Abstract
Kombucha is a traditional refreshing beverage usually prepared by fermentation of sweetened black or green tea. In this study, it was aimed to investigate the effects of different raw material usage on composition and sensory properties of kombucha beverage. For this aim, cherry laurel, red raspberry, blackthorn fruits and black carrot juice concentrate were added to green tea infusion. After 40 h of fermentation at 28 ± 2 °C, beverages were stored at 4 °C for 10 days. During fermentation and storage; total acidity, pH, brix, colour, total phenolic matter content, antioxidant capacity (DPPH, FRAP and CUPRAC assays) and total monomeric anthocyanin content of the samples were analyzed. Furthermore, the changes in sensory properties were determined periodically. Total phenolic content and antioxidant capacities of the products were increased when compared to uncultivated substrates. The results demonstrated that use of anthocyanin rich raw materials together with green tea for fermentation contributed nutritional value, functional and sensory properties of the kombucha beverage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For centuries, humans have benefited from fermentation with the purpose of making the food sustainable. Fermentation is not only an efficient preservation method, but also health beneficial components are produced. Fermented products show variety according to the dietary cultures of each community. Due to their positive effects on human health, having beneficial microorganisms, protection of food, improving of nutritional value and antioxidant activity and positive contribution to immunological system; consumption of fermented foods and beverages has increased in recent years [1].
Kombucha tea is produced by fermenting sugared black or green tea with powerful symbiosis of acetic acid bacteria (Acetobacter aceti, Acetobacter pasteurianus and Glucobacter oxydans, Acetobacter xylinum, Bacterium gliconicum) and yeasts (Saccharomyces sp., Zygosaccharomyces, Torulopsis sp., Pichia sp., and Brettanomyces sp.) forming a cellulose-like pellicle (zoogleal mat) on its surface [2, 3]. The origin of this beverage is China. It has been intensely consumed for a long time worldwide for its prophylactical and curative features [4]. Regular consumption of kombucha leads to longevity because of the reversal of aging processes [5]. Kombucha beverages with different flavors are sold worldwide in markets [1, 6,7,8,9].
Kombucha contains organic acids (acetic, lactic, malic, tartaric, malonic, oxalic, succinic, pyruvic, 2-keto-d-gluconic, glucuronic and usnic acids), amino acids (lysine, methionine, arginine), sugars (sucrose, glucose, fructose), probiotics, biogenic amines, purines, pigments, ethanol, carbon dioxide, glycerol [10], phenolic compounds (orcinol, anthranin, orsellinic, slazine, lecaronic acids) [11], enzymes, B group vitamins (B1, B2, B6, B12), vitamin C, vitamin E and some minerals (potassium, manganese, zinc, copper, iron, nickel, cobalt and fluoride ions) [12, 13].
Kombucha has antimicrobial activity against a majority of microorganisms [14]. The vitamins B, C and E not only contribute to its nutritional value but also conduct antioxidant properties. The high antioxidant activity of the kombucha beverage has been associated with benefits such as cancer prevention, improvement of joint rheumatism and supporting of the immune system [15]. Consumption of kombucha was reported to be effective in rebalancing blood pH of cancer patients and it is thought to be helpful in preventing headache, nervousness, insomnia, geriatric depression and epilepsy crises [16].
Green tea has significant amount of phenolic substances such as gallic acid, caffeic acid, chlorogenic acids and some flavonols, and also has high antioxidant capacity compared to black tea and fermented black tea with kombucha culture [17, 18].
