Abstract
Microencapsulation of Lactobacillus Plantarum was carried out using sodium alginate (Alg), inulin (IL), and dextran (DT) to increase the survivability of probiotic cells. The effects of IL (0.5, 1, and 1.5%) and DT (0.5, 1, and 1.5 mg/L) additions were investigated on the encapsulation efficiency (EE) and cell survivability in the gastric and biliary conditions. Results showed that microencapsulation significantly increased the cell survivability. 1.5% of IL and 0.5 mg/L of DT were the best concentrations to obtain the highest EE (93.55%). The microencapsulated cells within the Alg-IL-DT system had higher count than cells entrapped within the Alg microspheres either in gastric or biliary conditions. Inclusion of free cells and microencapsulated cells increased the probiotic count of whey beverage (WB). Albeit, the WB with the microencapsulated cells had higher probiotic count. pH was significantly decreased in drinks with probiotic cells while acidity was significantly increased. 2,3-Pentanedione, 2-Heptanone, Acetoin, Propylene glycol, 2-butoxyethoxy-2-Propanol, Benzaldehyde, Butyric acid, 4-methylbenzaldehyde, Hexanoic acid, Ethyl acetate, and Benzoic acid were the most abundant volatile compounds in WBs. The sensory test exhibited that addition of probiotic cells either in free or microencapsulated types increased the scores of sensory properties such as flavor, color, odor, concentration, and total acceptability. Thus, the microencapsulation of L. plantarum by Alg-IL-DT system can be taken into an advantage to increase the survivability of probiotic cells in strong acidic and saline conditions. Furthermore, incorporation of the microencapsulated cells in food products can increase their health aspects and palatability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Production of functional beverages has recently been launched to present new ready-to-drink products with enhanced bioactive compounds [1,2,3]. The functionality of beverages is derived from the utilization of bioactive components including probiotic cells as the most celebrated ones are so-called lactic acid bacteria (LAB) which have a long history of safe use in the food industry [4, 5]. Probiotics are usually added to a wide range of food and beverage products such as sour milk, fruit juices, yogurts, fermented vegetables, muesli, etc. [3, 4, 6, 7]. The benefits of probiotics are associated with their numerous health impacts such as improving intestinal microbial balance, inhibition of pathogenic growth by production of antimicrobial substances, modulating the innate immune systems, indicating antimutagenic activities, and prevention of carcinogenesis [3, 4, 8]. The most important associated probiotics with the gastrointestinal tract (GIT) are Lactobacillus genera (plantarum, casei, etc.) [4, 5]. It has been reported some criteria to use probiotics in food and beverage products as following: (1) the viability during the industrial processes; (2) being survival during preparation and storage of the carrier foods; (3) remaining during the entrance through the GIT environment of the host; (4) show health benefits through fermentation process in the large intestine of the host [3, 4]. Therefore, gut microbes are closely associated with most human health. It has been reported that when probiotics are ingested orally, they can effectively regulate the composition and quantity of human intestinal microorganisms, which is beneficial to human health [9].
The whey beverages are so appreciated due to their nutritional quality which can increase the survival of probiotics during the storage time [3]. It should be noted that the functional and probiotic terms can be dedicated to a specific product which contains viable cells in range of 106-107 CFU/mL until expiration date. Additionally, the probiotics should survive during the passage through the GIT, which highlights the application of encapsulation techniques [1, 3, 10]. Probiotic cells which are incorporated in food and beverage products are sensitive to processing and environmental circumstances such as low pH and heat treatment [1, 7, 11]. Encapsulation increases the survivability of probiotic cells by establishment of cover surrounding the cells, which preserves them from the intensive conditions [1, 12,13,14].
