Abstract
The sarcoplasmic proteins from hake (Merluccius merluccius) were subjected to high-pressure treatments (200, 400 and 600 MPa for 6 min at 20 °C). The physicochemical changes and the emulsifying capacity of sarcoplasmic proteins were evaluated. High-pressure processing increased the surface hydrophobicity and the reactive sulfhydryl group content of sarcoplasmic proteins above 200 MPa. Likewise, conformational changes were induced on the secondary structure of sarcoplasmic proteins at 400 MPa. High-pressure denaturation of sarcoplasmic proteins promoted their aggregation. The electrophoretic patterns showed that some sarcoplasmic proteins (78, 73, 63, 58, 37, 32 and 22 kDa) were the most pressure labile. However, the carbonyl content of sarcoplasmic proteins was not affected by high-pressure treatments. The pressure-induced denaturation affected the emulsifying properties of sarcoplasmic proteins, which was dependent on pressure. In contrast to high-pressure levels (400 and 600 MPa), sarcoplasmic proteins treated at 200 MPa produced emulsions with enhanced stability, greater consistency index, and shear-thinning behavior. These results suggested that high-pressure denaturation can affect the functional properties of sarcoplasmic proteins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcoplasmic proteins are soluble proteins present in fish and meat sarcolemma. Enzymes, albumins and myoglobin are sarcoplasmic proteins, and they account for 30–35 % of the total muscle proteins [1, 2]. Sarcoplasmic proteins influence the functional properties of fish products. Enzymes of the glycolytic pathway and lysosomal enzymes influence the quality of fish muscle [3]. Firmness of rainbow trout muscle is associated with the stability of some sarcoplasmic proteins, such as glycogen phosphorylase, creatine kinase and triosephosphate isomerase B [4]. In addition, previous studies have reported that improving technological properties of minced fish is obtained by removing sarcoplasmic proteins because they can interfere with the gelation of myofibrillar proteins [5, 6]. However, more recent studies have indicated that the addition of sarcoplasmic proteins contributes to their gelation [7, 8]. Sarcoplasmic proteins also exhibit emulsifying properties and are suggested as a functional ingredient [9–11].
High-pressure processing is an emerging technology for food preservation as a main approach with a potential for wider applications. For example, pressure treatments (200–300 MPa) have been reported to kill larvae on hake muscle, but texture and color modifications were concomitant to the process as a consequence of protein denaturation [12]. At first, the understanding of protein denaturation in food muscles has been focused on studying myofibrillar proteins [13–15]. However, recent studies have highlighted the influence of high pressure on the aggregation of sarcoplasmic proteins in fish products [16, 17]. Sarcoplasmic proteins have been considered more susceptible to high-pressure processing than myofibrillar proteins. Sarcoplasmic proteins have also been associated with technological properties such as color and water-holding capacity, and they have been recently suggested as protein markers of product quality [17, 18]. High-pressure processing of fish products induces denaturation of sarcoplasmic proteins. In cod muscle, sarcoplasmic proteins are denatured at 300 MPa [19]. Solubility loss of sarcoplasmic proteins by high-pressure processing (100–608 MPa) has been reported in mackerel, turbot, salmon, sea bass and cod muscle [16, 17, 20–22]. Likewise, gelation of fish sarcoplasmic proteins at high concentration (>50 mg/ml) is promoted by high-pressure processing [23]. Nevertheless, to our knowledge, there is limited information about the extent of physicochemical changes of sarcoplasmic proteins by high-pressure processing and the consequences on their emulsifying properties. This study aimed to determine the effect of high-pressure processing on the structural and emulsifying characteristics of sarcoplasmic proteins from hake (Merluccius merluccius).
Materials and methods
Sarcoplasmic protein extraction
Hake (Merluccius merluccius) was obtained from a local market (24 h after catch from La Turballe). Minced fish muscles were vacuum packaged and frozen (−20 °C). Before protein extraction, fish muscles were thawed overnight at 4 °C. The sarcoplasmic proteins were extracted according to the method of Sikes et al. [24] with some modifications. The muscle was homogenized with extraction buffer (pH 7; 100 mM KCl, 50 mM Tris–HCl and 5 mM EDTA) at a ratio of 1:10 (w/v). The supernatant was collected after centrifugation (10,000g for 15 min at 4 °C) and filtration through gauze. The protein content was evaluated by the Biuret assay [25] and characterized with SDS-PAGE electrophoresis [26]. Experiments were performed in quadruplicate corresponding to four hakes.
High-pressure treatment
The high-pressure equipment is a 3-l pressure pilot unit (ACB, Nantes, France) that uses water as a transmitting fluid. The temperature is controlled by a temperature regulator device (Julabo, Seelbach, Germany) and a cooling water jacket around the vessel. The samples were high-pressure-treated (200, 400 or 600 MPa) for 6 min at 20 ± 3 °C. The compression rate was 3 MPa/s, and the pressure was released immediately (<2 s). Untreated samples were considered as controls.