It has been reported that antioxidant activity of kombucha tea after 15 days of fermentation was increased by about 70% [2]. Kombucha prepared with lemon balm showed antioxidant activity against DPPH radicals higher than kombucha prepared with black tea [19]. In another study, kombucha prepared with green tea showed the highest antioxidant activity [20]. Sun et al. [21] demonstrated that phenolic content of traditional kombucha was increased when it was supplemented with wheatgrass juice. Ayed et al. [22] determined the chemical composition and organoleptic and antimicrobial activities of the red grape juice kombucha beverage. Phenolic and anthocyanin contents and the antioxidant capacity of the fermented beverage were higher after fermentation, with the maximum increase observed on the sixth day of fermentation when values were approximately 2.47- and 1.59-fold higher than pre-fermentation values, respectively. It has been reported that different antioxidant activities depend on the tea composition as well as the difference in the amount of vitamin C and organic acids produced [23]. High levels of anthocyanins tend to improve the total antioxidant capacity [24, 25]. For this reason, the possibility of using different raw materials with high anthocyanin content such as cherry laurel, red raspberry, blackthorn and black carrot juice concentrate were used for kombucha beverage production to improve nutritional value, functional and sensory properites of kombucha beverage. The important properties of the raw materials used for kombucha beverages were summarized:
Cherry laurel (Prunus laurocerasus)
Cherry laurel belongs to the genus Prunus and the family Rosaceae. The fruit is rich in vitamin C with antioxidant activity as well as phenolic substances such as anthocyanins, which are functional food ingredients [26]. The rich antioxidant content is effective in the prevention of the formation and development of many diseases and exhibits various biological activities such as anti-inflammatory, antinociceptive, antioxidant, neuroprotective and antidiabetic. It is known as a medicinal plant and used in the treatment of stomach ulcers, digestive system disorders, bronchitis, eczema and hemorrhoids [27].
Raspberry (Rubus idaeus)
Raspberry (Rubus idaeus) is a plant species from the family Rosaceae that gives red sweet fruits in the summer and autumn seasons. Red raspberries possess a unique polyphenol profile that is characterized primarily by their anthocyanins. These pigments are important natural organic compounds having vital significance to prevent cardiovascular problems and tumourigenesis [25]. Raspberries are known to contain the highest antioxidant level among the fruits. Antioxidant activity of raspberries is primarily constituted by anthocyanins and ellagitannins which contributed about 25% and 52% to the total antioxidant activity [24].
Blackthorn (Prunus spinosa)
Blackthorn (Prunus spinosa), which belongs to the rose family (Rosaceae), is a perennial plant growing as a shrub on slopes of wild uncultivated areas. Fruits are blueish black, bloomy, globular drupe, 10–15 mm with green astringent flesh. This wild fruit is used to stop bleeding in traditional medicine. It has diuretic effect and increases body resistance [28, 29]. Fruits are rich in polyphenolic compounds, as well as in vitamin C that contribute to their high antioxidant activity [30].
Black carrot (Daucus carota L. ssp. sativus var. atrorubens Alef)
According to botanical classification of carrot seeds, they are separated in two groups. The anthocyanin group (Daucus carota ssp. Sativus var. Atrorubens Alef.) and the carotene group (Daucus carota ssp. Sativus var.). Although orange colored carrot varieties are more common, consumption of black or purple carrots (Daucus carota L. ssp. sativus var. atrorubens Alef.) is currently increasing in world due to its positive effects on health. Black carrot has attracted the attention of the scientific community due to their phenolic compounds, vitamin C and E which are significantly related to the antioxidant capacity [31].
This study aimed to investigate the possibility of using different raw materials containing significant amount of anthocyanins to produce kombucha beverage with high antioxidant capacity. At the same time, kombucha beverage flavor was improved with the use of these raw materials for receiving appreciation of wider group of consumers.
Materials and methods
Material
Green tea (Camellia sinensis), cherry laurel (Prunus laurocerasus), red raspberry (Rubus idaeus) and sucrose were purchased from local market in Bursa. Blackthorn (Prunus spinosa) was obtined from Giresun province. Black carrot (Daucus carota L. ssp. sativus atrorubens Alef.) juice concentrate was obtained from Aroma Bursa Fruit Juices and Food Ind. Inc.