Entrapment of probiotic cells with hydrocolloids has been attracted great attention due to the efficiency of method compared to other techniques [1, 12, 15, 16]. Indeed, the greatest advantage of microencapsulation of probiotic with hydrocolloids is that cells are embedded within the matrix during the formation of the spheres, while in other techniques such as spray, freeze, and fluidized bed drying, the cells are likely released into the product and exposed to the environmental condition [10, 15]. The most widely used matrix for microencapsulation is alginate (Alg) [1]. It forms gels which are susceptible to disintegration in the presence of excess monovalent ions, Ca2+ chelating agents, and harsh chemical environments, such as those of low pH [15,16,17]. Alginate is used along with emulsifiers such as Tween 80 and co-emulsifier such as glycerol and emulsified through gentil stirring [1]. Addition of divalent ions such as Ca2+ supports microbeads (microspheres) formation [1, 15, 18]. These microbeads efficiently preserve the entrapped probiotic cells against the harsh condition [15, 18, 19]. The effects of addition of other hydrocolloids also have been investigated and revealed improved survivability [15, 16, 18, 20, 21]. Addition of hydrocolloids such as inulin, galactooligosaccharide (GOS), pectin, chitosan, etc. have been studied and findingds exhibited increased encapsulation efficiency (EE) and survivability towards the GIT and model food conditions [13, 21,22,23]. Survivability of combined or individually encapsulated lactobacillus aciduphillus and lactobacillus reutrti in alginate or alginate-chitosan (Alg-CH) was studied when they were exposed to simulated GIT concditions [23]. Using Alg-CH improved the cell protection better than Alg and improved also lactobacilli survival during storage in milk, peach nectar, or blackberry jam set-style yogurt; lactobacilli counts were ≥ 107 CFU/g after 30 days, except for encapsulated combined lactobacilli in peach nectar [23]. Based on several research studies, using multiple hydrocolloids have confirmed higher EE, better protection, and survivability in GIT and model food systems [10, 13, 20, 24].
So far no studies were carried out using the emulsification technique to microencapsulate the lactobacillus plantarum (L. plantarum) by alginate, inulin, and dextran to verify the possibility of increasing the viability of probiotic cells towards the GIT and whey beverage conditions. Co-encapsulation of probiotics with prebiotics has been practiced as a novel alternative approach for further improvement of the oral delivery of viable probiotics toward their targeted release in the host intestine.
Materials and methods
Materials
Lactobacillus plantarum (L. plantarum) was purchased from Christian Hansen, Horsholm, Denmark. De Man Rogosa and Sharpe (MRS) broth, peptone water, inulin, dextran, alginate, glycerol, Tween 80, calcium chloride (CaCl2), simulated gastric fluid (SGF), simulated intestine fluid (SIF), chloridoid acid (HCl), and nutrient agar were supplied by Merck Co. (Germany). Pepsin was provided from Novozymes (Denmark). Whey protein and milk powder were purchased from Pegah dairy Co. (Tehran, Iran). Other chemicals used were analytical grade and were provided from Merck and Sigma-Aldrich Co (USA).
Preparation of L. plantarum
The lyophilized L. plantarum cells were added to 10 mL of MRS broth and incubated for 24 h at 37 °C to be activated. The obtained solution was added to 95 mL of MRS broth to reach the bacteria count at least 108 CFU/mL. The cultivated liquor was centrifuged at 1500×g and 25 °C for 5 min. Then, the cells were rinsed twice with 0.1% sterile peptone water [16].
Generation of alginate microsphere containing L. plantarum
The Alg microspheres incorporated with inulin and dextran were produced based on the developed method of Rodrigues et al. [25] with slight modifications. Alg solution was prepared at 2% w/v, which incorporated with inulin (IL) and dextran (DT) solutions at 0.5 to 1.5% w/v and 0.5 to 1.5 mg/mL, respectively. The prepared solutions were heated at 70 °C and mixed with 0.5% w/w glycerol and 0.1% w/w Tween 80. All solutions were sterilized at 121 °C for 15 min. The prepared cell culture (1% w/v) was added to the Alg-IL-DT solutions and mildly stirred at sterile condition. The obtained solutions were transferred into the syringe with a diameter of 1 mm and added to sterile CaCl2 solution (2% w/v) dropwise. The formed microspheres were kept for 10 min at ambient temperature and then rinsed with distilled water, followed by mixing with peptone water 0.1% w/w to keep at 5 °C for further tests. The produced microspheres based on their contents of IL and DT were called T1 to T13 (Table 1).