Protein surface hydrophobicity
The surface hydrophobicity of sarcoplasmic proteins was determined by the fluorescence probe method [27]. The samples (1 mg/ml) were diluted in extraction buffer (pH 7; 100 mM KCl, 50 mM Tris–HCl and 5 mM EDTA) to obtain protein concentrations between 0.02 and 0.2 mg/ml. Samples (2 ml) were mixed with 10 µl of 8 mM 1-anilinonaphthalene-8-sulfonic acid (ANS). Diluted samples without ANS were used as blanks. The fluorescence (excitation wavelength of 355 nm and emission wavelength of 460 nm) was measured after 15 min using a fluorescence plate reader (Fluostar Optima, BMG Labtech, Ortenberg, Germany). The slope (R 2 > 0.95) of the fluorescence intensity versus protein concentration was the relative fluorescence intensity for each sample.
Reactive sulfhydryl group content
The reactive sulfhydryl group content of sarcoplasmic proteins (1 mg/ml) was determined according to the Ellman method [28]. The samples (0.5 ml) were mixed with 2 ml of 0.1 M phosphate buffer (1 mM EDTA, pH 8) for determination of reactive sulfhydryl groups, and 30 µl of 10 mM 5,5′-dithio-bis 2-nitrobenzoic acid (DTNB) was then added and mixed. To determine the total sulfhydryl groups, 0.1 M phosphate buffer (1 mM EDTA, pH 8) containing 8 M urea was used. After 15 min, the absorbance at 412 nm was measured, and the sulfhydryl group content was obtained using the extinction coefficient of 13,600 M−1 cm−1 [28]. The sulfhydryl group content was expressed as nanomoles per milligram of sarcoplasmic proteins.
Carbonyl group content
The carbonyl groups of sarcoplasmic proteins (4 mg/ml) were evaluated with the DNPH method [29]. Samples (100 µl) were precipitated with 10 % (w/v) trichloroacetic acid (TCA). Pellets were treated with 0.2 % (w/v) 2,4-dinitrophenylhydrazine (DNPH) in 2 M HCl (800 µl). For the HCl control, pellets were treated only with 2 M HCl. Samples were incubated under agitation (Disruptor Genie, Scientific Industries, Bohemia, NY, USA) for 1 h at room temperature. The samples were then precipitated with 10 % TCA and centrifuged for 5 min (10,000g) at 4 °C. Pellets were washed three times with 1 ml of ethanol-ethyl acetate (1:1). Dried pellets were dissolved in 1 ml of 6 M guanidine hydrochloride (2 mM sodium phosphate buffer, pH 6). After 30 min of agitation (1000 rpm) at room temperature, insoluble residues were removed (10,000g for 5 min at 4 °C). The spectrum of the DNPH sample was corrected with the HCl control. Protein absorption at 280 nm was used to calculate protein concentration. The carbonyl groups were estimated using the extinction coefficient of 21,000 M−1 cm−1 at 370 nm [29] and were expressed as nanomoles of DNPH fixed per milligram of sarcoplasmic proteins.
Turbidity measurements and protein solubility
The turbidity of sarcoplasmic proteins solutions was evaluated by measuring the absorbance at 660 nm [30]. The protein solubility of sarcoplasmic proteins was measured following the procedure of Jung et al. [31]. Samples were centrifuged (14,000g for 20 min at 4 °C), and the protein concentration of supernatants was determined using the Biuret assay [25] with bovine serum albumin as a standard.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Soluble sarcoplasmic proteins were analyzed by SDS-PAGE [26] using a PhastSystem separation and development unit (GE Healthcare, UK). The samples were diluted with a denaturation buffer (0.125 M Tris–HCl containing 4 % SDS, 1 % 2-mercaptoethanol and 0.02 % bromophenol blue; pH 6.8) and heated at 100 °C for 5 min. The soluble sarcoplasmic proteins were adjusted to the same concentration. Proteins (0.5 mg/ml) were loaded on 20 % polyacrylamide gels. The gels were stained with nitrate silver. Protein markers (GE Healthcare, UK) were used to determine the molecular weight (phosphorylase b, 97 kDa; albumin, 66 kDa; ovalbumin, 45 kDa; carbonic anhydrase, 30 kDa; trypsin inhibitor, 20.1 kDa; and alpha-lactalbumin, 14.4 kDa).
Far UV circular dichroism spectra
Circular dichroism measurements were performed to evaluate the secondary structure of sarcoplasmic proteins. The protein solutions (0.2 mg/ml) in extraction buffer (pH 7; 100 mM KCl, 50 mM Tris–HCl and 5 mM EDTA) were analyzed using a Jasco 810 spectropolarimeter (Jasco, Tokyo, Japan) at room temperature. Spectra at the far UV (190–250 nm) were obtained from samples in a quartz cell with a 0.02-cm path cell length. Each spectrum was the average of three scans (scan rate of 50 nm/min, response time of 1 s and bandwidth of 2 nm), and the buffer signal was subtracted. A mean residue weight of 110 g/mol was considered to estimate the mean residue ellipticity (deg cm2 dmol−1). The secondary structure was estimated by CDpro software (CDSSTR method) [32].
