Abstract
This study was conducted to analyze the impact of sodium benzoate (SB), potassium sorbate (PS), and citric acid (CA) on the physicochemical properties, bioactive components, antioxidant activity, and color of Blackberry juice during storage at 4 °C. The total content of phenol (14 mg/100 g), anthocyanin (32.26 µg/100 g), proanthocyanidin (203.01 mg/100 g), and antioxidant activity (723.62 mmol/100 g) of juice prepared with preservatives (SB + PS + CA) substantially increased on the first day than those of the juice made with preservatives (SB + PS) and without preservatives. On the other hand, total phenol content and color values (L* and a*) increased while the amount of anthocyanin, flavonoid, and antioxidant activity declined for all samples over the storage period. A strong correlation between anthocyanin and flavonoid, anthocyanin and DPPH, anthocyanin and total sugar, phenol and acidity were observed. Therefore, preservatives (sodium benzoate, potassium sorbate, and citric acid were used to manufacture Blackberry juice, a practical approach to maintain nutritional quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Blackberry (Syzygium cumini L.) are belonging to the berry family because of having a tiny size and soft structure and also known as java plum, malabar plum, and black plum. Blackberries contain different vitamins, dietary fiber, and minerals along with phenolic compounds such as ellagitannins and anthocyanins [1]. Phenolic compounds have essential antioxidants (free radical scavenging and metal chelating) that can contribute to reduced cardiovascular disease, cancer, diabetes, and other diseases [2]. Due to high moisture content Blackberry is a very perishable fruit. It spoils within 24 h after harvest. Several methods such as coating [3] and juice making [4] have been applied to make phenolic compounds available to consumers for a long time. Juices are easy to prepare and also a convenient way of consuming fruits. Now a day’s consumers like to have beverages that provide bioactive compounds with healthful effects. The popularity of fruit drinks is on the rise because they are suitable for our well-being [5]. Fruit juices are typically preserved with heat treatments to avoid bacterial and microbial spoilage [4]. These procedures usually lead to unintended changes, such as loss of vitamins and minerals and the product’s fresh color and taste [6]. Much researches have been carried out to inhibit the changes and to preserve its nutritional value. Non-thermal processing is one of them [4]. Thermal technology such as ultrasound and natural antimicrobials has demonstrated useful tools to improve shelf-life and maintain the nutritional and functional properties of fruits and vegetables [7]. As ultrasound and microwave processing sometimes cause harm to the human body if it exits the limit.
To improve fruit juices shelf life in the food industry, sodium benzoate, potassium sorbate, and citric acid have been used [8]. Sodium benzoate prevents the growth of microorganisms, deterring spoilage, and improves the durability of processed foodstuffs, beverages, and other personal care items. It is particularly effective in acidic foods. Similarly, potassium sorbate is a widely used preservative. It is a popular preservative as it is effective and does not change the qualities of a product such as taste, smell, or appearance. Potassium sorbate is found in many products (soft drinks and juices, apple cider, baked goods, canned fruits and vegetables, cheeses, dried meats and fruits, ice cream, pickles, wine, yogurt, etc.). Citric acid is used as a flavoring, a preservative, an acidulant, and to provide pH control in foods and beverages [9]. It is applied in foods such as cookies, cake, ready sauces, cheese, baby foods, chewing gum, fizzy lemonade, margarine, juice and drinks [10]. In addition, sugar has now been applied to juices production to prevent polymerization and milk is also added to improve flavor.
Food and Drug Administration (FDA) and The European Food Safety Authority (EFSA) have established that sodium benzoate, potassium sorbate, and citric acid are “Generally Recognized as Safe” (GRAS), excessive addition of these preservatives in food products may present adverse health effects to consumers. The maximum permitted level for benzoates in soft drinks is 150 mg/L, expressed as benzoic Acid according to the European Food Safety Authority (ESFA). As a food additive, potassium sorbate is allowed in fruit juice and beverages at a concentration of 300 mg/L when used singly or 250 mg/L when used combined with benzoates. Usually, potassium sorbate ingests as a food additive, it passes through our digestive system harmlessly as water and carbon dioxide [11]. It does not accumulate in our bodies [12]. Considering the effectiveness of sodium benzoate, potassium sorbate, and citric acid have been used in this study to preserve the Blackberry juice. In this study, we tried to find out a way to make Blackberry juice in such a way which is both cost-effective and useful for preserving nutrients without causing any harmful effect. The goal of this study was therefore to analyze the effects of preservatives such as sodium benzoate (SB), potassium sorbate (PS), and citric acid (CA) on the physicochemical properties, bioactive compound, and color parameter of Blackberry juice during storage 4 °C.
