (substrate). After mixing the sample and the reagent, the initial absorbance was read after 1, 2 and 3 min at 405 nm using spectrophotometer. The ALP activity was subsequently calculated.
Determination of liver superoxide dismutase (SOD) activity
Superoxide dismutase was carried out as described by the method of Misra and Fridovich [39]. Briefly, 0.1 ml of the sample was diluted in 0.9 ml of distilled water. An aliquot of 0.2 ml of the diluted sample was added to 2.5 ml of 0.05 M carbonate buffer pH 10.2 to equilibrate in a cuvette and 0.3 ml of 0.3 M of adrenaline was added. The reference cuvette contained 2.5 ml of carbonate buffer, 0.3 ml of substrate (adrenaline) and 0.2 ml of distilled water. The increase in absorbance at 480 nm was monitored every 30 s for 150 s.
Determination of liver catalase activity
The liver homogenate was assessed for catalase activity according to the method of Asru, [40]. Briefly, 0.1 mL of sample was mixed with 4.9 mL of distilled water to give 1:50 dilution of samples. The assay mixture contained 2 mL of H2O2 solution and 2.5 mL of phosphate buffer in test tubes (arranged in duplicates). Then, 0.5 mL of properly diluted samples was rapidly added to the reaction mixture by a gentle swirling and the reaction proceeded at room temperature. A 1 mL portion of the reaction mixture was withdrawn and blown into 2 mL dichromate/acetic acid reagent at 60 s intervals for 3 min. the H2O2 contents of the withdrawn solution were subsequently determined.
Determination of tissues’ Lipid peroxidation content
Lipid peroxidation assay was carried out using the modified method of Ohkawa et al., [41]. Briefly, 300 µl of tissue homogenates (liver), 300 µl of 8.1% SDS (Sodium dodecyl sulphate), 500 µl of Acetic acid/HCl pH 3.4 and 0.6% TBA (Thiobarbituric acid) were added together and the mixture was placed in the water bath at 100 °C for 1 h. Thereafter, the thiobarbituric acid reactive species produced was measured at 532 nm using a spectrophotometer. MDA (Malonaldehyde) level was calculated from the absorbance according to the method of Adam-Vizi & Seregi, [42] and Lipid peroxidation was expressed as units/mg protein or gram tissue was computed with a molar extinction (Molar absorptivity) of 1.56 × 105 M−1Cm−1 for MDA equivalent.
Determination of total thiol content
Determination of the of the total thiol content in the tissue homogenate was done following the method of Ellman, [43]. The reaction mixture was made up of 270 µL of 0.1 M potassium phosphate buffer (pH 7.4), 20 µL of homogenate, and 10 µL of 10 mM DTNB. This was followed by 30-min incubation at room temperature, and absorbance was measured at 412 nm using a spectrophotometer. The total thiol content was subsequently calculated and expressed as (µmol/mg protein).
Determination of non-protein thiol content
Determination of the Non-protein thiol content in the liver homogenate was done following the method of Ellman, [43]. The reaction mixture containing 270 µL of 0.1 M potassium phosphate buffer (pH 7.4), 20 µL of homogenate, thereafter, 10% TCA was added. The mixture was centrifuged or allowed to settle after which 100 µL was taken from the supernatant and 300 µL of 10 mM DTNB was added. This was followed by 30-min incubation at room temperature, and absorbance was measured at 412 nm using a spectrophotometer. The total thiol content was subsequently calculated and expressed as (µmol/mg protein).
Determination of nitric oxide (NO) level
NO content of the liver was estimated in a medium containing 400 µL of 2% vanadium chloride (VCl3) in 5% HCl, 200 µL of 0.1% N-(l-naphthyl) ethylenediamine dihydrochloride, 200 µL of 2% sulphanilamide (in 5% HCl). After incubating at 37 °C for 60 min, nitrite levels were determined spectrophotometrically at 540 nm, based on the reduction of nitrate to nitrite by VCl3 [44]. Serum and kidney nitrite and nitrate levels were expressed as nanomole of NO/milligram of protein.
Data analysis
The results of replicate readings were pooled and expressed as mean ± standard deviation. One-way analysis of variance was used to analyse the results and Duncan’s New Multiple Range Test was used for the post hoc [45]. Statistical package for Social Science (SPSS) 15.0 for Windows was used for the analysis.