Methods
Preparation and cultivation of kombucha
Sweetened green tea was prepared as shown in Fig. 1. First, 60 g of sucrose were dissolved in 1 L of hot tap water (98 °C) and pasteurized for 15 min. Then, 10 g of green tea was infused with this water at 95 °C for 12 min and then filtered. According to the results of preliminary tests, mash of cherry laurel, blackthorn and red raspbery fruits were added as 10% (v/v) and black carrot juice concentrate was added as 1% (v/v) to cooled green tea infusion, separately, then inoculated with kombucha culture and fermented at 28 ± 2 °C. Green tea infusion containing other raw materials was inoculated with kombucha culture [10% (v/v)]. The kombucha culture was obtained from previous fermentation (14 days) of green tea. Fermentation was monitored at 28 ± 2 °C. The green tea kombucha beverage was analyzed as control sample.
Analyses
Water soluble dry matter, total acidity, pH and color
Water soluble dry matter (brix°) was measured by using RA-500 model KEM refractometer (Kyoto Electronics Manufacturing Co. Ltd., Japan); total acidity was determined by potentiometric method, where samples were titrated with 0.1 N NaOH to pH 8.1 and the total acidity value was calculated as g/100 mL in terms of acetic acid [32]. The pH analysis weas conducted by pH meter (Mettler Toledo Sevencompact pH/Ion pH meter, Canada) Shimadzu (UV 1208) spectrophotometer (Japan) was used for total phenolics, total anthocyanins and antioxidant capacity analyses. Color analysis was done by Konica Minolta Chroma Meter, CR-5, Japan. The samples were filled into crystal quartz glass tubes of 6.3 cm in diameter and 4.3 cm in height so that no air space was left and L*, a* and b* values were read; hue and chroma values were calculated. L value indicates the change from vertical luminous intensity to darkness, (+ a) redness, (− a) greenness, (+ b) yellowness, (− b) blue, hue value [hue (hab = arctan (b*/a*))] reflects the tone and is expressed in degrees on a 360° scale; and chroma [C*ab = (a*2 + b*2)1/2] defines the chromaticness, being a measure of colour intensity or saturation and ranges from 0 (completely unsaturated) to 100 or more (pure colour). All analyses were performed in three replicates. All results are given as mean ± standard deviation.
Total phenolic matter content
The total phenolic matter content was determined by the Folin–Ciocalteu method [32]. Briefly, 0.2 mL of extract, 2.3 mL of distilled water and 0.15 mL of Folin–Ciocalteu (FC) reagent were added into capped glass scoops, and vortexed for 15 s. After 5 min, 0.3 mL of saturated Na2CO3 (35%) solution was added and the tube contents were agitated and allowed to stand in the dark condition for 2 h. At the end of the period, the absorbance of the sample was measured at 725 nm and the result was calculated as “mg gallic acid equivalent/100 g dry weight”.
Antioxidant capacity
Antioxidant capacity was analyzed both in raw material and in beverages, employing the DPPH [33], FRAP [34] and CUPRAC [35] assays. In the DPPH assay, 0.1 mL specimen was added to 3.9 mL of DPPH solution and vortexed (Vortex Mixer Classic, Velp Scientifica, Usmate, Italy) for 30 s. Test tubes stood in the dark at room temperature for 30 min to let the reaction occur. A trolox calibration curve (R2 = 0.9997) was obtained by measuring the reduction in absorbance of the DPPH solution in the presence of different concentrations of trolox (10–100 µmol/L). In FRAP assay, 3 mL of daily prepared FRAP reagent was mixed with 300 µL of distilled water and 100 µL of the sample or blank. The test samples, FRAP solution and blank were incubated at 37◦C for 60 min. At the end of the incubation, absorbance was measured immediately at 595 nm. The results were calculated from calibration curve as µmol trolox/mL for beverages (R2 = 0.9934). In CUPRAC assay, (1 × 10−2 M) CuCl2, (7.5 × 10−3 M) neocuproine, and (1 M) ammonium acetate were mixed and the final absorbance was measured at 450 nm after 30 min (R2 = 0.9933). Results were given as trolox equivalents [36].