Materials and methods
Materials
Ripe, mature, and alike size Blackberries were collected from the local market. Other items such as sugar, black salt, and powder milk were purchased from nearby supermarkets. Blackberries were cleaned with tap water, and pulps were produced using a locally developed mechanical pulping machine. Pulps were stored at − 18 °C before juice preparation.
Chemical and reagents
Sodium benzoate, potassium sorbate, citric acid, Follin Ciocalteu’s reagent, 2,2-diphenyl-1-picryl hydrazyl (DPPH), gallic acid, sulphuric acid, catechin, trichloroacetic acid, methanol, ethanol, sodium carbonate, sodium nitrite, aluminium chloride, sodium hydroxide, hydrochloric acid, vanillin, and ferric chloride were purchased from Merck (Darmstadt, Germany).
Preparation of juice
Raw pulps were thawed out for 5–6 h at room temperature (28 ± 1 °C). Based on 100 g total weight, the juice was prepared by following two distinct steps. At first 20 g pulps were blended with a blender for 3 min. Then different preservatives (sodium benzoate, potassium sorbate, and citric acid) and pulps were added to the mixture containing water (boiled water cool at room temperature), icing sugar, milk powder, black salt, and xanthan gum according to the Table 1. Then the juice was further blended for 2 min. The prepared juice was heated for 5 min at 80 ± 2 °C using a shaking water bath (VS-1205SW1). Hot juice was cooled at room temperature (28 ± 1 °C, for few minutes) and then filled in pre-sterilized (ringed with boiled water for 1 min) food grade plastic bottles. The juice bottles were stored at 4 to 6 °C throughout the storage period. A sensory test (data not shown) was carried out for the determination of various preservative concentrations. Colors of blackberry juice were not retained at room temperature so we didn't preserve it at room temperature.
Determination of chemical evaluation of the fruit juice
In order to determine the pH and TSS of Blackberry juice samples, the pH-meters (HANNA, 2211, pH/ORP meter, China) and refractometer (HANNA, HI 96814, China) were used at room temperature (28 ± 1 °C). The titratable acidity of Blackberry juice was calculated with a titration against 0.0212 N sodium hydroxide (NaOH).
Determination of total sugar content
In order to calculate total sugar Dubois et al. [13] procedure was adopted. A beaker containing 1 g of juice and an additional 15 mL of 80% ethanol solution was applied to the sample. The mixture was then mixed for 4 min in a magnetic agitator (VS-130 SH, Korea) and was filtered with the filter paper using Whatman No. 41 and centrifuged at 2000 g for 20 min. 2 mL of supernatant was added with 1 mL of 5% phenol solution. Subsequently, 5 mL of 95.5% H2SO4 solution was added to the sample. Then the test tube was allowed to stand for 10 min and vortex (KMC-1300 V) for 30 s. The sample was kept in a shaking water bath (VS-1205SW1) at room temperature (28 ± 1 °C) for the development of color. Finally, the absorbance was read at 490 nm employing a spectrophotometer (UV/VIS, UV-1800) against a blank. Standard curve for the total sugar content determination was constructed by using glucose solutions and expressed as g/100 g of sample.
Estimation of total phenol content
Total content of phenol was calculated by Singleton and Rossi [14] and Saikia et al. [15]. 5 g of sample and 10 mL of 80% ethanol was dissolved and stirred for 2 min and then filtrate through Whatman No. 41. Then 0.5 mL of filtrated sample along with 0.5 mL of Folin Ciocalteu’s reagent was taken in a 25 mL falcon tube and mixed thoroughly. The solution was allowed to react with 1 mL of 7.5% saturated sodium carbonate (Na2CO3) to the falcon tube for neutralization and then vortexed (KMC-1300 V) for 30 s. After the mixture was allowed left in a dark place for 35 min at room temperature and centrifuged at 4000 g for 10 min. The absorption of the sample was read by a visible spectrometer (UV/VIS, UV-1800) at of 725 nm. Gallic acid was used to execute a standard (calibration) curve. The findings have been shown to be equal mg/100 g of Gallic acid per 100 g of juice.