Results and discussion
Determination of HDL, total cholesterol, triglyceride and LDL in the plasma
The biochemical effect of cassava/ wheat flour composite bread on the Lipid profile (HDL, total cholesterol, triglyceride and LDL) of the plasma of the normal rats are indicated in Table 1. From the result, it was discovered that there was a significant (p < 0.05) increase in the value of high density lipoprotein (HDL-C) in the plasma content of the normal rats fed with 100% CF bread + FL (165.96 h ± 0.70 mg/dl), 100% CF bread (158.65 g ± 0.98 mg/dl), 50% CF + 50% WF bread + FL (156.87f ± 2.55 mg/dl), 20% CF + 80% WF bread + FL (151.34e ± 1.79 mg/dl) and commercial bread (150.35d ± 2.17 mg/dl) as compared with the control bread, 100% WF bread (148.18c ± 1.17 mg/dl). It was also discovered from the result that, an increased substitution of cassava in composite bread fed by the normal rats contributed to the increase the HDL-C value in the plasma. However, total cholesterol (100% CF bread + FL = 17.98a ± 2.31 mg/dl), triglycerides (100% CF bread + FL = 69.71a ± 0.91 mg/dl) and Low density lipoprotein cholesterol (LDL-C) (100% CF bread + FL = 3.10a ± 0.07 mg/dl) revealed significant (p < 0.05) reduction in their values in all the composite bread viz-a-viz their values with normal rats fed with 100% WF bread control; [total cholesterol (100% WF bread = 25.88d ± 0.00 mg/dl) = 43.94%, triglycerides (100% WF bread FL = 112.27g ± 0.20 mg/dl) = 61.05% and Low density lipoprotein cholesterol (LDL-C) (100% WF bread = 15.28f ± 0.16 mg/dl) = 392.90%. Similarly, an increase in the content of the cassava flour supplementation in the composite bread fed with normal rats led to the significant decrease in the value of total cholesterol, triglycerides and low-density lipoprotein cholesterol (LDL-C) contents of the plasma in the normal rats. The percentage increase in the level of high-density lipoprotein (HDL-C) as compared to the control is as follows; 100% CF bread + FL = 10%, 10% CF bread alone = 7.07%, 50% CF + 50% WF bread + FL = 5.86%, 20% CF + 80% WF bread + FL = 2.13% and commercial bread = 1.5% increases.
According to other studies, HDL and LDL are important for assessment of lipid profile. Hypercholesterolemia is usually characterized by both increase leading to abnormal serum, hepatic triglyceride and cholesterol levels [46]. All the composite bread produced have good lipid profile which may alleviate the risk of developing coronary heart disease. It is worth noting that 100% CF bread + FL had an outstanding hypolipidemic action on the plasma lipid level of the rats. The result was in agreement with the one earlier reported by Alsoodeeri et al. [47] on cinnamon extract consumption of serum lipid profiles in Albino rats and Vadivel, et al. [48] where phenolic contents with high antioxidant activities could function against oxidative stress could be a precursor to cholesterol dysfunction [Fig. 1].
Determination of AST, ALT and ALP in the plasma
The biochemical effect of the cassava/ wheat flour composite bread on the Liver function biomarkers; aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were assessed in Table 2. The results showed a significant (p < 0.05) reductions in the values of AST in the plasma of the normal rats fed with 50% CF + 50% WF bread + FL (97.19a ± 0.73 U/L) < 100% CF bread + FL (97.54a ± 0.82 U/L) < 100% WF bread (98.88b ± 0.35U/L) < 20% CF + 80% WF bread + FL (99.12c ± 1.12 U/L) < 10% CF + 90% WF bread + FL (99.30c ± 0.65) < 100% CF bread + FL (101.58d ± 1.37U/L) < commercial bread (105.79e ± 1.57U/L) as compared to the control, [100% WF bread + FL (105.09e ± 0.59U/L)]. More so, the result in Table 2 revealed that there were significant (p < 0.05) decrease in the value of the ALT showing that 10% CF 90% WF bread + FL < 100% WF bread + FL < 20% CF + 80% WF bread + FL < 100% CF bread + FL < 50% CF + 50% WF bread + FL < 100% CF bread + FL < commercial bread as compared to the control 100% WF bread, with these corresponding values: 326.32a ± 0.47 U/L, 329.12b ± 0.07 U/L, 333.68c ± 1.27 U/L, 336.32d ± 1.42 U/L, 338.42e ± 2.33 U/L, 346.14f ± 0.89 U/L, 351.05 g ± 2.01 U/L. However, there were no significant (p > 0.05) difference in the values of the ALP in the plasma of the normal rats fed with composite bread as compared to the control.