Emulsion stability and protein adsorption
Emulsion stability and protein adsorption were evaluated according to the method of Hemung et al. [11] with some modifications. The sarcoplasmic protein solution (2 mg/ml) in extraction buffer (pH 7; 100 mM KCl, 50 mM Tris–HCl and 5 mM EDTA) and sunflower oil (proportion of 1:1) were homogenized for 1 min at 13,000 rpm (Ultra-Turrax T 25, IKA, Staufen, Germany). Emulsions were stored at 35 °C for 7 days. The emulsion phase was estimated as a percentage of the total volume. The protein adsorption was estimated by recovering the aqueous phase of emulsions separated from cut frozen tubes (−20 °C). The fraction of adsorbed sarcoplasmic proteins was the difference of total protein content minus the amount of remaining proteins in the aqueous phase. The protein concentration in the aqueous phase was determined using the bicinchoninic acid assay (Sigma-Aldrich, Saint-Louis, MO, USA). The adsorbed sarcoplasmic proteins at the interface were presented as the percentage of total proteins.
Droplet size distribution of emulsions
The droplet size distribution was obtained using a Mastersizer 3600 (Malvern Instruments Ltd., Malvern, UK) by laser light diffraction (λ: 633 nm). The prepared emulsions were diluted (1/10) in a 0.05 M Tris–HCl buffer (pH 8) with 1 % SDS. The volume-weighted mean diameter (d 4,3) and the span were measured.
Flow behavior of emulsions
Flow measurements were performed in an AR 1000 rheometer (TA Instrument, New Castle, UK) equipped with a cone and plate (1.58° angle and 60 mm diameter) at 20 °C. The data were fitted to the Herschel–Bulkley model to determine the yield stress (Pa), the consistency index (Pa sn) and the flow behavior index.
Statistical analysis
Analyses were performed in triplicate. Analysis of variance and Tukey’s test with a confidence level of 95 % were performed using Statgraphics Centurion XVII 17.1.04 software (Statpoint Technologies, Warrenton, VA, USA).
Results and discussion
High-pressure denaturation of sarcoplasmic proteins
The impact of high-pressure processing on the solubility of sarcoplasmic proteins is shown in Fig. 1. The solubility of sarcoplasmic proteins decreased with pressure level. Solubility loss of sarcoplasmic proteins was a first indication of protein denaturation. Approximately 87 % of sarcoplasmic proteins remained soluble after a treatment at 200 MPa. The solubility of sarcoplasmic proteins was further reduced at 400 and 600 MPa with only 32 and 20 % of soluble sarcoplasmic proteins, respectively. Our results were in accordance to previous investigations [16, 17, 22]. The soluble sarcoplasmic proteins from frozen mackerel represented only 32 % of the total after treatment at 450 MPa. However, the sarcoplasmic protein solubility of frozen mackerel is not affected by a treatment at 150 MPa [17]. In chilled salmon, the solubility of sarcoplasmic proteins decreases with pressure treatments at low pressure levels (170–200 MPa) [22]. For sea bass fillets, the solubility of sarcoplasmic proteins is reduced by 4.5-fold after high-pressure treatments (400 MPa) [16]. Likewise, the pressure-shift freezing (140 MPa and −14 °C) of turbot fillets decreases the solubility of sarcoplasmic proteins during frozen storage [21].
After pressure treatment, the sarcoplasmic proteins in solution presented an increased turbidity as shown in Fig. 1. The turbidity of the sarcoplasmic protein solutions increased with pressure levels until 400 MPa without any additional increase at 600 MPa. The increase in turbidity reflects the protein aggregation [33]. Thus, our results suggested that aggregation of sarcoplasmic proteins occurred from 200 MPa, which was consistent with the solubility loss of sarcoplasmic proteins previously reported. The sarcoplasmic proteins involved in pressure-induced aggregation were identified through the electrophoretic profiles of soluble sarcoplasmic proteins shown in Fig. 2. No differences were found among the electrophoretic profiles of sarcoplasmic proteins from the four hakes analyzed. The soluble sarcoplasmic proteins presented molecular weights between 7 and 78 kDa. Untreated sarcoplasmic proteins exhibited 12 major protein bands of 78, 73, 63, 48, 42, 37, 32, 30, 22, 19, 12 and 7 kDa. The electrophoretic profiles were similar to the pattern of sarcoplasmic proteins from European hake [34]. After high-pressure treatment at 200 MPa, the sarcoplasmic protein bands presented the same profile as the untreated proteins. The identification of sarcoplasmic proteins involved in the solubility loss at 200 MPa was not possible; probably due to reversible dissociation and reassociation of oligomeric structures at low pressure levels [35, 36]. Nevertheless, sarcoplasmic proteins subjected to 400 MPa did not present a similar pattern to untreated proteins. The solubility of five individual proteins with molecular weights of 78, 73, 63, 37 and 22 kDa was affected. At the same concentration, minor sarcoplasmic proteins (58, 18 and 10 kDa) became major soluble proteins after pressure treatment at 400 MPa. Several sarcoplasmic proteins were further affected after a pressure treatment at 600 MPa. The protein bands with molecular weights of 58, 37, 32 and 12 kDa exhibited solubility loss at 600 MPa. In fact, the sarcoplasmic proteins