Determination of total anthocyanin content
According to the method described by Ranganna [16] anthocyanin content was determined. 1 g of juice sample was added to 15 mL of HCl-ethanol (1 mL HCl in 99 mL 95% ethanol) solution and was kept in a dark place for 3–4 h. Obtained extract was filtrated through a Whatman No. 41 filter paper and centrifuged at 4000 g for 20 min. Then the absorbance (diluted for 5 times) was measured using a spectrometer (UV/Vis, UV-1800) against a blank at 530 nm.
where, A = absorbance, W = weight of sample taken, MW = molecular weight of cyanidin-3-glucoside (449.20 g/mol), DF = dilution factor, € = molar absorptivity of cyanidin-3-glucoside (26,900 L/mol/cm).
Determination of total flavonoid content
A colorimetric method described by Kim et al. [17] was used to evaluate the total flavonoid content in the juice. Sample extraction was carried out by stirring up of 4 g Blackberry juice with 20 mL of 80% ethanol solution. Then the sample was filtered through Whatman No. 41. After that, 1 mL of extract along with 4 mL of water and 5% NaNO2 with 0.3 mL distilled water poured into a 20 mL of falcon tube for 5 min. Afterward, the 0.3 mL AlCl3 (10%) was applied to the reaction mixture and allowed to stand 1 min, followed by 2 mL of 1 M NaOH and centrifuged at 4000 g for 5 min. Then tubes were incubated for 15 min at room temperature, using spectrophotometer (UV/VIS, UV-1800) at 510 nm and expressed as mg/100 g.
Determination of proanthocyanidin content
A method described by Price et al. [18] with minor modifications was used for analyzing proanthocyanidin. Briefly, 1.5 g sample was applied in the falcon tube to 14 mL of 0.5% vanillin-HCl solution (0.5%, w/v vanillin in 4% concentrated HCl in methanol). The mixture was then transferred into a vortex for 1 min and filtered through Whatman No. 41. After incubation for 20 min, proanthocyanidin was quantified at room temperature (28 ± 1 °C) by a spectrophotometer (UV/VIS, UV-1800) at an absorbance of 500 nm against a blank. The results were expressed as g/100 g of juice.
Determination of free radical scavenging activity using DPPH
According to the Madhujith and Shahidi [19] was used to determine of free radical scavenging. Briefly, an aliquot of 0.1 mL of supernatant was taken in a falcon tube. Subsequently, 1.9 mL of 0.3 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenger in methanol was added with the sample. The mixture was allowed to rest for 30 min. DPPH (2,2-Diphenyl-1-Picrylhydrazyl) was calculated using a spectrophotometer (UV/VIS, UV-1800) with 517 nm and the results were expressed as mmol/100 g juice.
Determination of color parameter
The sample color was determined with the colorimeter (BIOBASE-Lab, Qingdao, China). L* (light/darkness), a* (roughness/greenness), and b* (yellowness/blueness) color were expressed.
Statistical analysis
The experiment was conducted in duplicate for each of the samples. All of the results were presented means ± standard deviation. A one-way variance analysis (ANOVA) was conducted to analyze the statistic (SPSS for Windows version 25). Duncan’s multiple range tests were also performed at p ≤ 0.05. Pearson’s coefficient correlation was done using SPSS version 25.
Results and discussion
Effect of preservatives on the chemical evaluation (pH, acidity and total soluble solids) of Blackberry juice during storage
The control sample (S1) had a maximum pH value compared to the other samples that were treated with additives (SB, PS, and CA) on the first day (Table 2). The pH of the control sample gradually decreased across the storage period. The sample S1 (control) pH is higher than that found by other authors in Blackberry cultivars. [20]. On the other hand, the pH of sample S2 and S3 declined at the end of the storage period in comparison with the initial day. A pH reduction could be associated with acidic components produced by samples of sugar and pectinic acid [21]. The maximum reduction of pH in the S3 sample because of increased hydrogen ions by adding citric acid [22].
