Abstract
The salt amounts of the brine samples decreased significantly and the salt values of brines varied between 4.47 (with flower honey) and 6.08% (with citric acid) depending on the additives added. The pH values of the brines ranged from 4.14 (with citric acid) to 4.98 (with glucose), while the titration acidity values of brines were found between 0.35 (plain salt) and 0.49% (with citric acid). Total carotenoid amounts of raw and fermented caperfruits were reported between 0.55 (with glucose) and 20.24 µg/g (control (raw)). The total flavonoid contents of raw and fermented capers were recorded between 234.52 (with flower honey) and 963.57 mgQE/100 g (with plain salt added), while the antioxidant activities of raw and fermented caperfruits are found between 6.49 (control) and 7.99 mmol TE/kg (with salt added). The dominant phenolic components of raw and fermented caperfruits were catechin, rutin, 3,4-dihydroxybenzoic acid, and gallic acid. Palmitic, stearic, oleic, linoleic and linolenic acids are the abundant fatty acids of the oils. P, K, Ca, Mg, S and Na were the most abundant minerals in raw (control) and fermented caper fruits. While caper fruits fermented in brine with added citric acid were most appreciated, followed by brined (only salty), sugared and flower honey, pine honey and glucose added fruit samples in decreasing order.
Graphical abstract
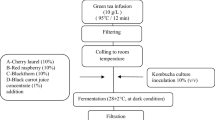
In this study, the effect of different additives (pine honey, flower honey, glucose syrup, citric acid and granulated sugar) into 10% brine and the desired composition properties and product qualities after 40 days of fermentation was investigated. Fermented caper fruits were also subjected to sensory analysis in accordance with their purpose, and the jury determined which of the caper fruits with additional additives was more delicious and high quality.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The caper, belonging to the Capparaceae family, is a shrub fruit, a perennial herb with medicinal and aromatic properties. In the world, the caper plant is known by various names such as “capers, galegrass, yum, thistle, gebre, şebellah, gemire, cedar grass, gevil, bubu, kebere, belt, menginik, caper, cat’s claw”, but it is generally called “caper”, “kebere” or “Gebre otu” in Turkey [1, 2]. The flower and fruits of caper plants have been stored in salt water and have become an expensive product in recent years. For human nutrition, young shoots, flower buds and fruits are used. All over the world, various organs of the caper plant are used according to the type and variety. Flower buds, roots, fruits, seeds and fresh shoots of caper plants are used as nutrition [3]. Capers are not a staple food consumed alone; it adds flavor to other foods or is used as a garnish [4]. Alternative composite flour mixtures affect the rheological and technological properties of dough, the overall quality and nutritional value of baked goods. Composite flours containing wheat and other grains and/or vegetable products have become popular in bakery products due to consumer interest in healthier foods. The brine process is applied to different parts of the caper plant. Plants are sources of valuable minerals, vitamins, phenolics and other important bioactive compounds. Inadequate intake of vitamins and minerals, which are the leading micronutrients, causes important problems in terms of public health and economy all over the world. For this reason, food enrichment, which is carried out by adding nutrients to foods in order to treat and prevent some diseases caused by malnutrition, is of great importance today [5,6,7,8]. Caper buds and caper fruits are used as flavor enhancers in foods such as salads, meat and bakery products, fish and milk products. In addition, Özcan [9] worked on pickled capers (Capparis spp.) and its storage. The same researcher also carried out the production of caper marmalade under laboratory conditions. There is limited information about the technological and sensory properties of caper paste [9,10,11,12]. Özcan [11] and Özcan [13] described the production process of caper marmalade and also determined its chemical properties, microbiological properties and mineral contents. The most used part of the caper plant and has an important international commercial value is the flower buds. In addition, fruit and shoot tips are also preserved in brine and vinegar, although not as much as seeds, and used for nutrition [14, 15]. Recently, the caper fruit has also started to be processed like the caper bud. The flower buds of the caper plant, which are rich in protein, vitamins and minerals, are collected and eaten by making pickles. The harvest of buds starts in May and continues until September [16, 17]. The formation of fruits takes place in July. It has a sour, salty, slightly burning and bitter taste and is appetizing [4]. Capparis spinosa L. buds and fruits, which are in bushy form, are one of the most important members of the genus economically, as they are widely used in the kitchen. This herb is of particular interest for its nutritional and medicinal properties [16, 18]. C. spinosa is consumed after processing the flower buds and fruits of capers, which are grown especially in the Mediterranean basin [19]. A study of the phenolic composition and antioxidant activity of flower buds has already been conducted [20, 21]. More comprehensive studies are needed to reveal the current properties of capers and to realize efficient production. The current level of studies on the brine processing of the caper plant is limited. The aim of this study is to determine the effect of different additives (pine honey, flower honey, glucose syrup, citric acid and granulated sugar) into 10% brine and the desired composition properties and product qualities after 40 days of fermentation. Fermented caper fruits were also subjected to sensory analysis in accordance with their purpose, and the panelists determined which of the caper fruits with additional additives was more delicious and high quality.
Materials and methods
Material
Fresh caper (Capparis ovata subsp. herbacea) fruits used in this study were collected from wild caper plants grown in Konya. After cleaning the fresh and small caper fruits, chemical analyzes of the fruits were carried out. While preparing the brine, commercially available water and clean non-iodized rock salt were used. In addition, citric acid, granulated sugar, pine honey, flower honey and glucose syrup were obtained from the market.