Total monomeric anthocyanin
The principle of this method is based on the fact that the monomeric anthocyanins are dominant in the colored oximes form at pH 1.0, while at pH 4.5, the colorless hemiketal form dominates. Accordingly, the difference in measured absorbance values when the media is at pH 1.0 and at 4.5 is directly proportional to the concentration of anthocyanin. Potassium chloride (KCl) buffer solution (0.025 M, pH 1.0) and sodium acetate (NaC2H3O2) buffer solution (0.4 M, pH 4.5) used in this method were prepared as indicated [37]. In this assay, 100 µL of the extract was used for total phenol determination and 400 µL of pH 1 (KCl 0.025 M) buffer was added. 100 µL of the extract was used in the determination of total phenolic compound and 400 µL of pH 4.5 (sodium acetate 0.4 M) buffer was added. Absorbance values measured at 512 nm and at 700 nm against pure water. The total amount of anthocyanin was calculated according to equation E (1), where MW is the molecular weight of the anthocyanine standard (449.2 for cyanidin-3-glucoside), df is the dilution factor, € is the molar absorptivity or absorbance coefficient (26,900 for cyanidin-3-glucoside) and l is the layer thickness of the spectrophotometer bath (cm) [38].
Anthocyanin (cyanidin-3-glucoside equivalent) (mg/kg)
Sensory evaluation
Sensory analysis was carried out at 0, 3, 5, 10 and 12 storage days. The products were evaluated for appearance, color, odor, sourness and sweetness. A nine-point hedonic scale was used. The selected terms for each choice in the scale were based on equal interval spaces, allowing the assignment of numerical values to the response options. The proposed categories were: (1) dislike extremely, (2) dislike very much, (3) dislike moderately, (4) dislike slightly, (5) neither like nor dislike, (6) like slightly, (7) like moderately, (8) like very much and (9) like extremely. Ten panellists evaluated the samples coded with three digits random numbers. A rating below the 5-point limit value indicates that the product will not be consumed [39].
Statistical analysis
The assessment was conducted using the SAS Statistical Program developed by SAS Institue, Inc. Data were analysed with the Analysis of Variance (ANOVA), and further analysis was conducted with the Least Square Difference (LSD), both at a 5% significant level [40].
Results and discussion
Total acidity, pH and Brix
Samples were evaluated for physicochemical properties during 40 h of fermentation at 28 ± 2 °C. Total acidity of kombuchas prepared with green tea, black carrot juice concentrate, cherry laurel, blackthorn and red raspberry reached 0.26 g/100 mL, 0.26 g/100 mL, 0.26 g/100 mL, 0.28 g/100 mL and 0.32 g/100 mL, respectively at the end of the second day of fermentation. All samples were accepted ready for consumption with these acidities and then stored at 4 °C. The variation of total acidity during fermentation and storage is given in Fig. 2. Although beverages stored at 4 °C, fermentation was continued and total acidity increased especially after 5th day of storage.
The pH of green tea kombucha beverage decreased during fermentation period (Fig. 3). It decreased rapidly from 7.23 to 3.97 within 2 days of fermentation and then it continued to decrease slightly up to 10th day. Other samples showed similar trend. Similar pH values were observed when kombucha analogues were prepared with medicinal herbs from the Lamiaceae family such as thyme, lemon balm, peppermint and sage [41]. pH of kombucha beverages decreased depending on the organic acid production. The decrease in pH can be beneficial in terms of preventing the chemical degradation of polyphenols and retaining beverage color. Under acidic conditions, anthocyanins retain their chemical structure and become more stable [42]. Vīna et al. [43] attributed the beneficial properties of Kombucha beverage primarily to its acidic composition. Ayed et al. [22] observed pH stabilization from day 8 of fermentation onward despite the increase in organic acid content. With the addition of sucrose to the green tea extract and culture inoculation, the acidity of the samples increased during fermentation period.
Carbon dioxide was released slowly at the beginning and much faster after 2–3 days of fermentation. This was the valid reason for slight decrease in pH during the fermentation process. The obtained water solution of carbon dioxide dissociates and produces the amphiprotic hydrocarbonate anion (HCO−3), which easily reacts with hydrogen ions (H+) from organic acids, preventing further changes in the (H+) concentration and contributing to a buffer character of the system [15]. These data are in harmony with the research findings of other studies [14, 44]. Depending on the fermentation of sugar in the samples, alcohol and carbon dioxide were produced and brix values were decreased rapidly during fermentation. The decrease in brix during storage shows that fermentation continues in cold storage (Fig. 4).