The acidity of sample S3 was substantially (p ≤ 0.05) higher than that of other samples during the storage era (Table 1). The sample S3 had a lower acidity than those recorded by other researchers in Blackberry cultivars [20]. This was due to the addition of citric acid to the sample, which raised acidity by increasing the hydrogen ion concentration [21]. A steady increase (0.704–0.80%) in acidity over the storage period was observed in sample S3, while the control sample (S1) and sample S2 increased over 30 days and then decreased for the remainder of the storage period. Acidity improved with storage time was also found in ready to serve blended Bael (Aegele marmelos) beverages reported by Singh et al. [23]. The degradation of pectin into pectic acid resulted in a percentage increase in acidity of dried apricot diet jam [24]. The decrease in titratable acidity was compatible with the findings of Tiwari et al. [25]. Total acidity decreases due to the copolymerization of organic acids with browning reaction materials [26].
The impact of additives (SB, PS, and CA) on the total soluble solids (TSS) of Blackberry juice during the storage period is shown in Table 2. The higher (20.95° Brix) TSS was observed in the control sample (S1) compared with other samples treated with preservatives on the primary day. The TSS of the control sample (S1) was also higher than those reported in Blackberry cultivars by other investigators [20]. The TSS of the sample S3 increased while the sample S1 decreased with an increase the storage period. In contrast, the TSS in the sample S2 has been reduced up to 30 days then increased the remaining period of storage. The rise in TSS may be due to the breakdown of polysaccharides into monosaccharides and oligosaccharides obtained by Imtiaz et al. [27]. The ascorbic acid reduction is also responsible for increasing TSS because the structural formula of ascorbic acid is similar to glucose; hence, ascorbic acid reduction led to an increase in glucose and increased TSS [28].
Effect of preservatives on the total sugar content of Blackberry juice during storage
The highest total sugar levels (2.66–2.95 g/100 g) were observed during the initial storage days for all samples (Table 2). The total sugar content of sample S2 and S3 gradually reduced over the storage period whereas control sample (S1) did not show the similar trend. This research's total sugar content is lower than that of other authors found during storage in Red Currant juice [29]. Different sample, processing, and storage condition can cause the lower total sugar content. The reduction of total sugar for all samples was also demonstrated in apple-jamun juice blend during storage [30]. The drop in total sugars may be due to the inclusion of sugars in browning reactions [31]. Other authors also detected an increase in total sugar of jamun ready-to-serve (RTS) drink and nectar during storage [32]. Conversion of starch and pectin into simple sugars may be responsible for the raising of total sugar [33].
Effect of preservatives on the total phenol content (TPC) of Blackberry juice during storage
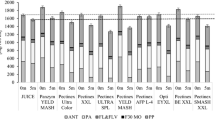
The impact of preservatives on the total phenol content (TPC) of Blackberry juice during storage are shown in Fig. 1. The control sample (S1) had a lower TPC than the treated sample (S2 and S3) at first days due to higher polyphenol oxidase levels [34]. The S3 sample had a significantly (p ≤ 0.05) higher total phenol content (14.87 mg/100 g) than the other samples (S1 and S2) during the storage period. The values documented here are consistent with those stated during storage in blackberry juice [35] and lower than other reporters' findings in Iranian wild Blackberry species [36]. Although there was no significant (p ≤ 0.05) difference between 30 and 60 days of storage in the value of the S3 sample. Sample S3 had a higher total phenol content because citric acid lowers the pH that inhibits the deterioration of polyphenol by suppressing the enzyme polyphenol oxidase [37]. Other authors have previously identified and documented a potential increase in total phenol content associated with reactions between oxidized polyphenols and the development of new antioxidant compounds during juice storage [38]. The highest (14.87 mg/100 g) total phenol content was observed at 30 days and then decreased (14.77 mg/100 g) for the rest of the storage period for all samples. However, the percentage decrease of total phenol content was higher in sample S2 contrast with other samples (S1 and S2) at the end of the storage days. The decrease in total phenol content in sample S2 (treated with SB and PS) can be attributed to the role of total phenol content in the production of polymeric compounds, the complex formulation and eventual precipitation of protein phenols [39]. In this study a strong correlation (r = 0.892) was observed between total phenol content and acidity (Table 3).