Methods
Processing of raw caper fruits in brine in different additive media
After adding 5% granulated sugar, pine honey, flower honey, glucose syrup and 0.5% citric acid separately into each fermentation vessel in brine (10%), it was mixed with the control group (only 10%) in brine was left to ferment for 40 days. Analyzes were made on fermented caper fruits and brine after the fermentation process was completed.
Measurement of pH
pH measurement was performed using a pH-meter (pH 211, Hanna Instruments, Portugal) [22].
Acidity
For titration acidity, each brine sample was titrated with 0.1 N NaOH solution using phenolphthalein indicator. Results are given in % [23].
Determination of salt
The salt content of the brine samples was determined by titration of the prepared samples with 0.1 N AgNO3 [24].
Moisture content
The water contents of the samples were determined in an oven (Nüve FN055 Ankara, Turkey) at 105 °C until a constant weight [25].
Determination of oil
Caper fruit samples for oil determination were dried and ground into powder after drying under normal atmospheric conditions. Then, approximately 10 g of ground fruit sample was extracted with petroleum ether in Soxhelet apparatus for 6 h. After extraction, the solvent was evaporated at 40 °C. Fat content is given on % dry matter [26].
Determination of Carotenoid
Extraction process for carotenoids in caper fruits was determined according to method described by da Rocha et al. [27]. After weighing 2 g of sample, 25 ml of acetone was added on it. After filtration process, the filtrate was fractionated with 20 ml of petroleum ether and washed with 100 ml of distilled water to remove acetone. After analytical procedures, the volume of the extracts was made up to 25 ml with petroleum ether. Absorbance was measured at 450 nm via a spectrophotometer.
Extraction process
For extraction, 20 ml of methanolic water (80:20 v/v) was added to approximately 2 g of sample and shaken in a shaking water bath at room temperature for 3 h. 20 ml of Hexane was added to the remaining extract from the filtered samples and after phase separation was achieved in the separating funnel, the remaining methanol phase was carefully taken into the tubes and used in the analysis [28].
Determination of total phenolic and flavonoid contents
Total phenol content of extracts was determined by using the Folin–Ciocalteu (FC) according to method stated by Yoo et al. [29]. 0.5 ml of extract was mixed with 2.5 ml of Folin–Ciocalteu reagent and 1.5 ml of sodium carbonate solution. The absorbance values of the samples were measured at 725 nm. The total phenolic content of fruits was calculated using the calibration chart prepared from different concentrations of gallic acid solutions [30].
Total flavonoid content was determined according to study described by Dewanto et al. [31] with some modification. After processing, the absorbance of the resulting pink solution was measured at 510 nm against the blank.
Determination of antioxidant activity
The antioxidant activities of the samples were determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH). 0.1 ml of extract was mixed with 2 ml of DPPH solution. The absorbance values of the samples, which were kept in the dark and at room temperature for 30 min, were measured at 517 nm [32].
Phenolic compounds
Phenolic compounds were determined using Shimadzu-HPLC equipped with a PDA detector and an Inertsil ODS-3 (5 µm; 4.6 × 250 mm) column. 0.05% acetic acid (A) and acetonitrile (B) were used as the mobile phase. The flow rate of the mobile phase is 1 ml/min at 30 °C and the injection volume is 20 µl. Peaks were recorded at 280 nm with the PDA detector. Gradient program, 0–0.10 min 8% B; 0.10–2 min 10% B; 2–27 min 30% B; 27–37 min 56% B; 37–37.10 min 8% B; 37.10–45 min is 8% B. The total run time per sample was determined as 60 min.
Fatty acid composition
The fatty acid methyl esters of caper fruit oil esterified according to the method of ISO-5509 were analyzed in a gas chromatography instrument equipped with a flame ionization detector (FID) and a capillary column (Tecnocroma TR-CN100, 60 m × 0.25 mm, film thickness: 0.20 µm) [33]. Nitrogen was used as the mobile phase at a flow rate of 1.51 ml/min. The column temperature was programmed at 120 °C for 5 min and increased by 240 °C at 4 °C/min and held at 240 °C for 25 min.
Mineral analysis
About 0.5 g of dried and ground caper berries were incinerated in a closed microwave system (Cem-MARS Xpress) using 5 ml of 65% HNO3 and 2 ml of 35% H2O2. The volumes of the incinerated samples were made up to 20 ml with ultra-deionized water and the mineral contents were determined by ICP AES (Varian-Vista, Australia) [34].
Sensory analysis
Hedonic test was chosen to analysis of sensory properties. Sensory analyzes of the breads were presented to the panelists at the same time and with the same presentation by 7 experienced panelists at Selçuk University within 24 h after the bread was baked, and their scores were requested (1 = very bad, 2 = bad, 3 = fair, 4 = good, 5 = very good). Panelists evaluated the samples in terms of taste, smell, color, appearance and texture [35].
Statistical analysis
Research results were evaluated by analysis of variance and differences between groups were determined by Duncan Multiple Comparison Test [36].