Encapsulation efficiency (EE)
The EE% value of samples was measured by direct method and counting the viable cells in solutions before and after encapsulation, using the equation below [25]:
Survivability of probiotics
The viability of cells was assessed for Alg-IL-DT microspheres during 15 days of storage. 5 g of produced microspheres were mixed with 45 mL of sterile sodium citrate 2% adjusted to pH 6.0, at agitation condition of 45 °C for 10 min. After appropriate dilution in MRS broth, the cultivation was carried out based on the pour plate followed by incubating at 37 °C for 72 h. Also, total count of microorganisms was accomplished in nutrient agar at the similar condition [25]. The cells were stored at 4 °C for about a period of 15 days.
Survivability of probiotics
In SGF
SGF was prepared by dissolving 2 g NaCl, 2.92 g HCl, and 3 g pepsin in deionized water in a 1 L volumetric flask. The pH was adjusted to 2.1 by 0.1 N NaOH [23, 26]. 1 g of produced microspheres was mixed with 9 mL of prepared solutions and after 0, 30, 60, 90, and 120 min, they were separated and survivability of bacteria cells was assessed based on the counting method.
In biliary solution
1 g of produced Alg-IL-DT microspheres was mixed with 9 mL of bile solution including 10 g of bile salts and 2 g KCl, 2 g KH2PO4, 29.2 g Na2HPO4, and 80 g NaCl in a 1 L volumetric flask which had been sterilized before (the pH was adjusted to 7.8 using 0.1 N NaOH). After 0, 2, 4, and 6 h, the microspheres were separated and viability of cells was determined based on the counting method [27].
Production of probiotic beverage
The functional whey beverages were generated by selecting the optimum microsphere based on the higher EE% and survivability and then addition of it into the whey formula [2]. Whey solution of 11% w/v containing 80% protein was mixed with 2% w/w milk powder, and 10.3% w/w sugar, followed by homogenizing at 10,000 rpm for 10 min. Next, the solution was pasteurized at 75 °C for 30 min and then, cooled down to 40 °C. 20 g/L of encapsulated L. plantarum and free L. plantarum (108 CFU/mL) along with 0.3% v/v fermentation starter culture containing L. acidophilus and streptococcus thermophilus were added to the obtained pasteurized whey solution and kept at 4 °C for further test. The produced whey beverages were termed WB (control), whey beverage containing free lactobacillus plantarum (WBFLP), and whey beverage containing encapsulated lactobacillus plantarum (WBELP).
The survivability of probiotics
The survivability of cells (probiotics) and total count were determined based on the method described in Sect. 2.5.
pH and acidity determination
pH was determined by lab pH-meter and acidity of functional whey solutions was measured using 0.1 M NaOH based on the lactic acid and phenol phthalein as the indicator [28].
Volatile compounds determination
The volatile compounds in the produced whey beverage were determined based on the method suggested by Magalhães et al. [29] with brief modifications. The beverage was extracted with dichloromethane, and then analyzed by gas chromatography (Nexis, GC-2030) (Shimazu, Japan) equipped with a Split/Spitless injector and a flame ionization detector. A capillary column (50 m × 0.25 mm, id., 0.2 μm film thickness; Chrompack), coated with CP-Wax 57 CB was applied. The temperatures of the injector and detector were set to 250 and 280 °C, respectively. The oven temperature was held at 35 °C for 6 min, then programmed to run from 50 to 110 °C at 5 °C/min and held for 10 min. Next, the temperature was increased to 210 °C at 3 °C/min. Helium was used as the carrier gas at 3.33 psi, with a split vent of 15 mL/min. The volatile compounds were identified by comparing retention indices with those of standard compounds. The quantification of volatile compounds was conducted with the Varian Star Chromatography Workstation software (version 6.41).