Color analysis
While L value of green tea kombucha increased with fermentation, it did not significantly change during storage. The a* value of the second day of fermentation showed (−) value, b value and chroma decreased, no significant change in hue value was determined (Table 1).
L values of black carrot and cherry laurel kombucha beverages were not significantly change while L value of blackthorn increased. Black carrot and raspberry kombucha samples had the highest a value and cherry laurel had the highest b value. During fermentation and storage, the lowest and highest chroma values were green tea and black carrot kombucha respectively; the lowest and highest values of hue were measured at red raspberry and green tea kombucha beverages.
Color is an important visual attribute of beverages for quality and acceptability. Results showed correlation with sensory acceptability of the products. Also pH has an important impact on the processing of functional beverages, as it influences the appearance, including color variations, in many food products. Another significant factor that affects the color of the sample is their anthocyanin content. During storage anthocyanins degraded and the color properties are affected negatively. The enzymes liberated by bacteria and yeasts were capable of biotransforming various phytochemicals existing in the teas. This biotransformation process is typically known to result in a decreased color [6,7,8,9].
Bioactive compounds
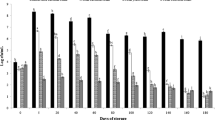
The health-promoting activities of kombucha beverage are also attributed to their phenolic content and their ability to act as antioxidants [22]. The amount of total phenolics of uncultivated substrates ranged from 645.726 to 1526.488 mg GAE/100 g (Fig. 5) (R2 = 0.9991). The substrates containing black carrot juice concentrate and green tea had significantly more phenolic compunds than others, aproximately twice the amount.
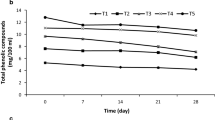
During fermentation, small molecules with higher antioxidant activities are released due to depolymerization of thearubigins and this is offered as explanation of increased total phenol content of kombucha [2]. Another reason of this increment is related to the enzymes liberated by bacteria and yeast during kombucha fermentation. That will be the reason for the degradation of complex polyphenols to small molecules which in turn results in the increase of total phenolic compounds [15, 45]. Nevertheless, more extended time of fermentation could lead to a reduction in polyphenols concentration [46]. Following the initiation of the fermentation process, total phenolic content of the fermented red grape kombucha increased significantly, showing a 40% increase by day 6 [22]. A study conducted on kombucha beverages fermented for 3 months indicated a decrease in the total phenolic content and the antioxidant potential [15]. Increase in total phenolic content among samples was observed in green tea, black carrot and blackthorn containing samples during storage. While total phenolics of cherry laurel kombucha increased during the fermentation, it was decreased during storage period (Fig. 6).
The result of total monomeric anthocyanins was calculated in terms of the product-specific dominant anthocyanin. Total monomeric anthocyanin content of the samples was calculated as cyanide 3-glycoside for green tea, cherry laurel and blackthorn containing kombucha beverages. However, this value was calculated as cyanide-3-galactoside [47] and cyanide 3-soforizide [31] for black carrot juice concentrate and red raspbery containing samples. The results of uncultivated substrates were given in Fig. 5. Fruit added kombuchas had higher results than the green tea kombucha due to their rich anthocyanin content. Red raspberry kombucha beverage has the highest anthocyanin content. Due to the increase of acidity depending on fermentation, significant decrease of anthocyanin contents (46.89–81.90%) was observed in both fermented and stored samples. In a study, monomeric anthocyanin reduction of black carrot juice was 52% in storage conditions [47]. Anthocyanins of black carrot juice concentrate and red raspberry containing samples were much more stable than others (Fig. 7).
The DPPH scavenging abilities of uncultivated substrates ranged from 64.83 to 74.28 µmol trolox equivalent/g t.s.s (total soluble solid) (Fig. 8). Slight increases were observed in antioxidant capacity determined by DPPH method of all samples with fermentation. This increase continued similarly during the storage (Fig. 9). The increased potential against DPPH radical might explain the phenomena that kombucha feding significantly reversed the chromate (IV) or lead induced oxidative injury in rats [48].
