Abstract
The lycopene pigment found abundantly in tomato peels has been proven to own antioxidant capacity and reduce risks of getting cancers. The present study aimed to investigate effects of enzymatic pretreatment to assist lycopene extraction from tomato peels using rice bran oil (RBO) as a green solvent. The peels were pretreated using Viscozyme L at different concentrations (0.5–2.5%), different incubation temperatures (30–70 °C), and incubation durations (30–150 min). The enzyme-assisted extraction conditions for lycopene from tomato peels were optimized using response surface methodology (RSM) based on Box–Behnken design with three levels of design factors (− 1, 0, and + 1). Pretreated peels were then extracted for 30 min at 25 °C using rice bran oil at a solid/oil ratio of 1:20 (w/v). Lycopene concentration were concurrently analyzed using Ultra Performance Liquid Chromatography system. The optimal extraction condition was 1.4% Viscozyme L incubated at 52 °C for 92 min resulted in a rice bran oil sample containing the highest concentration of lycopene (0.75 mg lycopene/100 ml rice bran oil or 399.6 mg lycopene/100 g dried tomato peels). Lycopene extraction using RBO along with Viscozyme L assistance could be a friendly extraction method to utilize the tomato-processing waste. RMS has been an effective tool for determining the optimal lycopene extraction conditions required to achieve a lycopene-containing oil product with both health and economic potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tomato (Lycopersicon esculentum L.) belonging to the Solanaceae family, is a commonly cultivated vegetable that originated in South America. The vegetable is a good source of nutritional compositions, from carbohydrate, proteins, lipids, to micronutrients such as vitamins A, vitamin C, thiamine, pyridoxine, folic acid, riboflavin, niacin [1], nitrogen, calcium, magnesium, phosphorus, potassium, sulfur, zinc, manganese, boron and others [2]. Moreover, significant amounts of phenolic acids, flavonoids, lycopene, and β-carotene are obtained greatly in ripened tomato fruits and tomato products that bring their great benefit to human health [3]. Lycopene is considered as a protector against cancer and other degenerative diseases caused by free radical reactions due to its high degree of conjugation [4]. Heat and light induce the isomerization that converts all-trans isomers to cis-isomers due to additional energy input, thereby changing the lycopene content [4]. Lycopene from tomato products is also used as a food additive in the food industry to improve storage stability and nutritional properties [5].
By-products from the production of tomato-based products such as tomato pomace including peels and seeds, on the other hand, are also rich sources of lycopene but seems to be neglected and treated as wastes since it is considered to be indigestible and low in nutrients. However, the dry pomace contains more than 50% tomato peels which occupies a lycopene content about 5 times greater than in the pulp [6,7,8]. The current challenge is to figure out how to take advantage of this low-cost lycopene source while also determining the best method for extracting and preserving lycopene. Several studies on extraction of lycopene using solvents, supercritical carbon dioxide, enzymatic hydrolysis, and supersonic-assisted treatments have been reported. Since lycopene is fat soluble, it is more commonly extracted with organic solvents [4, 9, 10]. However, organic solvents are generally poisonous, and even trace amounts of the extracting solvents in finished products must be considered [11]. Moreover, using solvent extraction solely is reported to be less efficient in lycopene extraction due to the plant tissue's compactness, which prevents solvent penetration to the lycopene-containing chromoplasts [12].