Sensory analysis
The sensory properties including taste, color, odor, concentration, and total acceptability were analyzed based on scoring the beverage sample from 1 to 5 using the 5-point hedonic test [30, 31]. 15 semi-taught panelists were used to score the beverage sample. The highest score (5) indicated the highest desirability and the lowest score (1) indicated the lowest desirability.
Statistical analysis
All experiments were done at three replications and data were reported by mean and standard deviation. The analysis of variance (ANOVA) was used to study of statistical difference and Duncan test was used to differentiate the data which had statistically difference at a confidence level of 95% (p < 0.05).
Results and discussion
EE (%)
The effects of IL and DT were studied on the EE% values of lactobacillus plantarum entrapped within the Alg-IL-DT microspheres. Alg has been well-known to form microbead or microspheres which have been efficiently used to protect the biological components by increasing EE value. On the other hand, utilization of other encapsulating agents or carriers has shown increasing effects on the EE values.
ANOVA statistical analysis of effects of IL and DT were presented in Table 2. The model used to investigate the effects of IL and DT was significant (p < 0.05). Also, linear IL and DT effects along with their interaction term were significant on the EE values (p < 0.05). Thus, the addition of IL and DT was elucidated that can significantly affect the EE values. The EE values varied from 58.98% (T1) to 93.55% (T11) (Table 1). The results based on Table 1; Fig. 1A indicated that inulin concentration had a significant effect on the EE% (p < 0.05). When it was increased from 0 to 1.5%, EE% of entrapped lactobacillus plantarum was significantly increased (p < 0.05). By considering the DT concentration constant, when IL concentration was increased from 0 to 0.5, 1, and 1.5%, EE values were obtained 60.70%, 68.52%, and 82.03%, respectively (Table 1). On the other hand, DT concentration indicated increasing effect on EE% up to 1 mg/mL (Fig. 1B). By considering IL constant (0.5%), when DT increased from 0 to 0.5, 1, and 1.5 mg/mL, EE values were increased to 63.75% and 68.06%, and decreased to 66.48%, respectively. According to Table 1, EE% values for T10, T11, T12, and T13 were obtained 82.03%, 93.55%, 89.41%, and 85.49%, respectively, which showed that at the highest concentration of IL (1.5%), the highest EE% value can be achieved when DT was used 0.5 mg/mL. Hence, it could be suggested that IL had increasing effect on EE% value at the concentrations used while DT alongside the IL had synergistic effect at 0.5 mg/mL [32].
IL as a Fructooligosaccharides (FOS) possibly formed an integrated layer surrounding the microspheres produced by Alg [1, 32]. This layer increased the EE% values and protected the probiotic cells from the environmental stresses [33, 34]. IL as a prebiotic has been reported to increase the cell viability by increasing the EE% [1, 32, 34, 35]. Lactobacillus acidophilus was entrapped within Alg microspheres which were incorporated with IL, rice bran, and resistant starch (hi-maize). The highest EE% (96.75%) was obtained when IL was added at 10% w/w to Alg solution with 2% w/w [18]. Similar results were found by the other researchers [20, 36].
DT is a complex branched glucan (polysaccharide derived from the condensation of glucose), originally derived from wine. It has been defined as a branched poly-α-d-glucosides of microbial origin having glycosidic bonds predominantly C-1 → C-6”. Dextran chains are of varying lengths (from 3 to 2000 kilodaltons) [37, 38]. DT has been introduced as an efficient capping agent in the enhanced delivery of curcumin by nanoparticles in breast cancer cells [38]. DT has large molecules which can either formation of a stable emulsion by steric hinderance role or formation of a layer with entrapping effects [38, 39]. Possibly, using a concentration higher than 0.5 mg/mL of DT led to depleted flocculation, thus decreasing the EE% values [1, 10]. Using combined IL and DT as capping agents at appropriate concentrations surrounding the Alg microspheres exhibited improved EE% values which can preserve the lactobacillus plantarum cells instead of GIT harsh conditions.