Effect of preservatives on total phenol content, anthocyanin, flavonoid and proanthocyanidin of Blackberry juice during storage at 4 oC. Different letters (a to c) indicate significant differences (p ≤ 0.05) among treatments of the samples. Different letters (A to E) indicate significant differences (p ≤ 0.05) among storage times
Effect of preservatives on the total anthocyanins content of Blackberry juice during storage
The influence of food preserving agents (SB, PS, and CA) on the stability of anthocyanins of the Blackberry juice during the storage period are presented in Fig. 1. Sample S3 showed a higher (32.26 µg/100 g) anthocyanin value in comparison with other samples (S1 and S2) on the initial day. The findings of anthocyanins are contradictory to those of Iranian wild Blackberry species and berry juices processed at different temperatures [36, 40]. This opposition may be due to the different in variety, treatment, packaging or storage temperature. The retention of total anthocyanin was improved in the sample S3 as the citric acid used to stabilize the pH in the juice [39]. The anthocyanin content of all samples (S1, S2, and S3) decreased throughout the storage period. However, control sample (S1) had higher (22.21 µg/100 g) anthocyanin content as compared to other samples at the end of the storage. The decrease in anthocyanin levels could be due to the rise of hydroxyl groups in the anthocyanin nucleus B-ring [41]. The loss of anthocyanin may also be caused by residual enzyme activity or anthocyanin condensation reactions with other phenolics [42]. In this study, there was an extensive correlation between the content of flavonoid and anthocyanin content (r = 0.890) and total sugar and anthocyanin content (r = 0.832) (Table 3). Muniyandi et al. [43] also showed the correlations between anthocyanin and flavonoid in rubus fruit. Shi et al. [44] were also observed similar positive results in the case of bayberry fruit between anthocyanin and total sugar.
Effect of preservatives on the total flavonoids content of Blackberry juice during storage
The contents of flavonoids (Fig. 1) were higher (2.114 mg/100 g) in control sample (S1), while the contents of flavonoids were lower (0.798 mg/100 g) in S2 sample during the storage period. The analysis of findings does not resemble those of other authors obtained during the shelf-life study of commercial tomato juices and the assessment of Blackberry wines [45, 46]. The diversity of samples, storage temperature, juice processing and treatment can be related to the different findings. Flavonoid content of all samples diminished throughout the storage period. The reduction of flavonoid content could be due to the oxidative cleavage of phenolic compounds and their protein polymerization may also result in the reduction of flavonoid content during storage [47]. Same decline in flavonoid content was observed during storage in the carrot-grape juice blend [48]. Lower levels of anthocyanin may be the other reason for lower levels of flavonoid content in the S2 sample. Furthermore, in this study there was a significant correlation (r = 0.90) of flavonoid content with the anthocyanin content (Table 3). Muniyandi et al. [43] found a significant correlation between anthocyanins and flavonoid content (r = 0.753).
Effect of preservatives on the proanthocyanidin content of Blackberry juice during storage
The impact of preservatives on the proanthocyanidin content of Blackberry juice across the storage period are shown in Fig. 1. The proanthocyanidin content of the sample S1 and S3 was reduced at the final storage days compared to sample S2. On the other hand, the proanthocyanidin content of the sample S2 increased (173.38 mg/100 g) throughout the storage period. The results of this proanthocyanidin content analysis are much greater than those of other Blackberry wines and commercial blackcurrant juices collected by other researchers [46, 49]. Proanthocyanidin content reduction may be due to the efficacy of the respective combination of preservatives in precipitating tannins in juice which reduced proanthocyanidin content in samples S1 and S3. The proanthocyanidin content also increased with an increase in storage time when the pomegranate juice was kept at cool temperatures [50]. Furthermore, maillard reaction, ascorbic acid depletion, and tannin oxidation and condensation [51,52,53] are the relevant reactions responsible for non-enzymatic browning in juice products that increase proanthocyanidin content.