Since tomato peel is a highly structured plant material containing several different polysaccharides such as cellulose, hemicelluloses, and pectins [13], effects of mixed enzyme preparations with pectinolytic, cellulolytic, and hemicellulolytic activities were investigated to improve lycopene extraction [12]. With the view to making use of more lycopene in the tomato pomace with shorter duration of treatments, and saved production costs, Viscozyme L, a multi-enzyme complex including arabanase, cellulase, beta-glucanase, hemicellulase, and xylanase, is selected. There are several studies reporting on the effective uses of this enzyme for the extraction of polyphenol compounds from plant sources, such as berries [14] and oat bran [15]. Focusing on those promising beginnings, optimal enzyme concentration, incubation time, and temperature are studied onwards to obtain the highest yields of lycopene content from tomato peels. In addition, vegetable oils such as olive oil [16], almond, and sunflower seed oil [17] eventually become the green solvent of choice for many researchers in order to satisfy the need for a solvent that is ideal for lycopene, protect it from oxidation, and have no negative health effects. Along with the trend of utilizing food industrial waste, rice oil, also known as rice bran oil (RBO), a vegetable oil recovered as a by-product of rice production is high in bioactive phytonutrients including phytosterols, -oryzanol, squalene, and triterpene alcohols, as well as vitamin E (both tocopherols and tocotrienols) which contribute to high antioxidant, anti-inflammatory, hypocholesterolemic, antidiabetic and anticancer activities [18].
In spite of having many reasons above, this study used RBO as a solvent to identify the appropriate enzymatic treatments for extracting lycopene from tomato peels. Since the enzyme concentration, the incubation time, and the temperature were three focused factors that directly affected the enzyme-assisted treatment, the response surface methodology (RSM) was used as an effective tool for the optimization process. In addition, Box–Behnken design was conducted due to its cost-effective design that could reduce the number of experimental trials [19]. With the presence of lycopene, this lycopene enriched oil would be a potential nutritional product and help to diversify the product for the edible oil industry.
Materials and methods
Materials
Sixty kg sound and ripe tomatoes was collected from Thu Duc agricultural product market, Ho Chi Minh city, Vietnam. Tomatoes utilized for this experience were ‘red’ according to USDA color grading standards [20].
Methanol was purchased from Merck Company, Germany. Tetrahydrofuran was purchased from Honeywell Riedel-de Haën company, Germany. Natural lycopene standard, butylated hydroxytoluene, sodium citrate dihydrate, and citric acid were purchased from Sigma Chemical Company, U.S.A. Viscozyme L with enzyme activity 100 (FBG/g), density 1.21 (g/ml), optimum temperature (40–50 °C), and pH (3.3–4.5) was purchased from Novozymes Co., Denmark. Simply pure rice bran oil was obtained from CALOFIC (Cai Lan oils and fats industries Company, Vietnam).
Sample preparation
Tomatoes came through a washing process with clean water to remove impurities before measuring moisture content of selected tomatoes. They were steamed for three minutes with boiling water (100 °C) after applying an adequate X-shape insertion on the bottom of each and the skin was then manually peeled. Cold breaking processes at 65 °C in 24 min applied to the tomato skins to enhance enzyme activity before incubating with enzymes to recover the lycopene [21] after grinding the peels for 1 min using blender (Philips HR2221/00). After that, fresh ground peels were store at − 4 °C. The moisture content of ground peels was taken with an infrared moisture analyzer (Kett FD720, Japan).
Effects of enzyme concentration
The enzymatic assisted extraction of lycopene [4] was conducted with some modifications. Briefly, tomato peels were added to 100 mM citrate buffer (pH 5) at a ratio of 10:1 (v/w) containing different enzyme concentrations: 0.5; 1; 1.5; 2; 2.5 (%). The mixture was then placed in a shaking incubator (IKA KS 4000, Germany) for 90 min at 40 °C. Lycopene content was determined after the extraction step.
Effects of enzymatic incubation duration
To test the effect of enzymatic time reaction, ground tomato skin was incubated at 40 °C and 2% of enzyme concentration with citrate buffer (pH 5) at a ratio of 10:1 (v/w). The mixture was then incubated for five-time intervals (30, 60, 90, 120, 150 min).
Effects of enzymatic incubation temperature
In order to find out the optimum temperature for lycopene extraction using Viscozyme L, variable temperature points (30, 40, 50, 60, 70 °C) were examined. The mixture of tomato peels and 100 mM of citrate buffer (pH 5) was incubated in the fixed duration at 90 min with 2% of Viscozyme L.