Survivability of probiotics
The viability of lactobacillus plantarum cells was investigated for about a period of 15 days at 4 °C and results are presented in Table 3. As reported for EE%, the higher viability was obtained for T11 during the 15 days of storage. Using IL significantly increased the cell viability so that higher concentration (1.5% w/w) led to higher viability (p < 0.05). On the other side, entrapment of Alg microspheres by DT at concentrations up to 1% mg/mL increased the cell viability (T7 and T8) and decreased when 1.5 mg/mL was used. It was while that using IL at 1.5% w/w without addition of DT (T10) led to significant increase in the cell viability (p < 0.05). Despite, addition of 0.5 mg/mL of DT to microspheres entrapped with 1.5% w/w of IL significantly increased the cell viability (p < 0.05).
Storage of probiotic cells significantly decreased the cell viability (Table 3) (p < 0.05). However, the cells entrapped within the Alg microspheres capped with IL and DT had high survivability compared to entrapped cells within the Alg microspheres without encapsulating agents (IL and DT) (Table 3). Especially, high concentration of IL (1.5% w/w) and low concentration of DT (0.5 mg/mL) caused remarkable retaining of viable cells compared to samples with lower IL and higher DT. IL and DT might possibly construct a building block around the cells and maintained them from the environmental stresses [40, 41]. The number of probiotic cells also were retained using IL and DT due to their prebiotic impacts. Indeed, IL and DT can be consumed by the probiotic cells through the fermentation process [13, 16, 40, 42]. The survival of lactobacillus rhamnosus GG was increased by addition of inulin into the microcapsules produced by chitosan and alginate [13]. The survival of microencapsulated probiotics in alginate microbeads coated with chitosan and incorporated with inulin and galactooligosaccharides was increased [16]. It has been reported that addition of prebiotics increased the resistance of the probiotic cells to low pH, heat treatment, and also bile salt. Generally, prebiotic compounds provide carbon and nitrogen sources for the growth of probiotic bacteria, resulting in high number of the cells being survived [43, 44]. Addition of inulin has increased the survival ability of L. casei, which had been microencapsulated in high resistance starch-alginate using emulsion techniques, after subjecting to freeze-drying [36, 45]. Besides that, double coating on encapsulated probiotics could also improve their survivability through gastrointestinal digestion [34, 46].
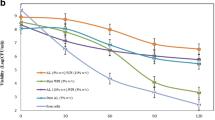
Survivability of probiotics at SGF
Viability of lactobacillus plantarum cells was studied at SGF condition and results are presented in Table 4. Accordingly, the higher IL addition and lower incorporation of DT increased the cell viability during the passing SGF (120 min). The highest viability of probiotic cells was obtained at 0 min when the cells did not pass the SGF condition. Moreover, T11 had the highest cell survivability (7.77 log CFU/g) at 0 min, which decreased (6.35 log CFU/g) at 120 min (p < 0.05). However, this reduction indicated high maintenance effects of IL and DT on the sensitive probiotic cells which number remarkably decreased when low concentration of IL (0 and 0.5% w/w) and high concentration of DT (1.5% w/w) were incorporated (Table 4). High EE% can preserve the probiotic cells due to the embedded structure that IL and DT constructed [47, 48]. SGF condition has an intensive acidic condition that can affect the cells membrane of probiotic cells and disrupt the structure and lead to diffusion of internal cell contents [12]. IL and DT can increase the viscosity of surrounding media and subsequently increase of the cell resistance to the acidic condition [1, 49]. Co-encapsulation of probiotics and prebiotics has been known as a novel technique to increase the viability of cells when they are passing the intensive acidic and basic conditions [49,50,51]. It has been reported that bacteria grow more poorly in monosaccharide fructose in comparison with that in oligosaccharide composed of fructose moieties. It has been demonstrated that there is a specific substrate transport mechanism that is most efficient at transporting indigestible oligosaccharides than simple sugars. Chicory which is rich in IL and FOS with a degree of polymerization of 10 may act as a potential prebiotic for probiotic strains. Sugarbeet and chicory have been found to be an efficient prebiotic with their fermentation rate and potent in co-encapsulation with probiotics in Alg matrix. It has been also reported high survival rate by immobilizing different probiotics with FOS, as a growth promoter [20, 32, 51]. Co-encapsulation of L. plantarum with Alg, IL, and DT led to high protection of probiotic cells when exposed to the drastic acidic conditions (Table 4). The higher cell mortality occurred for samples without addition of IL and DT. By addition of IL and DT, the cell death was decreased at SGF which can be attributed to the prebiotic impacts that maintained the cell growth and cell fermentation which provided enough energy even at harsh conditions.