Increase in these marker enzymes in the serum/plasma had been reported to be leakage from the liver to the blood stream indicating hepatopathy [49]. Normally, AST and ALP are enzyme located in the liver cells and leak out, making their way into general circulation as liver cells are damaged [50]. The results showed that cassava/wheat flour composite bread has hepatoprotective effect and this may not be farfetched from its antioxidant properties which was in agreement with the previous findings of Adefegha et al., [51] on the ability of spices to restore the level of liver biomarker enzymes in diabetic rat models.
Determination of superoxide dismutase and catalase in the liver
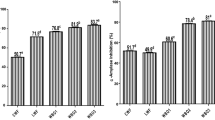
The effect of cassava/ wheat flour composite bread on the liver endogenous antioxidant enzymes [superoxide dismutase (SOD) and catalase (CAT)] are represented in Fig. 2 and Fig. 3. Figure 2 shows that there was a significant (p < 0.05) increase in SOD enzyme activity in the liver of the normal rats fed 100% CF bread + FL, 50% CF + 50% WF bread + FL and 100% CF bread when compared with the control. The trend of increase in the SOD enzymes activity is as follows for all the composite bread, 100% CF bread + FL > 50% CF + 50% WF bread + FL > 100% CF bread > commercial bread > 20% CF + 80% WF bread + FL > 10% CF + 90% bread + FL > 100% WF bread + FL. Similarly, catalase activity in the liver of the normal rats fed with composite bread was significantly (p < 0.05) higher in 100% CF bread + FL, 50% CF + 50% WF bread + FL, 100% CF bread as compared with the 100% WF bread control. The trend of increment in the catalase activity in the liver of the normal rats fed with composite bread is as follows; 100% CF bread + FL > 50% CF + 50% WF bread + FL > 100% CF bread > 20% CF + 80% WF bread + FL > commercial bread > 10% CF + 90% WF bread + FL + 100% WF bread + FL.
SOD activity of liver in normal rats fed with composite breads. Values represent mean ± standard deviation (n = 5). *Value are significantly (p < 0.05) different from normal and control group. Group I—Normal rats fed with commercial bread; Group II—Normal rats fed with 100% WF Bread; Group III—Normal rats fed with 100% CF bread; Group IV—Normal rats fed with 100% CF bread + FL (cocoa powder); Group V—Normal rats fed with 50% CF + 50% WF bread + FL; Group VI—Normal rats fed with 20% CF + 80% WF bread + FL; Group VII—Normal rats fed with 10% CF + 90% WF bread + FL; Group VIII—Normal rats fed with 100% WF bread + FL
Catalase activity of liver in normal rats fed with composite breads. Values represent mean ± standard deviation (n = 5). *Value are significantly (p < 0.05) different from normal and control group, Group I—Normal rats fed with commercial bread; Group II—Normal rats fed with 100% WF Bread; Group III—Normal rats fed with 100% CF bread; Group IV—Normal rats fed with 100% CF bread + FL (cocoa powder); Group V—Normal rats fed with 50% CF + 50% WF bread + FL; Group VI—Normal rats fed with 20% CF + 80% WF bread + FL; Group VII—Normal rats fed with 10% CF + 90% WF bread + FL; Group VIII—Normal rats fed with 100% WF bread + FL
Endogenous antioxidant enzymes [SOD and CAT] are equipped to prevent oxidative stress promotion cause by the free radical by various mechanism such as scavenging oxygen and nitrogen species, transition metal ion chelation and breaking free radical chain reaction [52]. Results showed the outstanding increase of these endogenous enzymes (SOD and CAT) in the liver of the normal rats resulted from 100% CF bread + FL which was in agreement with an earlier report by Cohen and Heikkila [53] where an abrupt reduction in the activities of these enzymes was reported in numerous pathophysiological process and diseases.