Lycopene extraction
Following the incubation process, the treated peels were heated to 90 °C for 5 min to inactivate enzymes prior extraction. The control sample was prepared by combining tomato peels and 100 mM citrate buffer (pH 5) at a ratio of 10:1 (v/w), and the mixture was then incubated at 40 °C for 90 min. Lycopene was recovered by adding rice bran oil to a flask containing peels collected in the enzyme inactivation step at a 20:1 (v/w) ratio and agitating the system for 30 min at 25 °C before centrifuging for 10 min at 9000 rpm and 4 °C. Finally, the topmost layer was collected, and the tests were carried out.
Lycopene extraction, storage, and analysis is took place in a regulated environment to prevent oxidative degradation and isomer formation, and lycopene exposure to light was prohibited [22].
UPLC analysis of lycopene
A method was based on the procedure of Sathish et al. with slight modifications [23]. The samples were resolved using UPLC system (Acquity UPLC H-Class/FD, UV, Waters, USA) with a C18 column (Acquity UPLC BEH, Waters, USA) (1.7 µm; 2.1 × 150 mm) and a mobile phase of methanol (Merck, Germany). Prior to use, the mobile phase was filtered over a 0.45 µm membrane and ultrasonically degassed. The column temperature was maintained at 40 °C, flow rate at 0.3 ml/min, and detection wavelength at 470 nm. The lycopene standard (Sigma-Aldrich, USA) and pigment-enriched oil were prepared by dissolving them in 10 ml of methanol/tetrahydrofuran mixture (50:50, %, v/v) with butylated hydroxytoluene (0.1% w/v) (Sigma-Aldrich, USA).
Box-Behnken experimental design
Box-Behnken design was employed to optimize the enzyme-assisted extraction for lycopene from tomato peels using rice bran oil. Three factors namely enzyme concentration (%, X1), incubation time (min, X2) and incubation temperature (°C, X3), consisting of 15 randomized runs with 3 center points (Table 1).
The equation (Eq. 1) from second order polynomial model of Montgomery was used as a reference to achieve the relationship between lycopene and three independent variables recalled enzyme concentration, incubation time, and incubation temperature:
where β0: constant number, β1, β2, β3: linear regression coefficient, β11, β22, β33: quadratic regression coefficient, β12, β13, β23: regression coefficient of interactions between factors
Statistical analysis
The analysis of results was performed with the statistical software Minitab®, version 19 (Minitab Inc., State College, PA, USA); Design Expert, version 12 (Stat-Ease Inc., Minneapolis, USA). The mean differences for all treatments were tested with one-way ANOVA and statistical significance differences between the mean values were established (P < 0.05) using Tukey's test. The results were expressed as mean ± standard deviation.
Results and discussion
In this study, the yields of lycopene extraction were evaluated as mg of lycopene per 100 ml oil, mg of lycopene per 100 mg of fresh tomato peels (FW) and mg lycopene per 100 mg of dried tomato peels (DB). The peel fraction of tomato had an average lycopene concentration of 114.6 ± 7.4 mg/100 g DB and a moisture level of 87.67 ± 0.45 wt%.
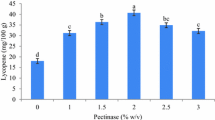
Effects of enzyme concentration
The results in Table 2 illustrate how the enzymatic treatment accelerated lycopene recovery. The maximum lycopene content was attained by adding enzyme at a concentration of 1.5% (40.3 ± 3.1 mg/100 g FW).