Results and discussion
Some chemical analyzes of brine
Some chemical analyzes of caper (Capparis ovata Desf var. herbacea (Coss.)) fruits fermented in brine by adding sugar, pine honey, flower honey, glucose and citric acid at different concentrations are given in Table 1. The pH values of the brines ranged from 4.14 (with citric acid) to 4.98 (with glucose), while titration acidity values are found between 0.35% plain salt) and 0.49% (with citric acid). The salt content of the brine samples decreased significantly and the salt values varied between 4.47 (with flower honey) and 6.08% (with citric acid) depending on the additives added. Generally, pH values of pine honey and glucose added brine were found to be high. The pH values of the salt and granulated sugar-containing brines were similar and there was no statistical difference between them. The degradation of glucose by lactic acid bacteria was limited and the titration acidity was found to be so low. The lowest pH was determined in citric acid brine and naturally the titration acidity value was determined at the highest level. A linear relationship was observed between the titration acidity and pH values of the brines. However, partial fluctuations were detected between titration acidity values. These differences may possibly be due to the diversity of microorganisms involved in spontaneous fermentation, the availability of microorganisms from the added additives, ambient conditions and inhibitory substances in caper fruits. The salt content of the salt and citric acid added brines was found to be slightly higher than the other added brines, and the salt contents of the other added brines were found to be close to each other and statistically significant (p < 0.05). The probable reason for the high salt content of the brine samples with citric acid and plain salt addition may be due to the low diffusion of salt to the fruit by creating hardness of salt and citric acid in caper fruits. On the other hand, since the texture of honey and sugar added brine is a bit soft, it may have caused a decrease in the salt content of the brine. Salt contents of sugar and glucose-containing brines were similar and not statistically significant. In general, citric acid added caper brine had an advantage over the others in terms of pH, titration acidity and salt content. Özcan [9] found that the dry matter, protein content, ash content and pH values of C. spinosa and C. ovata fruits were respectively 17.3–17.59%, 18.33–23.67%, 6.31-6.25% and 4.32–4.28. Argun [37] reported that acidity, pH and salt values in the brine of caper buds, which were subjected to fermentation for 42 days, varied between 0.350–0.401%, 4.94–4.59 and 5.595–5.702%. The mean pH values before the start of fermentation were 4.3 in caper fruits and 7.3 in brine. The pH of brines during fermentation, ranged from 7.1 (d 0) to 3.5 (d 45) for both trials [19]. The results showed partial similarities with the results of previous studies. But, there are some differences. These differences may have resulted from the fermentation environment such as the composition of the fermented material, the additives added and the temperature.
Bioactive components and antioxidant properties of raw and fermented caper fruits
Water, crude oil, total carotenoid, total phenolic substance, total flavonoid contents and antioxidant activity values of caper fruits, which were subjected to fermentation in brine containing salt, sugar, pine honey, flower honey, glucose and citric acid, are presented in Table 2. The water contents of raw (control) and fermented caper fruits ranged from 73.63% (with flower honey) to 78.49% (with citric acid), while the crude oil content of raw and fermented caper fruits was determined between 2.12 (with glucose) and 6.45% (control). In addition, the total carotenoid and total phenolic substance contents of raw and fermented caper fruits were detected between 0.55 (with glucose) and 20.24 µg/g (control (raw) to 116.79 (with flower honey) and 282.74 mg GAE/100 g (with flower honey), respectively. The total flavonoid contents of raw and fermented caper fruits were found to be between 234.52 (with flower honey) and 963.57 mgQE/100 g (with plain salt added), while the antioxidant activity values of raw and fermented capers were measured between 6.49 mmol TE/kg (control) and 7.99 mmol TE/kg (10% salt). It was observed that there was a partial increase in the amount of water of caper fruits fermented in brine, to which other additives were added, except pine and flower honey, when compared to the control. The water content of caper fruits fermented in brine with added pine and flower honey was slightly lower when compared to the control. Here, the water absorption of the fruits was partially decreased with the addition of honey. Crude oil content of caper fruits fermented with the addition of various additives was found to be significantly reduced compared to the control and it was found to be statistically significant (p < 0.05). This decrease in the oil content of the fruits may possibly be due to the deformation of the cell walls of the fresh caper fruit seeds as they ferment, thereby transferring the oil from the fruit to the brine. Only the fat contents of capers fermented in salt and citric acid brine were control (raw), citric acid, salt, pine honey, flower honey, sugar and glucose fermented caper fruits in brine, in decreasing order. The total carotenoid amounts of fermented capers were significantly reduced, approximately 19 times, when compared to the control. This decrease is probably due to some biochemical reactions occurring during the fermentation of fruits. In addition, it may have been caused by the deterioration in the structures of carotenoids by fermentation. The total phenolic contents of capers fermented in brine with control (raw), plain salt, sugar, glucose and citric acid were found to be close to each other although they were quantitatively slightly different, and the total phenolic content of capers fermented in brine with pine and flower honey was significantly higher. The total phenolic content of caper fruits fermented in salt, sugar and glucose added brine was higher than the total phenolic content of the raw fruit (control). The possible reason why the total phenolic content of caper fruits fermented in brine with flower and pine honey was lower than the control may be due to the deterioration of the structure of phenolic substances in the caper fruits by the enzymes in the honey. It was observed that this situation was related to the decrease in the amount of phenolic compounds with fermentation. In addition, fermentation was effective on the total flavonoid contents of caper fruits fermented in brines to which various additives (salt, sugar, pine and flower honey, glucose, citric acid) were added. In general, total flavonoid contents of capers fermented in salt, sugar, citric acid and glucose added brines were higher than fermented capers in both raw and pine and flower honey added brines. The highest total flavonoid content was determined only in the fermented