Determination of lipid peroxidation in the liver
Figure 4 presented the effect of cassava/ wheat flour composite bread on the MDA level of the liver in the normal rats. The result reveals that the boiling of the isolated rat’s liver in the boiling water bath in the presence of Fe2+ cause a significant (p < 0.05) decrease in the MDA levels in the liver of the normal rats fed with 100% CF bread + FL which was the highest while that of the commercial bread was the lowest. The result of the MDA level order of lipid peroxidation follows this order of decrease: 100 CF bread + FL < 50% CF + 50% WF bread + FL < 20% CF + 80% WF bread + FL < 10% CF + 90% WF bread + FL < 100% CF bread < 100% WF bread + FL < commercial bread.
MDA level of liver in normal rats fed with composite breads, Values represent mean ± standard deviation (n = 5), *Value are significantly (p < 0.05) different from normal and control group. Group I—Normal rats fed with commercial bread; Group II—Normal rats fed with 100% WF Bread; Group III—Normal rats fed with 100% CF bread; Group IV—Normal rats fed with 100% CF bread + FL (cocoa powder); Group V—Normal rats fed with 50% CF + 50% WF bread + FL; Group VI—Normal rats fed with 20% CF + 80% WF bread + FL; Group VII—Normal rats fed with 10% CF + 90% WF bread + FL; Group VIII—Normal rats fed with 100% WF bread + FL
Malondialdehyde (MDA) is a stable and critical end product of lipid peroxidation. Excess free radicals, resultant effect of oxidative stress couple with an elevation of MDA result to the long-term vascular complication in coronary heart disease [54]. In this study, the ability of the composite bread to significantly (p < 0.05) reduce the MDA level in the liver of the normal rats as compared to the control may be attributed to the high phenolic and antioxidant activities earlier reported by Ajani et al. [55] on the free phenolic contents and antioxidant activity of bread produced from an improved variety cassava-wheat composite flour.
Determination of total thiol and non-protein thiol in the Liver
The effect of cassava/ wheat flour composite bread on the total thiols and non-protein thiols of the liver of the normal rats are revealed in Fig. 5 and 6. Results showed that there was a significant (p < 0.05) increase in the total thiol value in the liver of the normal rats fed with composite bread while 100% CF bread + FL had the highest and the rat fed with 100% WF bread + FL had the least value. The trend of the increment of the total thiol in the liver of the normal rats is as follow: 100% CF bread + FL > 50% CF + 50% WF bread + FL > 20% CF + 80% WF bread + FL 100% CF bread + FL > 10% CF + 90% WF bread + FL > commercial bread > 100% WF bread + FL. Similarly, the results revealed a significant (p < 0.05) increase in the level of the non-protein thiol in the liver of the experimental rats fed with the cassava/ wheat flour composite bread as compared to the rats fed with 100% WF bread control. The sequential increase in the level of non- protein thiol in the liver of the normal rats fed with composite bread is in this order; commercial bread < 100% CF bread < 100% WF bread + FL < 10% CF + 90% WF bread + FL < 20% CF + 80% WF bread + FL < 50% CF + 50% WF bread + FL < 100% CF bread + FL.
Total thiol level of liver in normal rats fed with composite breads, Values represent mean ± standard deviation (n = 5). *Value are significantly (p < 0.05) different from normal and control group. Group I—Normal rats fed with commercial bread; Group II—Normal rats fed with 100% WF Bread; Group III—Normal rats fed with 100% CF bread; Group IV—Normal rats fed with 100% CF bread + FL (cocoa powder); Group V—Normal rats fed with 50% CF + 50% WF bread + FL; Group VI—Normal rats fed with 20% CF + 80% WF bread + FL; Group VII—Normal rats fed with 10% CF + 90% WF bread + FL; Group VIII—Normal rats fed with 100% WF bread + FL
Non-protein thiol level of liver in normal rats fed with composite breads. Values represent mean ± standard deviation (n = 5). *Value are significantly (p < 0.05) different from normal and control group. Group I—Normal rats fed with commercial bread; Group II—Normal rats fed with 100% WF Bread; Group III—Normal rats fed with 100% CF bread; Group IV—Normal rats fed with 100% CF bread + FL (cocoa powder); Group V—Normal rats fed with 50% CF + 50% WF bread + FL; Group VI—Normal rats fed with 20% CF + 80% WF bread + FL; Group VII—Normal rats fed with 10% CF + 90% WF bread + FL; Group VIII: Normal rats fed with 100% WF bread + FL
R-SH may serve as an important buffer for ROS and may thereby contribute to protect against the detrimental effects of reduced insulin signaling [56]. Various experimental studies reported that alteration of the thiol/disulphide ratio leads to changes in cellular status. It has been shown that the increase in the GSH/GSSG redox state leads to proliferation while the decrease in the GSH/GSSG redox state leads to apoptosis [57, 58]. The results in this study revealed that the sequential increase in the values of total thiol and non-protein thiol in the liver of the normal rats suggest the antioxidative ability of the composite bread.