involved depend on the pressure level. From previous studies, it was also noted that the types of sarcoplasmic proteins affected by high-pressure processing vary among the fish species. In the case of salmon, high-pressure processing (170–200 MPa) promotes partial loss of a protein band (29 kDa) that is identified as phosphoglycerate mutase [22]. The electrophoretic profile of sarcoplasmic proteins extracted from pressure-treated sea bass also shows a reduced solubility of two proteins (52.6 and 24.6 kDa) at 250 MPa, and 14 protein bands (158.8, 146.9, 84.3, 76, 54, 52.6, 48.3, 41, 24.7, 18.8, 15.6, 14.1, 13.6 and 10.8 kDa) present a reduced band intensity at 400 MPa [16]. Pazos et al. [17] found significant changes in the electrophoretic profile of sarcoplasmic proteins from frozen Atlantic mackerel at 300–450 MPa. They identified four involved sarcoplasmic proteins as creatine kinase (theoretical molecular weight at 42.9 kDa), fructose-bisphosphate aldolase A (39.6 kDa), glycogen phosphorylase (97.2 kDa) and beta-enolase (47.1 kDa). At 450 MPa, triosephosphate isomerase B (26.5 kDa), phosphoglucomutase (60.8 kDa) and phosphoglycerate kinase (44.1 kDa) are also implicated. In general, the electrophoretic profiles of sarcoplasmic proteins show that more proteins are affected with increasing pressure level and the species-specific patterns should affect in a different manner the quality of treated fish products. The selective denaturation of sarcoplasmic proteins will modify the protein gelation and the water-holding capacity of fish products [7, 17].
The changes in surface hydrophobicity of extracted sarcoplasmic proteins are presented in Fig. 3. The surface hydrophobicity of sarcoplasmic proteins at 200 MPa was increased by twice in comparison with native proteins. However, there was no statistically significant difference. High-pressure processing significantly enhanced the surface hydrophobicity of sarcoplasmic proteins at 400 MPa. The majority of hydrophobic groups are buried in globular proteins [37]. The extent of the exposure of hydrophobic groups of proteins could be indicative of a high content of globular structures. Our results were in accordance with an earlier study demonstrating that the surface hydrophobicity of sarcoplasmic proteins from treated chicken meat increases with pressure level [38].
The modifications of the surface hydrophobicity of sarcoplasmic proteins reflect the effect of high pressure on their quaternary and tertiary structures. Firstly, the slight increase in surface hydrophobicity of sarcoplasmic proteins at 200 MPa may have resulted from oligomeric dissociation. Glyceraldehyde-3-phosphate, lactate dehydrogenase and pyruvate kinase, which are sarcoplasmic proteins [39], dissociate and reassociate under pressure and after release of pressure (≤200 MPa) [35, 40]. In fact, the quaternary structure of proteins, which is stabilized by hydrophobic interactions, is sensitive to low pressure levels (<150–200 MPa) [36]. Secondly, the greater exposure of hydrophobic residues observed at 400 and 600 MPa may represent significant changes in the tertiary structure of sarcoplasmic proteins. Pressure-induced denaturation of sarcoplasmic proteins could be due to the weakening of hydrophobic interactions and to the exposure of the hydrophobic core, which is a consequence of decreasing volume under pressure [41]. Therefore, the solubility loss of sarcoplasmic proteins with pressure level increase might be the result of enhanced protein–protein interactions due to the increase in surface hydrophobicity.
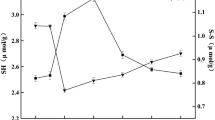
The effects of high-pressure processing on the total sulfhydryl and reactive sulfhydryl content are shown in Fig. 4. High-pressure processing increased the reactive sulfhydryl content of sarcoplasmic proteins at 400 MPa without further increasing at 600 MPa. At these pressure levels, the exposure of sulfhydryl groups from the hydrophobic core of sarcoplasmic proteins is probably favored. In addition, high-pressure processing reduced the total sulfhydryl content of sarcoplasmic proteins at 600 MPa. Therefore, the induced aggregation at 600 MPa could imply the contribution of disulfide bond linkages. The protein unfolding at 400 MPa increased the reactivity of sulfhydryl groups, which may promote protein–protein interactions. However, the sulfhydryl groups were probably not close enough to form disulfide bonds. The oxidation of sulfhydryl groups is associated with the inactivation mechanism of glycolytic enzymes (lactate dehydrogenase and pyruvate kinase) by high pressure [40, 42]. In contrast to our results, the ratio of reactive and total sulfhydryl contents of sarcoplasmic proteins from beef muscle is not affected after high-pressure treatment (551 MPa, −35 or 3 °C) although it is enhanced for total soluble proteins [43]. Sarcoplasmic proteins from different species might not be affected by high pressure in the same manner as noted for myofibrillar proteins. The total sulfhydryl content of actomyosin from threadfin bream decreases at 200 MPa [15], and the total sulfhydryl content of actomyosin from tilapia decreases at pressures greater than 200 MPa [44]. However, for myofibrils from hake, the sulfhydryl content is not affected by treatments at 250 and 500 MPa [14].