sprouts in salt-added brine. The possible reason for the low total flavonoid content of fermented caper fruits in pine and flower honey brine may be due to the biochemical reactions of flavonoid compounds in honey and flavonoids in caper fruits, enzyme activity or new metabolite products they formed. In addition, the antioxidant values of caper fruits fermented in different additive brines increased significantly compared to the control (except pine and flower honey), and the differences between each other were found to be statistically significant. As seen in Table 2, a linear relationship was observed between the total phenolic content, total flavonoid contents and antioxidant values of fermented capers. This relationship may be entirely due to the deterioration of the structures of bioactive substances as a result of the biochemical reactions occurring during the fermentation of capers. Total phenol, flavonoid amounts and antioxidant activity values of caper fruits varied between 6.5–11.1 mg GAE/g and 2.42–4.6 mgQE/g and 0.98–1.48 gTE/100 g, respectively [38]. Total phenolic and flavonoid amounts and antioxidant activities were found by in vitro antioxidant analyzes DPPH and ABTS+%, it was observed that the values were slightly higher after fermentation [38]. According to the size of the capers, the antioxidant activity values are 5.2–6.0 mM Trolox (thin), 12.5–15.5 (medium) and 13.3–16.1 mM Trolox (thick) [39]. Total phenol and total flavonoid contents of capers in different sizes were 61.5–86.2 mg GAE/100 g and 39.5–51.2 mg eq.rutin/100 g (thin), respectively; It ranged from 41.2–81.6 mg GAE/100 g to 67.8–101.3 mg eq.rutin/100 g (medium) and 66.8–119.2 mgGAE/100 g and 47.6–66.2 mgeq.rutin/100 g (thick) [39]. Significant differences were observed when the results were compared with previous study results. These fluctuations may be due to the type and variety of the material, the location and climatic factors where it is grown, whether the material is fresh or not, the harvest time and analytical procedures.
Phenolic compounds of raw and fermented caper fruits
The phenolic constituents and quantitative values of caper fruits fermented in brine with added pine and flower honey, only salt, sugar, glucose and citric acid are given in Table 3. It was observed that fermentation had a significant effect (p < 0.05) on the phenolic component amounts of caper fruits compared to the control, depending on the added additives (salt, sugar, pine and flower honey, glucose syrup and citric acid), and their amounts decreased. Accordingly, the dominant phenolic components were catechin, rutin, 3,4-dihydroxybenzoic acid, and gallic acid. The amounts of other components were detected at lower levels (Fig. 1). The gallic acid contents of fermented and raw caper fruits in brine with additives were found to be between 12.76 (with citric acid) and 34.03 mg/100 g (crude (control)), while the 3,4-dihydroxybenzoic acid values of caper fruits are found between 0.38 0 g (with additives) and 40.21 mg/100 g (control). The catechin and rutin values of raw (control) and fermented caper fruits ranged from 1.57 mg/100 g (with flower honey) to 97.93 (control) and 0.38 mg/100 g (with floral honey) to 143.01 mg/100 g (control), respectively. The caffeic acid contents of the caper fruits fermented in raw (control) and different additive brines were determined between 0.39 (with flower honey) and 9.42 mg/100 g (control), while the syringic acid contents of the caper fruits were found to be between 0.21 (with flower honey) and 8.69 mg/100 g (control). However, the p-coumaric and ferulic acid contents of raw and fermented caper fruits in brine with different additives were 0.04 (pine honey) and 2.43 mg/100 g (control) to 0.14 (salt only) and 2.35 mg/ 100 g (control), respectively. In addition, the resveratrol contents of raw and fermented caper fruits were detected between 0.08 (with pine and flower honey) and 1.12 mg/100 g (control), while the kaempferol contents of the samples were identified between 0.16 (glucose) (control) and 2.78 mg/100 g (control). The quercetin and cinnamic acid amounts of raw and fermented caper fruits were determined between 0.24 (with pine honey) and 0.68 mg/100 g (with citric acid) to 0.05 (with flower honey and glucose added) and 0.31 mg/100 g (with control), respectively. The highest phenolic components were determined in raw capers and their amount decreased significantly with fermentation. In general, the greatest decrease was detected in caper fruits fermented in brine with added pine and flower honey. The possible reason for this decrease may be due to the softening of fruit tissues during fermentation and the negative effects of enzymes such as polyphenoloxidase enzymes on phenolic compounds. In addition, since the fermentation process is a spontaneous fermentation, it may also be caused by some biochemical reactions that take place. Among these phenical components, phenolics that were partially resistant to fermentation activities were gallic acid, 3,4-dihydroxybenzoic acid and catechin. Although the quantitative values of these compounds decreased significantly during fermentation, they were partially higher than the amounts of other phenolic compounds. In addition, it is seen how rich the raw caper fruit is in rutin (143.00 mg/100 g) content, but it is understood from the results that it is a very sensitive compound to the fermentation process. p-Coumaric, ferulic, caffeic and cinnamic acid hydroxynamic acid group, gallic, 3,4-dihydroxybenzoic acid and syringic acid hydroxybenzoic acid group, and catechin and resveratol, which are the phenolic components of caper fruits fermented in raw and different additive brine, are the main compounds forming the flavanol group. In previous study, the flavone glycoside robinin was previously reported in Capparis spinosa [40]. The epicatechin concentration was 0.98 ± 0.06 mg/g DE (dry extract) in S1 while the concentration in processed fruits was 0.4–0.5 mg/g DE, representing an approximate 50% reduction in epicatechin concentration after fermentation [36]. In a previous study, lower amounts of epicatechin after a fermentation process have been previously reported in other food samples [41]. Francesca et al. [19] identified a total of five polyphenols in caper fruits and found that all samples contained high concentrations of rutin. Among the phenolic compounds, quercetin and rutin were also found in caper fruits [42]. However, rutin can also be directly hydrolyzed by hesperidinase to produce quercetin [43]. Results from this study show that fermented capers are characterized by a different polyphenolic profile than unprocessed fruits. The phenolic components and quantitative values of raw and fermented caper fruits were observed to be related to the concept that phenolic components decreased with fermentation in previous caper fruit studies. However, differences were observed in the amount of phenolic components. These differences may have been caused by the type and variety of capers, the maturity level of the fruits, harvest time and growing conditions.