Determination of no level in the liver
The effect of cassava/wheat flour composite bread on the NO level in the liver of the normal rats is observed in Fig. 7. Results showed that 100% CF bread + FL had the highest value of NO level while 100% WF bread had the least value. The values obtained for each composite bread follow this order: 100% CF bread + FL > 50% CF + 50% WF bread + FL > 20% CF + 80% WF + FL > 10% CF + 90% WF bread + FL > commercial bread > 100% CF bread > 100% WF bread + FL. Acetylcholine enhances endothelial nitric oxide synthase activities for NO production [59]. Different studies have suggested that reduced NO content is associated with impairment of endothelial function which has been indicted in development of vascular disease [60, 61]. The significant (p < 0.05) increase in NO level in this study revealed that interaction of compound constituents of cassava/ wheat flour composite bread enhanced NO production therefore promote vasodilation in the rats fed with composite bread.
NO level of liver in normal rats fed with composite breads, Values represent mean ± standard deviation (n = 5). *Value are significantly (p < 0.05) different from normal and control group. Group I—Normal rats fed with commercial bread; Group II—Normal rats fed with 100% WF Bread; Group III—Normal rats fed with 100% CF bread; Group IV—Normal rats fed with 100% CF bread + FL (cocoa powder); Group V—Normal rats fed with 50% CF + 50% WF bread + FL; Group VI—Normal rats fed with 20% CF + 80% WF bread + FL; Group VII—Normal rats fed with 10% CF + 90% WF bread + FL; Group VIII—Normal rats fed with 100% WF bread + FL
Coronary Heart disease (CHDs) are the frontline cause of morbidity and mortality globally [2, 3]. High LDL-C is of long-standing clinical and research interest as it is an independent risk factor for the occurrence of cardiovascular event and CHDs [62, 63]. Previous studies have clearly indicated high levels of LDL-C as atherogenic and have affirmed a connection between elevated level of LDL-C and cardiovascular events [64, 65].
Conclusion
In this study, supplementation of wheat flour with cassava in composite bread production showed high hypolipidemic effect and antioxidant potentials in rats. These products may serve as functional foods and provide good health benefits against oxidative stress, hyperlipidemia and related complications which pose threat to life. However, 100CF bread + FL had highest HDL-cholesterol level as well as the best antioxidant potentials. Further studies are needed from clinical trials of the composite bread in humans, to validate the effect of the composite bread.
References
World Health Organization (WHO) (2014): /Global status Report on Non-communicable Diseases/World Health Organization, Geneva, Switzerland.
R. Gupta, I. Mohan, C. Narula, Trends in coronary heart disease epidemiology in India. Annals. Global. Hlth. 82(2), 307–315 (2016)
D. Mozaffarian, E.J. Benjamin, Go, et al., Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 133(14), e38–e360 (2016)
M. Zhao, I. Vaartjes, I. Graham et al., Heart 103(20), 1587–1594 (2017)
O. Melander, J. Int. Med. 278(5), 433–446 (2015)
R.H. Nelson, Hyperlipidemia as a risk factor for cardiovascular disease. Primary Care clin. Off. Pract. 40(10), 195–211 (2013)
C.-F. Lin, Y.-H. Chang, S.-C. Chien, Y.-H. Lin, H.-Y. Lin, Inter J. Geront. 12(1), 2–6 (2018)
Perkumpulan Endokrinology Indonesia (PERKENI): Konsensus Pengelolaan Dyslipidemia di Indonesia, Pusat Penerbitan ilmu Penyakit Dalam Fakultas Kedokteran UI (2015).