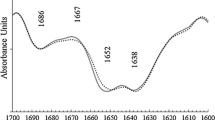
The influence of high-pressure processing on the secondary structure of sarcoplasmic proteins is shown in Fig. 5. The native secondary structure of sarcoplasmic proteins was characterized by a high contribution of alpha-helix structures (44 %) followed by unordered structures (26 %), beta-turns (18 %) and beta-sheets (12 %). The dominance of alpha-helix structures was in accordance with the results of Tadpitchayangkoon et al. [45] for sarcoplasmic proteins (pH 7) from striped catfish. The secondary structure of the sarcoplasmic proteins was not altered when they were subjected to 200 MPa. A previous report also did not find any influence of pressure treatment at 210 MPa on the secondary structure of a glycolytic enzyme (pyruvate kinase) [40]. Nevertheless, sarcoplasmic proteins treated at 400 MPa exhibited a conformational transition, which promoted beta-sheet and beta-turn structures, and the alpha-helix structures of sarcoplasmic proteins were greatly reduced and became a minor contribution to the secondary structures of proteins. The disruption of alpha-helix structures and the formation of beta-sheet structures of sarcoplasmic proteins are involved in protein aggregation [45]. The unordered structures were slightly favored by treatments at 600 MPa in comparison with untreated sarcoplasmic proteins. The conformational transition of sarcoplasmic proteins from hake induced by high-pressure processing was similar to that observed in treated myofibrillar proteins from hake. The pressure denaturation of myofibrillar proteins results in the decrease in alpha-helix and the increase in beta-sheet and beta-turn structures [14]. However, the protein unfolding promoted by high pressure can also be related to the main formation of unordered structures as reported by previous studies on beta-lactoglobulin and ribonuclease A [46, 47]. The differences between beta-sheet and unordered structures formation in the denaturated state will depend on native structure of proteins. The unordered structures are favored after pressure treatment of proteins containing high content of beta-sheet structures [46, 47], but sarcoplasmic and myofibrillar proteins contain a major fraction of alpha-helix structures.
Moreover, no significant differences were found in the secondary structures of sarcoplasmic proteins treated at 400 and 600 MPa. Sarcoplasmic proteins are less susceptible to pressure-induced changes in comparison with effects reported on the secondary structure of myofibrillar proteins from hake, as alpha-helix structures of myofibrillar proteins from hake decrease at high-pressure treatments at 150 MPa [14]. In general, high-pressure processing at a high level (greater than 300 MPa) is well known to change the secondary structure of proteins, leading to a non-reversible denaturation [36]. The predominant role of internal cavities in the changes of protein structure by water penetration has been suggested [48]. Likewise, water structure changes and their impact on the hydrophobic effects that stabilize folded proteins are indicated as the main factor of protein denaturation [41]. The unfolding of sarcoplasmic proteins under high pressure is a consequence of a mechanism accompanying volume reduction. The volume occupied by unfolded proteins is smaller than the volume of native proteins. Therefore, pressure can result in unfolded proteins with greater compressibility due to solvation and it can also induce an unfolded state with reduced compressibility as consequence of void volume and solvent exclusion [35]. The latter state was probably reached at 400 MPa; thus, increasing the pressure did not promote further denaturation of sarcoplasmic proteins.
Despite the structural modifications previously observed, high-pressure processing did not significantly promote protein carbonylation of extracted sarcoplasmic proteins (Table 1). In contrast to our results, previous studies have noted the generation of protein carbonyls by sarcoplasmic proteins due to the processing of pork and beef products [49, 50]. Moreover, loss of sulfhydryl groups is also considered as a consequence of protein oxidation [51], which was observed in the pressure-treated sarcoplasmic proteins (Fig. 4). Thus, high-pressure processing may cause protein oxidation of sarcoplasmic proteins with cysteine as the main target. For example, the oxidation of sulfhydryl groups has been suggested during the pressure treatment of pyruvate kinase, which has nine free cysteine residues per subunit [40]. These structural modifications could affect the nutritional value of fish products because the sulfhydryl oxidation has been reported to reduce digestibility of fish proteins [52]. For these reasons, further studies will be necessary for understanding the mechanisms of protein oxidation induced by pressure in fish products, as well as the role of lipid oxidation in this mechanism.