Fatty acid compositions of the oils of raw and fermented caper fruits
The fatty acid composition of the oils obtained by the Soxhlet method from caper fruits fermented in brine with raw and different additives (salt, sugar, pine honey, flower honey, glucose and citric acid) are given in Table 4. Palmitic, stearic, oleic, linoleic and linolenic acids are the dominant fatty acids of the oils (Fig. 2). Compared to the palmitic, stearic and linolenic acid control, it was observed that the oil content of fermented caper fruits increased in the added brine, while the linoleic acid content decreased. It was determined that the oleic acid content of the oils of the fermented caper fruits was also increased compared to the control (except for sugar and pine honey). Palmitic acid amounts of raw and fermented caper fruit oils were determined between 9.45 (control) and 14.95% (flower honey), while stearic acid contents of caper fruit oils were between 2.27 (control) and 4.45% (flower honey). The oleic and linoleic acid contents of raw and fermented caper fruit oils were determined between 20.79 (pine honey) and 24.79% (only salt) to 41.42 (with pine honey) and 61.40% (control), respectively. Linolenic acid amounts of raw and fermented caper fruit oils were determined between 2.78 (control) and 10.59% (with pine honey). Arachidic acid contents of caper fruits ranged from 0.56 (control) to 4.67% (with pine honey), while arachidonic acid amounts of oil samples were found between 0.01 (control) and 5.07% (with pine honey). Behenic acid contents of raw and fermented caper fruit oils were determined between 0.65 (control) and 0.98% (with flower honey). Lauric acid could not be detected in caper fruit oils fermented in brine with sugar and citric acid. As seen in Table 4, there were partial differences in the fatty acid composition of fermented caper fruit oils due to additives. In the studies carried out on the leaf, bud, fruit and seed lipids of the caper plant, it was determined that the seeds contain up to 30% oil, and the fatty acid contents are 57% oleic acid, 21% palmitic acid, 11% linoleic acid, respectively [44,45,46,47]. Fatty acid content in the flower bud oils of two C. spinosa cultivars grown in Spain were 31.9–32.4% palmitic acid, 4.1–4.9% stearic acid, 8.1–10.2% oleic acid, 17.9–18.2% linoleic acid, 35.0–37.5% linolenic acid [48]. When the results were compared with the literature data, they showed differences in the amount of fatty acids, but they had similarities in terms of fatty acid diversity. The possible reason for these differences may be due to the fact that the main components of the caper fruits are not sufficiently fermented during fermentation, there is a gravimetric change depending on the amount of dry matter, material type and variety differences, and the ripening stages of the fruits.
Mineral contents of raw and fermented caper fruits
Mineral contents of raw (control) caper fruits fermented in brine with different additives are shown in Table 5. It was observed that the mineral contents of the fermented caper fruits were significantly decreased when compared to the control (raw) and there were statistically significant differences between them (p < 0.05). P, K, Ca, Mg, S and Na were the most abundant minerals in raw (control) and fermented caper fruits. Fe, Cu, Zn, Mn, B and Ni macroelements were the following microelements in decreasing order. P and K amounts of raw and fermented capers were determined between 974.94 (with pine honey) and 3363.92 mg/kg (with flower honey) to 2487.86 (with citric acid) and 9942.45 mg/kg (salt), respectively. The Ca content of caper fruits fermented in raw and added brine was found to be between 2495.39 (with sugar) and 4606.95 mg/kg (with citric acid), while the Mg contents of capers are found between 502.94 (with pine honey) and 2065.69 mg/kg (with citric acid). In addition, the S amounts of capers were determined between 1461.62 (with flower honey) and 10,508.02 mg/kg (with citric acid), while the Na amounts of raw and fermented capers are found between 260.62 (with flower honey) and 70,525.36 mg/kg (glucose). In terms of microelement content, Fe and Cu amounts of raw and fermented caper fruits has been detected between 36.47 (with sugar) and 56.56 mg/kg (with flower honey) to 12.59 (with sugar) and 21.86 mg/kg (with flower honey), respectively. The Mn contents of raw and fermented caper fruits ranged from 5.83 (control) to 20.84 mg/kg (with flower honey), while the Zn contents of caper fruit samples are measured between 9.32 (with pine honey) and 39.63 mg/kg (flower honey). In addition, the Ni and B contents of capers fermented in raw and different additive brine has changed between 0.87 (with pine honey) and 1.98 mg/kg (with flower honey) to 0.93 (with pine honey) and 19.53 mg/100 g (with citric acid), respectively. In general, the mineral contents of caper fruits differed depending on the additives used. Sometimes the mineral contents of the fermented capers were lower when compared to the control, and sometimes they were higher. The possible reason for these differences may be due to the additives used, as well as the fact that these additives harden the product, reduce the solubility and slow down the mineral transition to the brine. In particular, Ca and S were the most abundant elements in caper fruits fermented in citric acid brine. This may be due to the fact that capers are grown in calcareous soils and have more sulfur compounds in their composition. In terms of P, Mg, Fe, Cu, Mn, Ni and Zn, capers fermented in flower honey brine had the richest mineral content when compared to other samples. The results sowed some differences depending on ingredient added for fermantation. The probable reason for these differences may be due to the ingredient, maturation position of caperfruits, climatic factors, soil element concentration, fermantation conditions, and analytical conditions.