R. Garg, S. Aggarwal, R. Kumar, S. Sharma, J. Nat. Sci. Biol. Med. 6(1), 163–168 (2015)
S. Fujikawa, R. Iguchi, T. Noguchi et al., Acta. Urolog. Japon 61(3), 99–102 (2015)
D. Glustarmi, I. Dalle-Donne, D. Tsikas, R. Rossi, Critic. Rev. Clin. Lab. Sci. 46, 241–281 (2009)
K.P. Poulianiti, A. Kaltsatou, G.I. Mitrou, A.Z. Jamurtas, Y. Koutedakis, M. Maridaki, I. Stefanidis, G.K. Sakkas, C. Karatzaferi, Oxid. Med. Cell. Longev. 2016, 8598253 (2016)
X.C. Ling, K. Kuo, Ren. Replace. Ther. 4, 53 (2018)
O.U. Boxin, H. Delijah, A. Maureen, K.D. Elizabeth, J. Agri Food Chem. 5, 223–228 (2002)
K.G. Vivek, K.S. Surendra, Nutra. Produc. Radia. 5, 326–334 (2006)
A. Dembinska- Kiec, O. Mykkanene, B. Kiec-Wilk, H. Mykkanene, British J. Nutr. 99, 109–117 (2008)
H.P.Y. Sin, D.T.L. Liu, D.S.C. Lam, Acta Opthalmol. 91, 6–11 (2013)
L.M. Willis, B. Shukitt-Hale, J.A. Joseph, Curr. Opinion Clin. Nutr. Metab. Care. 12(1), 91–94 (2009)
R.K. Nema, S. Yadav, M. Sulekha, J. Chem. Pharmac. Res. 1, 102–104 (2009)
D. Boskou, G. Blekas, M. Tsimidou, Curr. Topic. Nutraceut. Res. 3, 125–136 (2005)
G. Yuan, B. Sun, J. Yuan, Q. Wang, Food Chem. 118(3), 774–781 (2010)
M.W. Dysken, M. Sano, S. Asthana et al., JAMA 311, 33–34 (2014)
J. Hillmann, Food Rev. 37(1), 18–19 (2010)
H. Abramovic, V. Abram, Acta Agric. Slovenica 87(2), 255–261 (2006)
M. Azizkhani, P. Zandi, World Aca. Sci. Eng. Tech. 49, 93–96 (2009)
N.I. Krinsky, E.J. Johnson, Mol. Asp. Med. 26, 459–516 (2005)
International Institute of Tropical Agriculture (IITA) (2017). Available online: http://www.iita.org/news-item/nigeria-releases-cassava-higher-pro-vitamin-fight-micronutient-deficiency (accessed on 27 January, 2017).
R.M. Omodamiro, E. Oti, H.A. Etudaiye, C. Egesi, B. Olasanmi, U.J. Ukpabi, Adv. Appl. Sci. Res. 3, 2566–2572 (2012)
E. Alos, J. MariaRodrigo, L. Zacarias, Sub-cellular biochemistry Carotenoids in nature (Spinger, Berlin Germany, 2016)
N. Arlappa, N. Balakrishna, A. Laxmaiah, P. Raghu, V. Vikas Rao, K. Medhavan Nair, G.N. Braham, Annals Human Biol. 38(2), 131–136 (2011)
S.O. Oluwajoba, O. Malomo, O.A.B. Ogunmoyela, O.E.O. Dudu, A. Odeyemi, J. Microb. Biotech. Food Sci. 2(1), 42–68 (2012)
O.A. Olaoye, A.A. Onilude, African J. Food Agric Nutr. Devlop. 8(2), 192–203 (2012)
J.A. Ayo, V.A. Ayo, C. Popoola, M. Omosebi, C. Joseph, African J. Food Sci Techn. 5(1), 21–28 (2014)
N.A.V. Belle, G.D. Dalmolin, G. Fonini, M.A. Rubim, J.B.T. Rocha, Brain Res. 1008, 245–251 (2004)
C.C. Allain, L.S. Poon, C.S.G. Chan, W. Richmond, P.C. Fu, Enzymatic determination of total serum cholesterol. Clin. Chem. 20(4), 470–475 (1974)
N.W. Tietz, Fundamentals of clinical chemistry (W.B. Saunders Company, Philadelphia, 1982), pp.562–698
W.T. Friedewald, R.I. Levy, D.S. Fredrickson, Clinical Chem. 18, 499–502 (1972)
M.F. Lopes-Virella, P. Stone, S. Ellis, J.A. Colwell, Clin. Chem. 23(6), 265–270 (1977)
H.P. Misra, I. Fridovich, J. Biol. Chem. 247, 3170–3175 (1972)
K.S. Asru, Analyt. Biochem. 47(1972), 389–394 (1972)