Corresponding to the research of Nguyen and Nguyen (2015), this enzyme level might completely break the fiber network and liberate lycopene from chromoplast fractions [24]. Moreover, the involvement of cellulase and hemicellulase in this enzyme system contributed to break down the primary wall's gel-like matrix, allowing for more efficient extraction [4]. Low enzyme concentrations (0.5 and 1%) were only able to open the pulp cells remaining connected to the peel, leaving the majority of the peel unaffected. Exceeding 1.5% Viscozyme L results in overall hydrolysis and lycopene inhibition [25]. A limited amount of substrates might come from an overabundance of enzymes since they were entirely utilized at lower enzyme concentrations, which prevented the production of lycopene. In addition, an excess amount of the enzyme may interact with the released lycopene and break down it as a result.
Effects of enzymatic incubation temperature
Enzyme implementation combined with temperatures higher than the typical ambient one at least doubled the quantity of lycopene produced compared to the untreated peels (Table 3). The amount of lycopene recovered steadily increased nearly 43% when the temperature was elevated from 30 to 50 °C. However, when the temperature rises, the rate of an enzyme-catalyzed process increases; unfortunately, this also caused adverse effects as many enzymes are detrimental to high temperatures [26]. At higher temperatures, the amount of lycopene released might be reduced owing to enzymatic denaturation, since the broken bonds in the active region of the enzyme were no longer able to assist cell destruction [27, 28]. Moreover, thermal deterioration stimulated lycopene isomerization of the all-trans form to cis-isomers and hence degradation of cis-isomers, resulting in a considerable drop in overall lycopene concentration [29].
Effects of enzymatic incubation duration
According to findings in Table 4, after the first 30 min of incubation at 40 °C, with each consecutive half an hour of incubation, the enhancement kept growing. The maximum lycopene content was attained in this investigation by incubating tomato peels within 90 min. The treatment was effective at short extraction times in which lycopene degradation is not likely to occur [30]. The enzyme, however, did not react enough with the sample after inadequate incubation (30 and 60 min) since lycopene crystals were observed firmly entrenched in the composite polysaccharides membrane structure of chromoplasts [31]. While the extraction time was prolonged than 90 min, the recovered lycopene was likely to be oxidized by the temperature and certain substrate or unwanted chemicals such as apo-lycopenals and apo-carotendials formed during the extraction or from the external environment [32].
Optimization of conditions for lycopene extraction
Table 5 presents the predicted and observed lycopene content in lycopene-enriched oil, as a result of the combined influence of all three significant factors within the specified ranges. The process variables and their ranges were selected based on the preliminary experimental results. Whilst the above screening tests were successful in achieving adequate conditions, each condition was insufficient to demonstrate the whole impact of a variable, the individual effect as an independent variable, and the interaction with other variables. Therefore, it is necessary to conduct the full quadratic model of the response surface design in order to have an overview of the optimal conditions and to identify the lack of fit of the model.
The coefficients on the response variables were obtained by analysis of variance (ANOVA) (Table 6) and the regression model was predicted as follows:
Obviously, the individual variables namely enzyme concentration (X1) and incubation duration (X3) and other second-order interaction factors including (X1X2), (X1X3) had statistically significant effects. The linear terms X2 (p = 0.06) still involved in the model for hierarchical purposes instead of its insignificant effect on lycopene extraction.
In Eq. 2, the positive and negative coefficients of the factors demonstrate how the response varies in relation to these variables. The positive sign of the coefficients in the regression equations denotes a synergistic effect, whilst the negative values suggest an antagonistic influence on the lycopene concentration [33]. The linear term of duration (X3), with a p-value less than 0.0001, has the most beneficial influence on the extraction yield, according to the regression coefficients. In addition, during enzymatic extraction, the incubation temperature (X2) had no direct effect on obtained lycopene content (P > 0.05) and did not show a synergistic impact with incubation time on the extracted substance.