Maximum antioxidant capacity determined by DPPH assay was observed in red raspberry kombucha at the end of the fermentation. Darker colored raspberries have more effective antioxidant capacity [49]. The antioxidant capacity of raspberries is primarily constituted by anthocyanins and ellagitannins [24]. Anthocyanins have a strong antioxidative activity and protect cells and body from oxidation [25].
Recent studies demonstrated that kombucha had in vivo antioxidant activities. Antioxidant properties of kombucha mainly originated from polyphenols (especially catechins) from tea [50]. Amongst all types of tea, green tea is the richest in catechins. The antioxidant activity of green tea polyphenols is primarily attributed to the combination of aromatic rings and hydroxyl groups that assemble their chemical structure and consequently binding and neutralization of lipid free radicals by these hydroxyl groups. Several researches have reported that polyphenols and tea catechins are exceptional electron donors and effective scavengers of physiologically relevant reactive oxygen species in vitro. Catechins also exhibit antioxidant activity via chelating redox active transition-metal ions. Polyphenols possess hydroxyl and carboxyl groups, are able to bind particularly iron and copper. Transition metal ions typically have the ability to initiate free radical chain oxidations by decomposing lipid hydroperoxides by the hemolytic cleavage of the O–O bond and producing lipid alkoxyl radicals. Phenolic antioxidants, including tea catechins, inhibit lipid peroxidation by binding these lipid alkoxyl radicals. Green tea catechins also exhibit antioxidant activity through inhibiting pro-oxidant enzymes and inducing antioxidant enzymes [51]. While antioxidant capacity of all samples determined by FRAP assay increased at the end of fermentation, it decreased in cherry laurel kombucha. Black carrot juice concentrate kombucha demonstrated the higher results (Fig. 10). Özkan [47] reported that antioxidant activity of black carrot juice and concentrate is associated due to its high content of chlorogenic acid. FRAP analysis results of coconut kombucha fermented for 7 days were similar to our results. Their results were in the range of 120–180 µmol trolox equivalent/g t.s.s [46].
Kombucha reduces ferric ions by donating electrons, leading to the neutralization of the free radicals [52]. It is also able to neutralize DPPH radicals mainly by electron transfer and to a lesser extent by its hydrogen donating ability [53]. Significant increase (88.3%) in CUPRAC result was observed with fermentation of black carrot juice concentrate containing kombucha (Fig. 11). During fermentation of Cabernet Sauvignon and Merlot wines, CUPRAC value also increased indicating that alcoholic fermentation had a positive effect on the antioxidant capacity determined by CUPRAC assay [54].
Sensory evaluation
Appearance, color, odor, sourness and sweetness properties of kombucha samples were evaluated during storage (Fig. 12). All samples were highly appreciated for their appearance and color. A shorter fermentation time implies a higher content of sugar. This fact could be related to the higher acceptability for sweetness at beginning of storage. Kombucha products are distinguished by sour taste, which is considered a refreshing quality in functional beverage. The sensory perception of sourness is mainly attributed to the presence of hydrogen ions at higher concentrations [55]. According to the results of sensory analysis, the most desirable beverages were kombucha prepared with green tea and cherry laurel. Beverages which were prepared with red raspberry stored for 10 days, black carrot juice concentrate stored for 11 days and the other samples at the end of the 12 days storage were rejected for their sensory properties.
Conclusion
Kombucha beverages were successfully produced with green tea, cherry laurel, blackthorn, black carrot juice concentrate and red raspberry. Total phenolic content and antioxidant activities of the products increased when compared to uncultivated substrates. However, total monomeric anthocyanin content of the samples was reduced during storage. According to sensory analysis, the kombucha beverages made with red raspberry and black carrot juice concentrate lost their acceptability before other beverages and cherry laurel kombucha had the best scores.