Emulsifying properties of high-pressure-treated sarcoplasmic proteins
Pressure-induced denaturation of proteins may improve or reduce their emulsifying properties [53–55]. The characteristics of emulsions stabilized by sarcoplasmic proteins are presented in Table 2. Emulsions stabilized by sarcoplasmic proteins treated at 200 MPa exhibited different rheological properties in comparison with emulsions stabilized by native sarcoplasmic proteins. The consistency index is indicative of emulsion viscosity, which was enhanced by sarcoplasmic proteins treated at 200 MPa. The higher shear-thinning behavior (decrease in flow behavior index) and consistency index suggest the increase in flocculated droplets in the emulsion that alter the creaming rate of emulsions [56]. The emulsion stability was improved when sarcoplasmic proteins were treated at 200 MPa, which was probably due to trapped droplets in a network of aggregated droplets at a high oil droplet concentration [56]. In contrast, emulsions stabilized by sarcoplasmic proteins treated at 400 and 600 MPa showed a decrease in yield stress, consistency index and shear-thinning behavior. These characteristics showed a decrease in flocculated droplets in the emulsion. The yield stress is associated with the strength of attractive forces between droplets [57, 58]. Therefore, the attractive interaction between droplets was reduced when sarcoplasmic proteins were treated at 400 or 600 MPa before stabilizing the emulsions. In addition, the stability of emulsions containing sarcoplasmic proteins treated at 400 and 600 MPa decreased. During storage, the exposure of reactive groups of sarcoplasmic proteins by pressure denaturation may favor the attractive interactions between droplets leading to flocculation [56].
The droplet size distribution of emulsions stabilized by treated sarcoplasmic proteins was not different in comparison with emulsions stabilized by native sarcoplasmic proteins. Thus, the changes on the physicochemical properties of sarcoplasmic proteins did not affect the droplet size obtained during homogenization. In addition, protein adsorption results showed that sarcoplasmic proteins treated at 600 MPa were slightly better adsorbed than untreated sarcoplasmic proteins. A similar behavior has been reported after high-pressure treatment of soybean proteins [55, 59]. The percentage of adsorbed proteins increases after the treatment of soybean proteins, and it is related to the increased surface hydrophobicity of treated soybean proteins [55]. The better adsorption of sarcoplasmic proteins treated at 600 MPa could be due to the greater surface hydrophobicity of treated protein. However, the treatment did not reduce the droplet size, which may be explained by the interaction between the proteins in the aqueous phase and the proteins that were already adsorbed at the interface [59]. As a consequence, pressure denaturation of sarcoplasmic proteins at 400 and 600 MPa tended to enhance protein adsorption. Initially, the pressure denaturation may decrease attractive forces and restrict the interaction between droplets. Subsequently, the reactive groups of unfolded proteins could promote hydrophobic interactions between adsorbed proteins, which would destabilize these emulsions.
Conclusions
High-pressure processing induces the denaturation of extracted sarcoplasmic proteins from hake. Protein aggregation of sarcoplasmic proteins by pressure denaturation was induced at 200 MPa. Specific sarcoplasmic proteins (78, 73, 63, 58, 37, 32 and 22 kDa) were susceptible to protein aggregation. However, the structural characteristics of treated sarcoplasmic proteins depended on pressure level. Protein aggregates at 200 MPa were formed by weak forces, such as hydrophobic interactions, due to dissociation of some sarcoplasmic proteins. The aggregation process was increased at 400 MPa owing to the disruption of tertiary and secondary structures of sarcoplasmic proteins. At 600 MPa, protein aggregates may have been formed by hydrophobic interactions and disulfide bonds. Pressure denaturation of sarcoplasmic proteins promoted destabilizing interactions in emulsions that decreased the emulsifying capacity of sarcoplasmic proteins. Indeed, high-pressure processing modifies the functionality of sarcoplasmic proteins. Nevertheless, further research is needed to evaluate its implication on the gelation of myofibrillar proteins in treated fish or meat products.
References
Xiong YL (1997) In: Damodaran S, Paraf A (eds) Food proteins and their applications, 1st edn. Marcel Dekker Inc., New York
Hui YH, Cross N, Kristinsson HG, Lim MH, Nip WK, Siow LF, Stanfield PS (2012) In: Simpson BK (ed) Food biochemistry and food processing, 2nd edn. Wiley-Blackwell, Ames
Sikorski ZE, Kolakowska A, Sun Pan B (1990) In: Sikorski ZE (ed) Seafood: resources, nutritional composition, and preservation, 1st edn. CRC Press Inc., Boca Raton
Godiksen H, Morzel M, Hyldig G, Jessen F (2009) Contribution of cathepsins B, L and D to muscle protein profiles correlated with texture in rainbow trout (Oncorhynchus mykiss). Food Chem 113(4):889–896