H. Ohkawa, N. Ohishi, K. Yagi, Analyt. Biochem. 95, 351–358 (1972)
V. Adam-Visi, M. Seregi, Biochem. Pharmac. 31, 2231–2236 (1982)
G.L. Ellman, Arch. Biochem. Biophy. 82(1), 70–77 (1959)
K.M. Miranda, M.G. Espay, D.A. Wink, Biol. Chem. 5, 62–71 (2001)
J.H. Zar, Biostatistical Analysis, vol. 620P (Prentice-Hall Inc, USA, 1984)
Y.M. Wang, Z. Bei, X. Yong, L. Zhao-Jie, W. Jing-Feng, X. Chang-Hu, Y. Teruyoshi, Lipids Hlth. Disease. 9, 4 (2010)
F.N. Alsoodeeri, F.N. Alqubbani, N.M. Aldossari, J. Lipids. (2020). https://doi.org/10.1155/2020/84699830
V. Vadivel, J.N. Cheong, H.K. Biesalski, LTW-Food Sci. Tech. 47, 255–260 (2012)
D.S. Pratt, M.M. Kaplan, New Engl. J. Medic. 342, 1266–1271 (2000)
G. Oboh, J. Medicin. Food. 8, 560–563 (2006)
S.A. Adefegha, G. Oboh, O.M. Adefegha, A.A. Boligon, M.L. Athayde, J. Sci. Food Agric. 94, 2726–2737 (2014)
V.P. Veerapur, K.R. Prabhakar, B.S. Thippeswamy, Food Chem. 132, 186–193 (2012)
G. Cohen, R.E. Heikkila, J. Biochem. 249, 2447–2452 (1994)
S. Mishra, B.B. Mishra, Int. J. Appl. Basic Med. Res. 72(2), 88–93 (2017)
R.A. Ajani, G. Oboh, S.A. Adefegha, A.A. Akindahunsi, Trop. J. Nat. Prod. Res. 6(2), 227–235 (2022)
C.M. Cremers, U. Jakob, J. Biol. Chem. 288(37), 26489–26496 (2013)
W.G. Kirlin, J. Cai, S.A. Thompson, D. Diaz, T.J. Kavanagh, D.P. Jones, Free Radic. Biol. Med. 27(11–12), 1208–1211 (1999)
Y.S. Nkabyo, T.R. Ziegler, L.H. GU, W.H. Watson, D.P. Jones, 2002 Am J Physiol Gastrointest Liver Physiol. 283(6): 352–359
R.M. Lataro, C.A.A. Silva, C. Tefé-Silva, C.M. Prado, H.C. Salgado, Am. J. Hyperten. 28(10), 1201–1208 (2015)
S.B.A. Cau, R.C. Tostes, Hypertension 59(3), e21–e21 (2012)
G.V. Marinova, R.Y. Loyaga-Rendon, S. Obayashi, T. Ishibashi, T. Bota, M. Imamura, J. Pharmacol. Sci. 106, 385–393 (2008)
N.J. Stone, J.G. Robinson, A.H. Lichtenstein et al., J. Am. coll Cardiol. 63(25), 2889–2934 (2014)
R.S. Rosenson, H.B. Brewer, B.J. Ansell et al., Nat. Rev. Cardiol. 13(1), 48–60 (2016)
C.J. Girman, T. Rhodes, M. Mercuri et al., Am. J. Cardiol. 93(2), 136–141 (2004)
P.M. Ridker, M.J. Stampfer, N. Rifai, J. Am. Medic. Assoc. 285(19), 2481–2485 (2001)
Acknowledgements
The authors wish to acknowledge the Functional Food and Nutraceutical Research Laboratory Unit of the Department of Biochemistry, Federal University of Technology, Akure, Ondo State, Nigeria for granting the opportunity to carry out this research in their Laboratory.
Author information
Authors and Affiliations
Contributions
RAA: Functional Food and Nutraceutical Research, carried out the analysis. SAA: Functional Food and Nutraceutical Research, Co-supervisor. GO: Functional Food and Nutraceutical Research, Major supervisor.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ajani, R.A., Adefegha, S.A. & Oboh, G. Hypolipidemic effect and antioxidant properties of cassava-wheat flour composite bread in rats. Food Measure 17, 278–288 (2023). https://doi.org/10.1007/s11694-022-01600-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01600-1