This study demonstrated that the use of different raw materials combined with green tea as substrates for kombucha, improved the antioxidant capacity of the beverages. Black carrot juice concentrate, cherry laurel, blackthorn and red raspberry were alternative sources of substrates for producing functional fermented beverages and improved the acceptability of kombucha beverages. Considering the health benefits that have been associated with the consumption of anthocyanin-rich foods, these materials appears as an important sources for functional beverages with good nutritional value.
References
C. Dufresne, E. Farnworth, Food Res. Int. 5, 409–421 (2000)
S.C. Chu, C. Chen, Food Chem. 8(3), 502–507 (2006)
T. Sriharia, J. Funct. Foods 5, 1794–1802 (2013)
H. Battikh, A. Bakhrouf, E. Ammar, LWT Food Sci. Technol. 47, 71–76 (2012)
C.P. Kurtzman, C.J. Robnett, E. Basehoar-Powers, FEMS Yeast Res. 1, 133–138 (2001)
P.J. Blanck, Biotechol. Lett. 18, 139–142 (1996)
M.R. Roussin, Analyses of kombucha ferments: report on growers: information resources. (LC, Salt Lake City, 1996)
D. Cvetković, S. Markov, M. Djuric, D. Savic, A. Velicanski, J. Food Eng. 85, 387–392 (2008)
M.I. Watawana, N. Jayawardena, S.J. Ranasinghe, V.Y. Waisundara, J. Chem. 1–9 (2015)
A.L. Teoh, G. Heard, J. Cox, Int. J. Food Microbiol. 95, 119–126 (2004)
C.J. Greenwalt, K.H. Steinkraus, R.A. Ledford, J. Food Prot. 63, 976–981 (2000)
S.D. Kumar, G. Narayan, S. Hassarajani, Food Chem. 11, 774–788 (2008)
J.M. Leal, L.V. Suárez, R. Jayabalan, J.H. Oros, A. Escalante-Aburto, CyTA J. Food 16(1), 390–399 (2018)
G. Sreeramulu, Y. Zhu, W. Knol, J. Agric. Food Chem. 48, 2589–2594 (2000)
R. Jayabalan, P. Subathradevi, S. Marimuthu, M. Sathishkumar, K. Swaminathan, Food Chem. 109, 227–234 (2008)
R.O. Lobo, C.K. Shenoy, J. Food Sci. Technol. 52(7), 4491–4498 (2015)
S. Bhattacharya, R. Gachhui, P.C. Sil, Food Chem. Toxicol. 60, 328–340 (2013)
M.P. Fournier-Larente, D. Morin, Grenier, Arch. Oral Biol. 65, 35–43 (2016)
D. Velicanski, S.L. Cvetkovic, V.T. Markov, S.M. Tumbas, Savatovic, Acta Period. Technol. 38, 1–190 (2007)
F. Fu, Z. Yan, F. Cao, J. Xie, Lin, Food Sci. Technol. Campinas 34(1), 123–126 (2014)
T.Z. Sun, J.S. Li, C. Chinshuh, J. Food Drug Anal. 23(4), 709–718 (2015)
L. Ayed, S.B. Abid, M. Hamdi, Ann. Microbiol. 67, 111–121 (2017)
R.V. Malbasa, E.S. Loncar, J.S. Vitas, J.M. Canadanovic-Brunet, Food Chem 127, 1727–1731 (2011)
J. Beekwilder, P. Meesters, R.D. Hall, I.M. van der Meer, C.H. Ric, de Vos, J. Agric. Food Chem. 53, 3313–3320 (2005)
H. Teng, T. Fang, Q. Lin, L. Chen, Trends Food Sci. Technol. 66, 153–165 (2017)
E. Ergüney, Z. Gülsünoğlu, E. Fıratlıgil-Durmus, M. Kılıç-Akyılmaz, Akademik Gıda 13(2), 108–114 (2015)
A. Eken, B. Baldemir, E. Ünlü-Endirlik, S. Bakır, İlgün, J. Food Compos. Anal. 26, 1–4 (2017)