Okada M (1964) Effect of washing on the jelly forming ability of fish meat. Nippon Suisan Gakkaishi 30(3):255–261
Shimizu Y, Nishioka F (1974) Interactions between horse mackerel actomyosin and sarcoplasmic proteins during heat coagulation. Nippon Suisan Gakkaishi 40:231–234
Jafarpour A, Gorczyca EM (2009) Characteristics of sarcoplasmic proteins and their interaction with surimi and kamaboko gel. J Food Sci 74(1):N16–N22
Siriangkanakun S, Yongsawatdigul J (2012) Trypsin inhibitory activity and gel-enhancing effect of sarcoplasmic proteins from common carp. J Food Sci 77(10):C1124–C1130
Krasaechol N, Sanguandeekul R, Duangmal K, Owusu-Apenten RK (2008) Structure and functional properties of modified threadfin bream sarcoplasmic protein. Food Chem 107(1):1–10
Yongsawatdigul J, Hemung BO (2010) Structural changes and functional properties of threadfin bream sarcoplasmic proteins subjected to pH-shifting treatments and lyophilization. J Food Sci 75(3):C251–C257
Hemung BO, Benjakul S, Yongsawatdigul J (2013) pH-dependent characteristics of gel-like emulsion stabilized by threadfin bream sarcoplasmic proteins. Food Hydrocoll 30(1):315–322
Vidacek S, de las Heras C, Solas MT, Rodriguez Mahillo AI, Tejada M (2009) Effect of high hydrostatic pressure on mortality and allergenicity of Anisakis simplex L3 and on muscle properties of infested hake. J Sci Food Agric 89(13):2228–2235
Chapleau NJ, de Lamballerie-Anton M (2003) Changes in myofibrillar proteins interactions and rheological properties induced by high-pressure processing. Eur Food Res Technol 216(6):470–476
Cando D, Moreno HM, Tovar CA, Herranz B, Borderias AJ (2014) Effect of high pressure and/or temperature over gelation of isolated hake myofibrils. Food Bioprocess Technol 7(11):3197–3207
Zhou A, Lin L, Liang Y, Benjakul S, Shi X, Liu X (2014) Physicochemical properties of natural actomyosin from threadfin bream (Nemipterus spp.) induced by high hydrostatic pressure. Food Chem 156:402–407
Teixeira B, Fidalgo L, Mendes R, Costa G, Cordeiro C, Marques A, Saraiva JA, Nunes ML (2013) Changes of enzymes activity and protein profiles caused by high-pressure processing in sea bass (Dicentrarchus labrax) fillets. J Agric Food Chem 61(11):2851–2860
Pazos M, Méndez L, Gallardo JM, Aubourg SP (2014) Selective-targeted effect of high-pressure processing on proteins related to quality: a proteomics evidence in Atlantic mackerel (Scomber scombrus). Food Bioprocess Technol 7(8):2342–2353
Marcos B, Mullen AM (2014) High pressure induced changes in beef muscle proteome: correlation with quality parameters. Meat Sci 97(1):11–20
Angsupanich K, Ledward DA (1998) High pressure treatment effects on cod (Gadus morhua) muscle. Food Chem 63(1):39–50
Ohshima T, Nakagawa T, Koizumi C (1992) In: Bligh E (ed) Seafood science and technology. Fishing News Books, Oxford
Chevalier D, Sequeira-Munoz A, Le Bail A, Simpson BK, Ghoul M (2000) Effect of pressure shift freezing, air-blast freezing and storage on some biochemical and physical properties of turbot (Scophthalmus maximus). LWT-Food Sci Technol 33(8):570–577
Ortea I, Rodríguez A, Tabilo-Munizaga G, Pérez-Won M, Aubourg SP (2010) Effect of hydrostatic high-pressure treatment on proteins, lipids and nucleotides in chilled farmed salmon (Oncorhynchus kisutch) muscle. Eur Food Res Technol 230(6):925–934
Okazaki E, Nakamura K (1992) Factors influencing texturization of sarcoplasmic protein of fish by high pressure treatment. Nippon Suisan Gakkaishi 58(11):2197–2206
Sikes A, Tornberg E, Tume R (2010) A proposed mechanism of tenderising post-rigor beef using high pressure–heat treatment. Meat Sci 84(3):390–399
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the Biuret reaction. J Biol Chem 177(2):751–766
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Kato A, Nakai S (1980) Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim Biophys Acta 624(1):13–20
Ellman GL (1958) A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys 74(2):443–450
Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER (1987) Age-related changes in oxidized proteins. J Biol Chem 262(12):5488–5491
Benjakul S, Visessanguan W, Ishizaki S, Tanaka M (2001) Differences in gelation characteristics of natural actomyosin from two species of bigeye snapper, Priacanthus tayenus and Priacanthus macracanthus. J Food Sci 66(9):1311–1318
Jung S, de Lamballerie-Anton M, Ghoul M (2000) Modifications of ultrastructure and myofibrillar proteins of post-rigor beef treated by high pressure. LWT-Food Sci Technol 33(4):313–319
Sreerama N, Woody RW (2000) Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem 287(2):252–260
Mahler HC, Friess W, Grauschopf U, Kiese S (2009) Protein aggregation: pathways, induction factors and analysis. J Pharm Sci 98(9):2909–2934
Losada V, Piñeiro C, Barros-Velázquez J, Aubourg SP (2004) Effect of slurry ice on chemical changes related to quality loss during European hake (Merluccius merluccius) chilled storage. Eur Food Res Technol 219(1):27–31