The ANOVA results reveal that the models for lycopene content are significant, with an acceptable determination coefficient (R2 = 0.82) that has a good correlation with the predicted value (R2 = 0.72), meaning that the interaction between response and independent variables is adequate. In addition, the F-value (18.21) and P value (< 0.0001) of the model shown in Table 6 implying that it is significant. As shown in Table 7, adequate precision for responses is more than 4, indicating that the signals are appropriate [34]. Moreover, the model well describes the response while the experimental results are connected with a high degree of accuracy due to the low value of CV (less than 10) [35]. Furthermore, the lack-of-fit was not significant (P = 0.66), suggesting that the model goodness-of-fit is reliable. The effects of the operated factors and their interactions on the response of the analysis are well demonstrated in Table 6.
The 3-D response surface plot in Fig. 1 represents that the extracted carotenoid content in tomato increased when X1 and X2 increased in the range of 1–1.4%, and 40–52 °C, respectively; but surpassed 1.4% and 52 °C, the lycopene content gradually decreased. It means the fluctuations of both temperature and enzyme concentration became critical elements for enhancing the ideal substance and their interaction was consistent with the result in Table 6. Moreover, it might imply lycopene degradation as a result of the extended exposure of peels to high temperatures.
In Fig. 2, the concentration of lycopene is influenced by enzyme concentration and incubation time at a constant temperature of 50 °C. When the incubation time (X3) was prolonged in the range of 60–92 min, the extracted lycopene content in tomato increased, but when the temperature was raised above 92 min, the lycopene content steadily declined. This could be owing to the long-term exposure of lycopene to oxygen and light in the environment raises the chances of oxidation or breakdown [36].
The optimal extraction parameters for lycopene enzymatic extraction were achieved based on the desirability function methodology. The desired goal for each factor and response was chosen. The levels of enzyme concentration (1–2%), incubation temperature (40–60 °C), and incubation duration (60–120 min) were set. The optimization was carried out with the target of achieving 417.4 mg lycopene per 100 g tomato peels. In the range of 0–1, desirability d value illustrates the acceptance of response values. By seeking from 10 starting points in the response surface changes which have overall desirability of 1.00, the optimal extraction conditions were 1.4% enzyme, 52 °C, and 92 min of reaction time. The expected enzyme concentration under these conditions was 399.6 mg/100 g. A verification experiment was carried out under optimal conditions to confirm the adequacy of the model equation for predicting the optimum response value. The result demonstrated that the value (399.6 mg/100 g) predicted by the suggested model corresponded well with the observed value (400.2 mg/100 g) (P < 0.05). Therefore, the response model was suitable to reflect the expected optimation.
Conclusions
In this experiment, pretreatment of tomato peels with Viscozyme L can significantly enhance the extraction of lycopene from tomato peels, even with a short incubation time and mild temperatures. The results indicated that the application of BBD to optimize the extraction of lycopene from fresh tomato peels was successful with the development of a significant quadratic model for the prediction of lycopene extraction yield. Effect of independent variables including enzyme concentration and incubation duration on the responses was significant (P < 0.05). The optimal conditions for lycopene extraction were determined to be with 1.4% Viscozyme L at 52 °C, and incubation duration of 92 min in which approximately 399.6 mg lycopene/100 g tomato peels were achieved. The findings of this study would be a useful data for the discovery of potential natural carotenoid extraction from food processing waste and the development of a prospective lycopene-rich oil product.
Data availability
Not applicable.