R. Pinacho, R. Cavero, I. Astiasaran, D. Ansorena, M. Calvo, J. Funct. Foods. 19, 49–62 (2015)
T. Marakoğlu, D. Arslan, M. Özcan, H. Hacıseferoğulları, J. Food Eng. 68, 137–142 (2005)
E. Sikora, M.I. Bieniek, B. Barbara, Acta Sci. Pol. Technol. Aliment. 12, 365–372 (2013)
C. Alasalvar, J.M. Grigor, D. Zhang, P.C. Quantick, F. Shahidi, J. Agric. Food Chem. 49, 1410–1416 (2001)
B. Cemeroğlu, Gıda Analizleri, Gıda Teknolojisi Derneği Yayınları. (2007)
V.L. Singleton, J.A. Rossi, Am. J. Enol. Vitic. 16, 144–158 (1965)
V. Katalinic, M. Milos, T. Kulisic, M. Jukic, Food Chem. 94, 550–557 (2006)
I.F. Benzie, J.J. Strain, Anal. Biochem. 239(1), 70–76 (1996)
R. Apak, K. Güçlü, M. Özyürek, S.E. Çelik, Microchim. Acta 160, 413–419 (2008)
J. Lee, R.W. Durst, R.E. Wrolstad, J. AOAC Int. 88(5), 1269–1278 (2005)
L. Jungmin, R.W. Durst, R.E. Wrolstad, J. AOAC Int. 88(10), 1269–1278 (2005)
B.D. Vázquez-Cabral, N.E. Rocha-Guzmán, J.A. Gallegos-Infante, S.M. González-Herrera, R.F. González-Laredo, M.R. Moreno-Jiménez, Nutrafoods. 13, 169–178 (2014)
D. Granato, V.M.D.A. Calado, B. Jarvis, Food Res. Int. 55, 137–149 (2014)
D. Velicanski, S. Cvetkovic, Markov, Rom. Biotechnol. Lett. 8034–8042 (2013)
K. Torskangerpoll, O.M. Andersen, Food Chem. 89, 427–440 (2005)
P. I.Vīna, R. Semjonovs, I. Linde, Deniņa, J. Med. Food 17(2), 179–188 (2014)
C. Chen, B.Y. Liu, J. Appl. Microbiol. 89, 834–839 (2000)
R. Liamkaew, C. Janjira, D. Paiboon, Sci. Technol. RMUTT J. 2(6), 146 (2016)
M.I. Watawana, N. Jayawardena, B. Chaminie, Y. Gunawardhana-Viduranga, V.Y. Waisundara, Int. J. Food Sci. Technol. 51, 490–498 (2016)
M. Özkan, Ankara Üniversitesi Bilimsel Araştırma Projeleri, 2009
P. Dipti, B. Yogesh, A.K. Kain, T. Pauline, B. Anju, M. Sairam, Biomed. Environ. Sci. 16, 276–282 (2003)
M. Lui, X. Qi Li, C. Weber, C. Yong Lee, J. Brown, R. Hai Liu, J. Agric. Food Chem. 50(10), 2926–2930 (2002)
A. Mohammadshirazi, E.B. Kalhor, Renew. Sustain. Energy Rev. 55, 668–673 (2016)
S.P.J. Namal Senanayake, J. Funct. Foods 5(4), 1529–1541 (2013)
G.C. Yen, H.Y. Chen, J. Agric. Food Chem. 43(1), 27–32 (1995)
D. Huang, B. Ou, R.L. Prior, J. Agric. Food Chem. 53, 1841–1856 (2005)
B. Jiang, Z.W. Zhen-Wen, Zhang, Molecules 17(8), 8804–8821 (2012)
R.J. Clarke, J. Bakker, Front matter in wine flavor chemistry, (Blackwell, Oxford, 2007)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ulusoy, A., Tamer, C.E. Determination of suitability of black carrot (Daucus carota L. spp. sativus var. atrorubens Alef.) juice concentrate, cherry laurel (Prunus laurocerasus), blackthorn (Prunus spinosa) and red raspberry (Rubus ideaus) for kombucha beverage production. Food Measure 13, 1524–1536 (2019). https://doi.org/10.1007/s11694-019-00068-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00068-w