Sensory properties of fermented capers
The sensory properties (taste, smell, color, texture) and general appreciation of caper fruits fermented in salt, sugar, pine honey, flower honey, glucose and citric acid brine are given in Table 6. Taste and odor values of fermented capers were determined between 2.00 (with pine honey) and 4.40 (with citric acid) and 3.20 (with sugar and pine honey) and 4.00 (with citric acid), respectively. In addition, the color values of fermented capers were determined between 3.60 (with flower honey) and 4.60 (only salty and citric acid), while the texture values of the fruits were reported between 3.80 (with pine honey and glucose) and 4.80 (only salty). Overall, capers fermented in brine with added glucose received the least approval from the panelists. While caper fruits fermented in brine with added citric acid were most appreciated, followed by brined (only salty), sugared and flower honey, pine honey and glucose added fruit samples in decreasing order. It has been observed that the caper fruits fermented in citric acid added brine preserve the characteristics of the product in terms of both color and texture. In addition, it has kept its taste and smell at a level that will satisfy the consumer’s taste. Özcan [9] determined that 6% salty and 0.5% lactic acid samples showed the best effect on caper pickles produced using lactic acid in terms of sensory properties. It was determined that the differences in the sensory properties of fermented caper fruits differ depending on the additives added to the brine.
Conclusion
Our study was based on the use of fast, efficient and sensitive methods based on GC, HPLC and ICP-OES for brine analysis, identification and quantification of bioactive components, fatty acids, polyphenolic compounds and mineral contents from raw and fermented caper fruits, respectively. Our study represents the first report on the analysis of bioactive properties, fatty acids composition, polyphenols and minerals, and sensory evaluations from caper fruits. Generally, pH values of pine honey and glucose added brine were found to be high. The pH values of the salt and granulated sugar-containing brines were similar and there was no statistical difference between them. The degradation of glucose by lactic acid bacteria was limited and the titration acidity was found to be so low. The lowest pH was determined in citric acid brine and naturally the titration acidity value was determined at the highest level. Crude oil content of caper fruits fermented with the addition of various additives was found to be significantly reduced compared to the control and it was found to be statistically significant (p < 0.05). It was observed that the total carotenoid amounts of fermented capers were significantly reduced, approximately 19 times, when compared to the control. The total phenolic content of caper fruits fermented in salt, sugar and glucose added brine was higher than the total phenolic content of the raw fruit (control). The highest phenolic compounds were determined in raw capers and their amount decreased significantly with fermentation. In general, the greatest decrease was detected in caper fruits fermented in brine with added pine and flower honey. Palmitic, stearic, oleic, linoleic and linolenic acids are the dominant fatty acids of raw and fermented caper fruit oils. Compared to the palmitic, stearic and linolenic acid control, it was observed that the oil content of fermented caper fruits increased in the added brine, while the linoleic acid content decreased. In general, the mineral contents of caper fruits differed depending on the additives used. Sometimes the mineral contents of the fermented capers were lower when compared to the control, and sometimes they were higher. Overall, capers fermented in brine with added glucose received the least approval from the panelists. While caper fruits fermented in brine with added citric acid were most appreciated, followed by brined (only salty), sugared and flower honey, pine honey and glucose added fruit samples in decreasing order. As a result, the addition of citric acid, granulated sugar and flower honey to the brine in the fermentation of caper fruits is preferred in terms of both acidity value, bioactive properties, mineral contents and sensory properties.