References
M.T. Melfi, D. Nardiello, N. Cicco, V. Candido, D. Centonze, Simultaneous determination of water-and fat-soluble vitamins, lycopene and beta-carotene in tomato samples and pharmaceutical formulations: double injection single run by reverse-phase liquid chromatography with UV detection. J. Food Compos. Anal. 70, 9–17 (2018)
U.M. Sainju, R. Dris, B. Singh, Mineral nutrition of tomato. Food Agric. Environ. 1(2), 176–183 (2003)
B. Salehi, R. Sharifi-Rad, F. Sharopov, J. Namiesnik, A. Roointan, M. Kamle, P. Kumar, N. Martins, J. Sharifi-Rad, Beneficial effects and potential risks of tomato consumption for human health: an overview. Nutrition 62, 201–208 (2019)
R.C. Ranveer, S.N. Patil, A.K. Sahoo, Effect of different parameters on enzyme-assisted extraction of lycopene from tomato processing waste. Food Bioprod. Process. 91(4), 370–375 (2013)
J. Shi, M. Le Maguer. Degradation of lycopene in tomato processing. Paper presented at the VII International Symposium on the Processing Tomato 542. (2000)
M.M. Poojary, P. Passamonti, Extraction of lycopene from tomato processing waste: kinetics and modelling. Food Chem. 173, 943–950 (2015)
S. Machmudah, S. Winardi, M. Sasaki, M. Goto, N. Kusumoto, K. Hayakawa, Lycopene extraction from tomato peel by-product containing tomato seed using supercritical carbon dioxide. J. Food Eng. 108(2), 290–296 (2012)
E.H. Papaioannou, A.J. Karabelas, Lycopene recovery from tomato peel under mild conditions assisted by enzymatic pre-treatment and non-ionic surfactants. Acta Biochim. Pol. 59(1), 71–74 (2012)
T. Baysal, S. Ersus, D. Starmans, Supercritical CO2 extraction of β-carotene and lycopene from tomato paste waste. J. Agric. Food Chem. 48(11), 5507–5511 (2000)
M.J. Periago, F. Rincon, M.D. Agüera, G. Ros, Mixture approach for optimizing lycopene extraction from tomato and tomato products. J. Agric. Food Chem. 52(19), 5796–5802 (2004)
R. Ciriminna, A. Fidalgo, F. Meneguzzo, L.M. Ilharco, M. Pagliaro, Lycopene: emerging production methods and applications of a valued carotenoid. ACS Sustain. Chem. Eng. 4(3), 643–650 (2016)
R. Lavecchia, A. Zuorro, Improved lycopene extraction from tomato peels using cell-wall degrading enzymes. Eur. Food Res. Technol. 228(1), 153–158 (2008)
G.W. Cheng, D.J. Huber, Alterations in structural polysaccharides during liquefaction of tomato locule tissue. Plant Physiol. 111(2), 447–457 (1996)
N.T. Huynh, G. Smagghe, G.B. Gonzales, J. Van Camp, K. Raes, Enzyme-assisted extraction enhancing the phenolic release from cauliflower (Brassica oleracea L. var. botrytis) outer leaves. J. Agric. Food Chem. 62(30), 7468–7476 (2014)
N. Ratnasari, M. Walters, A. Tsopmo, Antioxidant and lipoxygenase activities of polyphenol extracts from oat brans treated with polysaccharide degrading enzymes. Heliyon 3(7), e00351 (2017)
M. Kehili, S. Sayadi, F. Frikha, A. Zammel, N. Allouche, Optimization of lycopene extraction from tomato peels industrial by-product using maceration in refined olive oil. Food Bioprod. Process. 117, 321–328 (2019)
M.H. Zuknik, N.N. Norulaini, A.M. Omar, Supercritical carbon dioxide extraction of lycopene: a review. J. Food Eng. 112(4), 253–262 (2012)
A. Ali, S. Devarajan, Nutritional and health benefits of Rice bran oil, in Brown rice. ed. by A. Manickavasagan, C. Santhakumar, N. Venkatachalapathy (Springer, Cham, 2017), pp.135–158
X. Guan, H. Yao, Optimization of Viscozyme L-assisted extraction of oat bran protein using response surface methodology. Food Chem. 106(1), 345–351 (2008)