Effect of preservatives on the free radical scavenging activity of Blackberry juice during storage
The free radical scavenging behavior of the S3 sample was of higher value compared to other samples on the primary day (Fig. 2). S3 sample retained higher (723.621 mmol/100 g) free radical scavenging activity than other samples (S1 and S2). The highest DPPH-free radical scavenging activity could be supported in addition to the increased anthocyanin in the sample S3. The enhanced activity of antioxidants through the use of citric acid in processed foodstuffs has also been found by other writers [54]. The DPPH free radical activity is lower than Blackberry wines and carrot-grape juice during storage studied by other authors [46, 48]. During each storage cycle, all three samples reduced antioxidant activity. Similar conclusions were also seen during the storage time in Blackberry juice recorded by Kopjar et al. [35]. The reduction of anthocyanin and phenolic compounds as well as changes in structure of formed compounds have led to the degradation of the antioxidant activity [35]. It was noteworthy that the association between the total content of flavonoids and activity of antioxidants was high (r = 0.802) (Table 3). The strong association of the flavonoid and antioxidant content in fruit also emerges from Calado et al. [55]. Moreover, there was a better connection between the anthocyanin and the DPPH (r = 0.889) (Table 3). The positive associations between anthocyanin and DPPH scavenging behaviors in pomegranate juice were also observed by Çam et al. [56].
Effect of preservatives on the color parameters of Blackberry juice during storage
The effect of the preservatives on L* (lightness or darkness), a* (green and red), and b* (blueness and yellowness) of Blackberry Juice are shown in Fig. 3 during storage. Color is a crucial factor in the evaluation of visual characteristics and an important parameter for the product selection. Color changes may be induced due to the acceleration of chemical reactions, increased diffusion rate, dispersion, aggregate formation, and particle breakdown [57]. The lightness (L*) and redness (a*) of sample S3 had a higher value in comparison with the control sample (S1) and sample S2 (treated with SB and PS) throughout the storage period. The value of L* and a* declined up to thirty days and then increased for rest of the storage period for all the sample. Increased lightness (L) of the juice may be caused by an increase attribution to partial precipitation of unstable suspended particles [58]. All the samples showed a fluctuation in b* values through the storage period. Changes in a* and b* coordinates may be related to changes in L* values [25]. The decreased L* and b* may be caused by oxidative browning [59]. An increase in L* value during the storage period similarly detected by del Socorro Cruz-Cansino et al. [57]. A reduction of the L* values also recorded by Daoudi et al. [60] after 30 days of storage at 4 °C. On the other hand, an increment in a* value in juices have also been found by Patras et al. [61]. As b* implies yellowness, the color of the blackberry juice is not very influential, since it is a purple fruit itself.
Conclusion
Juice treated with preservatives (SB and PS) and (SB, PS and CA) retained higher physicochemical properties (TSS, acidity) and bioactive compounds (total phenol) than those of the untreated Blackberry juice during storage. Treated juice did not prevent decreases in the amount of anthocyanin, proanthocyanidin, and free radical scavenging than the untreated sample. The lightness (L*) and redness (a*) of color had a higher value in treated samples as compared to the untreated samples at the end of the storage period. Therefore, the treated juice (SB, PS and CA) could also increase the shelf-life of Blackberry juice by maintaining a higher phenolic content and color values (L* and a*).
References
M.E. Schreckinger, J. Lotton, M.A. Lila, E.G. de Mejia, J. Med. Food 13, 233 (2010)
A. Panghal, R. Kaur, S. Janghu, P. Sharma, P. Sharma, N. Chhikara, Food Chem. 289, 723 (2019)
N.B. Gol, P.B. Vyas, T.V. Ramana Rao, Int. J. Fruit Sci. 15, 198 (2015)
L. Cassani, E. Gerbino, M. del Rosario Moreira, A. Gómez-Zavaglia, J. Funct. Foods 40, 128 (2018)
M.J. Rodríguez-Roque, B. de Ancos, C. Sánchez-Moreno, M.P. Cano, P. Elez-Martínez, O. Martín-Belloso, J. Funct. Foods 14, 33 (2015)
L. Cassani, B. Tomadoni, A. Ponce, M.V. Agüero, M.R. Moreira, Food Bioprocess. Technol. 10, 1454 (2017)
T. Bohn, G.J. McDougall, A. Alegría, M. Alminger, E. Arrigoni, A.-M. Aura, C. Brito, A. Cilla, S.N. El, S. Karakaya, Mol. Nutr. Food Res. 59, 1307 (2015)
S. Rani, J.A. Nasir, M. Ayub, U. Shahni, A. Zeb, Pure Appl. Biol. PAB 7, 103 (2018)
I.E. Sweis, B.C. Cressey, Toxicol. Rep. 5, 808 (2018)
Ş Türkoğlu, Mutat. Res. Toxicol. Environ. Mutagen. 626, 4 (2007)
P. Dehghan, A. Mohammadi, H. Mohammadzadeh-Aghdash, J.E.N. Dolatabadi, Trends Food Sci. Technol. 80, 123 (2018)
S.S. Deshpande, Handbook of Food Toxicology (CRC Press, Boca Raton, 2002).