References
M. Özcan, Composition and pickling product of Capers (Capparis spp.) flower buds. Ph.D. Thesis, Graduate School of Natural and Applied Sciences, Department of Food Engineering, Selçuk University, Konya, 1996, p. 102
B. Matthäus, M. Özcan, Glucosinolate composition of young shoots and flower buds of capers (Capparis species) growing wild in Turkey. J. Agric. Food Chem. 50, 7323–7325 (2002)
M. Özcan, A. Akgül, Pickling process of Capers (Capparis spp.) flower buds. Grasas y Aceites 50, 94–99 (1999)
A. Akgül, Baharat bilimi ve teknolojisi. Gıda Teknol Der Yay 15, 111–113 (1993)
M. Noorfarahzilah, J.S. Lee, M.S. Sharifudin, A.B. Mohd Fadzelly, M. Hasmadi, Applications of composite flour in development of food products. Int. Food Res. J. 21(6), 2061–2074 (2014)
R.J. Fletcher, I.P. Bell, J.P. Lambert, Public health aspects of food fortification: a question of balance. Proc. Nutr. Soc. 63, 605–614 (2004)
T. Gull, F. Anwar, B. Sultana, M.A.C. Alcayde, W. Nouman, Capparis species: a potential source of bioactives and high-value components: a review. Ind. Crops Prod. 67, 81–96 (2015)
F. Anwar, G. Muhammad, M. AjazHussain, G. Zengin, K.M. Alkharfy, M. Ashraf, A.H. Gilani, Capparis spinosa L.: a plant with high potential for development of functional foods and nutraceuticals/pharmaceuticals. Int. J. Pharm. 12, 201–219 (2016)
M. Özcan, Pickling and storage of Caperberries (Capparis spp.). Zeits. Lebens. Unt. Forsch. A 208, 379–382 (1999)
M. Özcan, Organoleptic quality and production of pickling Capers (Capparis spp.) paste. Obst. Gem. Kartoffelverarbeit 86, 122–124 (2001)
M. Özcan, Composition and pickling product of capers (Capparis spp.) young shoots. Obst. Gem. Kartoffelverarbeit 87(2), 20–22 (2002)
M. Özcan, Pickling and storage of caperberries (Capparis spp.). Eur. Food Res. Technol. 208(5–6), 279–382 (1999)
B. Matthäus, M. Özcan, Glucosinolates and fatty acid, sterol and tocopherol composition of seed oils from Capparis spinosa var. spinosa andCapparis ovata Desf. var. canescens (Coss.) Heywood. J. Agric. Food Chem. 53, 136–7141 (2005)
A. Akgül, Yeniden keşfedilen lezzet: Kapari (Capparis spp.). Gıda 21, 119–128 (1996)
M.S. Moghaddasi, Caper (Capparis spp.) importance and medicinal usage. Adv. Environ. Biol. 5(5), 872–879 (2011)
G. Barbera, Programme de recherche Agrimed le caprier (Capparis spp.) Commission des Communautaes Europeennes. Serie Agric EUR, 3617 (1991)
M. Özcan, A. Akgül, Physical and chemical properties of pickled capers (Capparis spp.) flower buds. Obst. Gem. Kartoffelverarbeit 85(4), 165–167 (2000)
A. Mollica, G. Zengin, M. Locatelli, A. Stefanucci, A. Mocan, G. Macedonio, E. Novellino, Anti-diabeticand anti-hyperlipidemicproperties of Capparis spinosa L.: Invivoand in vitroevaluation of itsnutraceuticalpotential. J. Funct. Foods 35, 32–42 (2017)
N. Francesca, M. Barbera, A. Martorana, F. Saiano, R. Gaglio, M. Aponte, L. Settanni, Optimised method for the analysis of phenolic compounds from caper (Capparis spinosa L.) berries and monitoring of their changes during fermentation. Food Chem. 196, 1172–1179 (2016)
R. Perez Pulido, N. Ben Omar, H. Abriouel, R. Lucas Lopez, M. Martinez Canamero, A. Galvez, Microbiological study of lactic acid fermentation of caperberries by molecular and culture-dependent methods. Appl. Environ. Microbiol. 71, 7872–7879 (2005)
I. Tagnaout, H. Zerkani, M. Mahjoubi, M. Bourakhouadar, F. Alistiqsa, A. Zouzoubaa, T. Zair, Phytochemical study, antibacterial and antioxidant activities of extracts of Capparis spinosa L. Int. J. Pharm. Phytochem. Res. 8, 1993–2006 (2016)
B. Cemeroğlu, Meyve ve Sebze İşleme Endüstrisinde Temel Analiz Metotları (Biltav Yayınları, Ankara, 1992)
AOAC, Official Methods of Analysis, 15 the Association of Official Analytical Chemists (AOAC, Washington, DC, 1990)
B. Cemeroğlu, Gıda Analizleri. Gıda Teknolojisi Derneği Yayınları 34, 657 (2010)
AACC, Approved Methods of the American Association of Cereal Chemists (AACC, St. Paul, 1990)
A. Akgül, M. Özcan, Some compositional characteristics of Capers (Capparis spp.) seed and oil. Grasas y Aceites 50, 49–52 (1999)
A. Silva da Rocha, E.K. Rocha, L.M. Alves, B. Amaral de Moraes, T. Carvalho de Castro, N. Albarello, C. Simoes-Gurgel, Production and optimization through elicitation of carotenoid pigments in the in vitro cultures of Cleome rosea Vahl (Cleomaceae). J. Plant Biochem. Biotechnol. (2013). https://doi.org/10.1007/s13562-013-0241-7