U.S.D.A. Tomatoes, Shipping point and market inspection instructionsUnited States (Department of Agriculture, Washington, 2005)
H. Kelebek, S. Selli, P. Kadiroğlu, O. Kola, S. Kesen, B. Uçar, B. Çetiner, Bioactive compounds and antioxidant potential in tomato pastes as affected by hot and cold break process. Food Chem. 220, 31–41 (2017)
S.M. Choudhari, L. Ananthanarayan, Enzyme aided extraction of lycopene from tomato tissues. Food Chem. 102(1), 77–81 (2007)
T. Sathish, D. Udayakiran, K. Himabindu, P.L.D. Sridevi, D. Kezia, P. Bhojaraju, HPLC method for the determination of lycopene in crude oleoresin extracts. Asian J. Chem. 21(1), 139 (2009)
T.M.T. Nguyen, M.T. Nguyen, Effect of enzymatic treatments on lycopene in vitro bioaccessibility in high pressure homogenized tomato puree and chromoplast fraction. Can Tho Univ. J. Sci. 01, 61–68 (2015)
I. Çinar, Effects of cellulase and pectinase concentrations on the colour yield of enzyme extracted plant carotenoids. Process Biochem. 40(2), 945–949 (2005)
V.P. Nam, Improvement of lycopene extraction from tomatoes by enzyme–assisted treatment. Vietnam J. Sci. Technol. 54(4A), 275–275 (2016)
M.R. Ladole, A.B. Muley, I.D. Patil, M. Talib, V.R. Parate, Immobilization of tropizyme-P on amino-functionalized magnetic nanoparticles for fruit juice clarification. J. Biochem. Technol. 5(4), 838–845 (2015)
A. Sahoo, P.S. Badhe, R. Adivarekar, M.R. Ladole, A.B. Pandit, Synthesis of glycinamides using protease immobilized magnetic nanoparticles. Biotechnol. Rep. 12, 13–25 (2016)
J. Chen, J. Shi, S.J. Xue, Y. Ma, Comparison of lycopene stability in water-and oil-based food model systems under thermal-and light-irradiation treatments. LWT Food Sci. Technol. 42(3), 740–747 (2009)
A. Zuorro, M. Fidaleo, R. Lavecchia, Enzyme-assisted extraction of lycopene from tomato processing waste. Enzyme Microb. Technol. 49(6–7), 567–573 (2011)
R.K. Saini, A.E.-D.A. Bekhit, S. Roohinejad, K.R. Rengasamy, Y.-S. Keum, Chemical stability of lycopene in processed products: a review of the effects of processing methods and modern preservation strategies. J. Agric. Food Chem. 68(3), 712–726 (2019)
C. Caris-Veyrat, A. Schmid, M. Carail, V. Böhm, Cleavage products of lycopene produced by in vitro oxidations: characterization and mechanisms of formation. J. Agric. Food Chem. 51(25), 7318–7325 (2003)
S. Das, S. Mishra, Box–Behnken statistical design to optimize preparation of activated carbon from Limonia acidissima shell with desirability approach. J. Environ. Chem. Eng. 5(1), 588–600 (2017)
R.K. Agarwal, S.J.D. Bosco, Optimization of viscozyme L assisted extraction of coconut milk and virgin coconut oil. Asian J. Dairy Food Res. 33(4), 276–284 (2014)
E.A. Abd El-Salam, N.F. Morsy, Optimization of the extraction of polyphenols and antioxidant activity from Malva parviflora L. leaves using Box-Behnken design. Prep. Biochem. Biotechnol. 49(9), 876–883 (2019)
G. Chen, Z. Djuric, Carotenoids are degraded by free radicals but do not affect lipid peroxidation in unilamellar liposomes under different oxygen tensions. FEBS Lett. 505(1), 151–154 (2001)
Acknowledgements
This research is funded by International University, VNU-HCM under grant number T2020-06-BT
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tran, Q.T.N., Nguyen, H.V.H. Optimization of enzyme-assisted lycopene extraction from tomato (Lycopersicon esculentum) peel using rice bran oil. Food Measure 17, 5154–5162 (2023). https://doi.org/10.1007/s11694-023-02029-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02029-w