M. Dubois, K.A. Gilles, J.K. Hamilton, P.T. Rebers, F. Smith, Anal Chem. 28, 350 (1956)
V.L. Singleton, J.A. Rossi, Am. J. Enol. Vitic. 16, 144 (1965)
S. Saikia, H. Dutta, D. Saikia, C.L. Mahanta, Food Res. Int. 46, 334 (2012)
S. Ranganna, Manual of Analysis of Fruits and Vegetables (McGraw-Hill, New Delhi, 1977).
D.-O. Kim, S.W. Jeong, C.Y. Lee, Food Chem. 81, 321 (2003)
M.L. Price, S. Van Scoyoc, L.G. Butler, J. Agric. Food Chem. 26, 1214 (1978)
T. Madhujith, F. Shahidi, J. Agric. Food Chem. 54, 8048 (2006)
D.P. Moraes, J. Lozano-Sánchez, M.L. Machado, M. Vizzotto, M. Lazzaretti, F.J.J. Leyva-Jimenez, T.L. da Silveira, E.F. Ries, M.T. Barcia, Food Chem. 322, 126783 (2020)
C. Phimpharian, A. Jangchud, K. Jangchud, N. Therdthai, W. Prinyawiwatkul, H.K. No, Int. J. Food Sci. Technol. 46, 972 (2011)
E. C. Kilel, J. K. Wanyoko, A. K. Faraj, and P. Ngoda, (2019). Food Nutr. Sci.
S. Singh, R.K. Godara, R.S. Saini, J.R. Sharma, Haryana J. Hortic. Sci. 34, 263 (2005)
F.M. Anjum, M.U. Din, I. Ahmad, A.R. Pasha, Pak. J. Food Sci 3, 21 (2000)
B.K. Tiwari, C.P. O’Donnell, P.J. Cullen, J. Food Eng. 93, 166 (2009)
M. Buglione, J. Lozano, J. Food Sci. 67, 1538 (2002)
H. Imtiaz, Z. Alam, A. Muhammad, World J. Dairy Food Sci. 6, 79 (2011)
A. Aslam, T. Zahoor, M.R. Khan, A. Khaliq, M. Nadeem, A. Sagheer, M.F.J. Chugtai, M.W. Sajid, J. Food Process. Preserv. 43, e13941 (2019)
B. Djordjević, K. Šavikin, G. Zdunić, T. Janković, T. Vulić, Č Oparnica, D. Radivojević, Plant Foods Hum. Nutr. 65, 326 (2010)
V. Mishra, U. Pradesh, K. Sharma, Int. J. Adv. Res. 4, 1210 (2016)
P.E. Shaw, J.H. Tatum, and R.E. Berry, in Development in Food carbohydrate, ed. by G.G. Birch, R.S. Shallenberger, vol. 1 (Applied Science Publishers Ltd., London, 1977), pp. 91–111
G. Rakesh, R. Singh, B.S. Yadav, Haryana J. Hortic. Sci. 39, 142 (2010)
A. Kesharwani, S.N. Dikshit, K. Kumar, P. Thakur, N. Chandel, Ecoscan 7, 379 (2015)
R. Nisar, W.N. Baba, F.A. Masoodi, Cogent Food Agric. 1, 1063797 (2015)
M. Kopjar, K. Jakšić, V. Piližota, J. Food Process. Preserv. 36, 545 (2012)
M. Garazhian, A. Gharaghani, S. Eshghi, Sci. Rep. 10, 1 (2020)
A. Altunkaya, V. Gökmen, Food Technol. Biotechnol. 49, 249 (2011)
H.E. Martínez-Flores, M.G. Garnica-Romo, D. Bermúdez-Aguirre, P.R. Pokhrel, G.V. Barbosa-Cánovas, Food Chem. 172, 650 (2015)
T.N. Hamid, P. Kumar, A. Thakur, Int. J. Curr. Microbiol. Appl. Sci. 6, 1067 (2017)
J. Hellström, P. Mattila, R. Karjalainen, J. Food Compos. Anal. 31, 12 (2013)
J. Fleschhut, F. Kratzer, G. Rechkemmer, S.E. Kulling, Eur. J. Nutr. 45, 7 (2006)
C. Brownmiller, L.R. Howard, R.L. Prior, J. Food Sci. 73, H72 (2008)
K. Muniyandi, E. George, S. Sathyanarayanan, B.P. George, H. Abrahamse, S. Thamburaj, P. Thangaraj, Food Sci. Hum. Wellness 8, 73 (2019)