A.F. Vinha, F. Ferreres, B.M. Silva, P. Valentao, A. Gonçalves, J.A. Pereira, M.B. Oliveira, R.M. Seabra, P.B. Andrade, Phenolic profiles of portuguese olive fruits (Olea europaea L.): influences of cultivar and geographical origin. Food Chem. 89(4), 561–568 (2005)
K.M. Yoo, K.W. Lee, J.B. Park, H.J. Lee, I.K. Hwang, Variation in major antioxidants and total antioxidant activity of Yuzu (Citrus junos Sieb ex Tanaka) during maturation and between cultivars. J. Agric. Food Chem. 52(19), 5907–5913 (2004)
I.M. Zijp, O. Korver, L.B.M. Tijburg, Effect of tea and other dietary factors on iron absorption. Crit. Rev. Food Sci. Nutr. 40(5), 371–398 (2000)
V. Dewanto, X. Wu, K.K. Adom, R.H. Liu, Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 50(10), 3010–3014 (2002)
S.K. Lee, Z.H. Mbwambo, H. Chung, L. Luyengi, E.J. Gamez, R.G. Mehta, A.D. Kinghorn, J.M. Pezzuto, Evaluation of the antioxidant potential of natural products. Comb. Chem. High Throughput Screen. 1(1), 35–46 (1998)
ISO-International Organization for Standardization, Animal and Vegetable Fats and Oils Preperation of Methyl Esters of Fatty Acids, ISO. Geneve, Method ISO 5509 (1978) , pp. 1–6
S. Skujins, Handbook for ICP-AES (Varıan-Vista). A short guide to Vista series ICP-AES operation. VarianInt (1998)
P. Kumar, H.N. Mishra, Mango soy fortified set yoghurt: effect of stabilizer addition on physicochemical. Sens. Textural Prop. Food Chem. 87(4), 501–507 (2004)
O. Düzgüneş, T. Kesici, O. Kavuncu, F. Gürbüz, Araştırma ve Deneme Metodları (İstatistik Metodları-II). Ankara Üniversitesi Ziraat Fakültesi Yayınları 1021, 295 (1987)
M. Argun, Kapari (Capparisovatadesf. var. canescens) çiçek tomurcuklarının fermantasyonu üzerine bazı baharat uçucu yağ ve ekstraktlarının etkisi. Yüksek Lisans Tezi, Selçuk Üniversitesi Fen Bilimleri Enstitüsü Gıda Mühendisliği Ana Bilim Dalı, Konya, 2012, p. 66
J. Jiménez-López, A. Ruiz-Medina, P. Ortega-Barrales, E.J. Llorent-Martínez, Phytochemical profile andantioxidant activity of caper berries (Capparis spinosa L.): evaluation of the influence of the fermentation process. Food Chem. 250, 54–59 (2018)
M. Grimalt, F. Hernández, P. Leguab, M.S. Almansa, A. Amorós, Physicochemical composition and antioxidant activity of three Spanish caper (Capparis spinosa L.) fruit cultivars in three stages of development. Sci. Hortic. 240, 509–515 (2018)
M. Maldini, M. Foddai, F. Natella, R. Addis, M. Chessa, G.L. Petretto, G. Pintore, Metabolomic study of wild and cultivated caper (Capparis spinosa L.) from different areas of Sardinia and their comparative evaluation. Mass Spectr. 51, 716–728 (2016)
M.J. Payne, W.J. Hurst, K.B. Miller, C. Rank, D.A. Stuart, Impact offermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacaobeans and cocoa ingredients. J. Agric. Food Chem. 58, 10518–10527 (2010)
F. Conforti, M.C. Marcotullio, F. Menichini, G.A. Statti, L. Vannutelli, G. Burini, F. Menichini, M. Curini, The influence of collection zone on glucosinolates, polyphenols and flavonoids contents and biological profiles of Capparis sicula ssp. sicula. Food. Sci. Technol. Int. 12(2), 87–97 (2011)
S. Tranchimand, P. Brouant, G. Iacazio, The rutin catabolic pathway with special emphasis on quercetinase. Biodegradation 21, 833–859 (2010)
A. Sen Gupta, M.M. Chakrabarty, composition of the seed fats of the Capparidaceae family. J. Sci. Food Agric. 15(2), 69–73 (1964)
Z.F. Ahmed, A.M. Rizk, F.M. Hammouda, M.M. Seif El-Nasr, Phytochemical investigation of Egyptian Capparis species. Planta Med. 21(02), 156–160 (1972)
R. Sushila, Oils and fats in arid plants with particular reference to Capparis decidua L. Transact. Indian Soc. Technol. 12(2), 99–105 (1987)
N. Pilone, Effetti dell’IBA sulla radicazione delle talee di Capparis spinosa in cassone riscaldato. Inf. Agrar. 46, 81–82 (1990)
M. Rodrigo, M.J. Lazaro, A. Alvarruiz, V. Giner, Composition of capers (Capparis spinosa): influence of cultivar, size and harvest date. J. Food Sci. 57(5), 1152–1154 (1992)
Funding
This study was supported by Selçuk Unıversity BAP-Office (Project Number: 21401036). Authors thank to SU-BAP staffs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conficts of interest are declared related to the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Özcan, M.M., Uslu, N. The effect of fermentation with different additives on bioactive compounds, antioxidant activity, phenolic component, fatty acid composition and mineral substance contents of capers fruits. Food Measure 17, 3896–3908 (2023). https://doi.org/10.1007/s11694-023-01909-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-01909-5