Boonyaratanakornkit BB, Park CB, Clark DS (2002) Pressure effects on intra- and intermolecular interactions within proteins. Biochim Biophys Acta Protein Struct Mol Enzymol 1595(1):235–249
Lullien-Pellerin V, Balny C (2002) High-pressure as a tool to study some proteins’ properties: conformational modification, activity and oligomeric dissociation. Innov Food Sci Emerg Technol 3(3):209–221
Damodaran S (1997) In: Damodaran S, Paraf A (eds) Food proteins and their applications, 1st edn. Marcel Dekker Inc., New York
Omana DA, Plastow G, Betti M (2011) The use of β-glucan as a partial salt replacer in high pressure processed chicken breast meat. Food Chem 129(3):768–776
Jafarpour A, Gorczyca EM (2012) Contribution of sarcoplasmic proteins to myofibrillar proteins gelation. J Food Sci 77(2):R73–R81
De Felice FG, Soares VC, Ferreira ST (1999) Subunit dissociation and inactivation of pyruvate kinase by hydrostatic pressure. Eur J Biochem 266(1):163–169
Grigera JR, McCarthy AN (2010) The behavior of the hydrophobic effect under pressure and protein denaturation. Biophys J 98(8):1626–1631
Schmid G, Lüdemann HD, Jaenicke R (1978) Oxidation of sulfhydryl groups in lactate dehydrogenase under high hydrostatic pressure. Eur J Biochem 86(1):219–224
Lowder AC, Waite-Cusic JG, DeWitt CAM (2014) High pressure–low temperature processing of beef: effects on survival of internalized E. coli O157: H7 and quality characteristics. Innov Food Sci Emerg Technol 26:18–25
Hsu KC, Hwang JS, Yu CC, Jao CL (2007) Changes in conformation and in sulfhydryl groups of actomyosin of tilapia (Orechromis niloticus) on hydrostatic pressure treatment. Food Chem 103(2):560–564
Tadpitchayangkoon P, Park JW, Mayer SG, Yongsawatdigul J (2010) Structural changes and dynamic rheological properties of sarcoplasmic proteins subjected to pH-shift method. J Agric Food Chem 58(7):4241–4249
Panick G, Malessa R, Winter R (1999) Differences between the pressure- and temperature-induced denaturation and aggregation of β-lactoglobulin A, B, and AB monitored by FT-IR spectroscopy and small-angle X-ray scattering. Biochemistry 38(20):6512–6519
Panick G, Winter R (2000) Pressure-induced unfolding/refolding of ribonuclease A: static and kinetic Fourier transform infrared spectroscopy study. Biochemistry 39(7):1862–1869
Roche J, Caro JA, Norberto DR, Barthe P, Roumestand C, Schlessman JL, Garcia AE, Garcia-Moreno BE, Royer CA (2012) Cavities determine the pressure unfolding of proteins. Proc Natl Acad Sci USA 109(18):6945–6950
Rowe LJ, Maddock KR, Lonergan SM, Huff-Lonergan E (2004) Influence of early postmortem protein oxidation on beef quality. J Anim Sci 82(3):785–793
Sun W, Zhao M, Yang B, Zhao H, Cui C (2011) Oxidation of sarcoplasmic proteins during processing of Cantonese sausage in relation to their aggregation behaviour and in vitro digestibility. Meat Sci 88(3):462–467
Lund MN, Heinonen M, Baron CP, Estevez M (2011) Protein oxidation in muscle foods: a review. Mol Nutr Food Res 55(1):83–95
Opstvedt J, Miller R, Hardy RW, Spinelli J (1984) Heat-induced changes in sulfhydryl groups and disulfide bonds in fish protein and their effect on protein and amino acid digestibility in rainbow trout (Salmo gairdneri). J Agric Food Chem 32(4):929–935
Galazka VB, Dickinson E, Ledward DA (1996) Effect of high pressure on the emulsifying behaviour of β-lactoglobulin. Food Hydrocoll 10(2):213–219
Chapleau N, de Lamballerie-Anton M (2003) Improvement of emulsifying properties of lupin proteins by high pressure induced aggregation. Food Hydrocoll 17(3):273–280
Puppo MC, Speroni F, Chapleau N, de Lamballerie M, Añón MC, Anton M (2005) Effect of high-pressure treatment on emulsifying properties of soybean proteins. Food Hydrocoll 19(2):289–296
McClements DJ (2009) In: Kasapis S, Norton IT, Ubbink JB (eds) Modern biopolymer science, 1st edn. Elsevier, San Diego
Paraskevopoulou A, Boskou D, Kiosseoglou V (2005) Stabilization of olive oil–lemon juice emulsion with polysaccharides. Food Chem 90(4):627–634
Hayati IN, Man YBC, Tan CP, Aini IN (2007) Stability and rheology of concentrated O/W emulsions based on soybean oil/palm kernel olein blends. Food Res Int 40(8):1051–1061
Puppo MC, Beaumal V, Chapleau N, Speroni F, de Lamballerie M, Añón MC, Anton M (2008) Physicochemical and rheological properties of soybean protein emulsions processed with a combined temperature/high-pressure treatment. Food Hydrocoll 22(6):1079–1089
Acknowledgments
We thank the IMPACT Core Facility Biogenouest (Nantes, France) for the technical assistance in the circular dichroism measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Human and animal rights
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Villamonte, G., Pottier, L. & de Lamballerie, M. Influence of high-pressure processing on the physicochemical and the emulsifying properties of sarcoplasmic proteins from hake (Merluccius merluccius). Eur Food Res Technol 242, 667–675 (2016). https://doi.org/10.1007/s00217-015-2574-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-015-2574-z