L. Shi, S. Cao, J. Shao, W. Chen, Y. Zheng, Y. Jiang, Z. Yang, J. Agric. Food Chem. 62, 10522 (2014)
F.J. García-Alonso, S. Bravo, J. Casas, D. Perez-Conesa, K. Jacob, M.J. Periago, J. Agric. Food Chem. 57, 6815 (2009)
D.A. Klarić, I. Klarić, A. Mornar, N. Velić, D. Velić, Foods 9, 1623 (2020)
H.-W. Huang, Y.H. Chang, C.-Y. Wang, Food Bioprocess. Technol. 8, 2483 (2015)
M. Nadeem, N. Ubaid, T.M. Qureshi, M. Munir, A. Mehmood, Ultrason. Sonochem. 45, 1 (2018)
P.H. Mattila, J. Hellström, G. McDougall, G. Dobson, J.-M. Pihlava, T. Tiirikka, D. Stewart, R. Karjalainen, Food Chem. 127, 1216 (2011)
C.-W. Hsieh, W.-C. Ko, LWT-Food Sci. Technol. 41, 1752 (2008)
N.E. Babsky, J.L. Toribio, J.E. Lozano, J. Food Sci. 51, 564 (1986)
B. Kacem, J.A. Cornell, M.R. Marshall, R.B. Shireman, R.F. Matthews, J. Food Sci. 52, 1668 (1987)
V. Cheynier, C. Owe, J. Rigaud, J. Food Sci. 53, 1729 (1988)
H.-D. Belitz, W. Grosch, P. Schieberle, Food Chemistry (Springer, New York, 2009).
J.C.P. Calado, P.A. Albertão, E.A. de Oliveira, M.H.S. Letra, A.C.H.F. Sawaya, M.C. Marcucci, Agric. Sci. 6, 426 (2015)
M. Çam, Y. Hışıl, G. Durmaz, Food Chem. 112, 721 (2009)
N. del Socorro Cruz-Cansino, E. Ramírez-Moreno, J.E. León-Rivera, L. Delgado-Olivares, E. Alanís-García, J.A. Ariza-Ortega, J. de JesúsManríquez-Torres, D.P. Jaramillo-Bustos, Ultrason. Sonochem. 27, 277 (2015)
P. Ghosh, S. Garg, I. Mohanty, D. Sahoo, R.C. Pradhan, J. Food Sci. Technol. 56, 1877 (2019)
B.K. Tiwari, A. Patras, N. Brunton, P.J. Cullen, C.P. O’donnell, Ultrason. Sonochem. 17, 598 (2010)
L. Daoudi, J.M. Quevedo, A.J. Trujillo, F. Capdevila, E. Bartra, S. Mínguez, B. Guamis, Int. J. High Press. Res. 22, 705 (2002)
A. Patras, N.P. Brunton, S. Da Pieve, F. Butler, Innov. Food Sci. Emerg. Technol. 10, 308 (2009)
Acknowledgements
The author wishes to thank the Bangladesh Bureau of Educational Information and Statistics (BANBEIS), Project No: LS 2018762, Ministry of Education, Government of the People’s Republic of Bangladesh, for their financial assistance in this report.
Author information
Authors and Affiliations
Contributions
Conceptualization, SA methodology, data curation, formal analysis, investigation, SA, MS and SPT; original draft preparation, SA; visualization and data curation, KAK.; supervision, visualization, writing—review & editing, MA. The published version manuscript was read and agreed by all authors.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmed, S., Mia, M.S., Shipon, T.P. et al. Physicochemical properties, bioactive compounds and total antioxidant activity of Blackberry (Syzygium cumini L.) juice retained by preservatives during storage. Food Measure 15, 3660–3669 (2021). https://doi.org/10.1007/s11694-021-00933-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00933-7