Survivability of probiotics in biliary solution
The viability of L. plantarum was investigated at biliary condition and results are presented in Table 5. When Alg-IL-DT microspheres were exposed to biliary condition, the number of probiotic colonies was significantly decreased (p < 0.05). The highest reduction was obtained for T1 which was not incorporated with IL and DT. Addition of IL and DT significantly increased the cell viability so that the highest viabilities were obtained for T11 and T12. Incorporation of 1.5 mg/mL of DT led to reduction of cell viability (T9). Thus, 0.5 and 1 mg/mL of DT could be considered the most appropriate concentrations for the maintenance of cell viability. On the other side, IL at the concentration of 1.5% w/w indicated the higher cell survivability at the biliary conditions. Comparing to the SGF, the cell enumeration exhibited a reduction at the biliary condition. The results of viability of encapsulated cells were in accordance with other reported works [23, 45, 52,53,54]. The protection of microencapsulation technique can lead to immobilize the probiotic cells which contribute to hypocholesterolemia through two mechanisms: decrease cholesterol absorption accompanied by enhanced cholesterol excretion via feces and the production of short chain fatty acids upon selective fermentation by intestinal bacterial microflora. These probiotics reduces bile production and also stimulates bile salt hydrolysate [51]. In the present study, IL and DT formed a tight network which increased the Alg microsphere resistance to the bile salts. Similar results were found regarding the increase in cell viability when encapsulated probiotic cells were exposed to the bile salts [53, 55, 56]. It was reported that the cell tolerance to the adverse environments such as high concentration of bile salts (1 and 2%) was increased by encapsulation technique (alginate–milk microspheres) [53]. It has been reported that the viabilities of microencapsulated probiotics containing 1.5% inulin and GOS was higher than those of without GOS when exposed to bile salts [16].
Whey beverage analysis
Volatile compounds in whey beverage
The volatile compounds of produced whey beverages (WB, WBFLP, and WBELP) were determined by GC-FID and are presented in Table 6; Fig. 2A-C. The volatile compounds which contribute to the flavor of dairy products are alcohols, esters, ketones, and aldehydes. The most abundant compounds were 2,3-Pentanedione, toluene, butyl acetate, 2-Heptanone, 2-Methyl-1-butanethiol, acetoin, propylene glycol, 2-butoxyethoxy-2-Propanol, 2-Nonanone, (2,5-dimethyloxan-2-yl) methanol, benzaldehyde, furan, butyric acid, 4-methylbenzaldehyde, anethole, hexanoic acid, octanoic acid, decanoic acid, ethyl acetate, butyl formate, and benzoic acid (Table 6). Regarding the WB which was not inoculated with probiotic bacteria, due to the presence of lactose and proteins, L. acidophilus and Streptococcus thermophilus as the starter culture fermented the mentioned available substrates and produced the volatile compounds shown in Table 6. These components are produced during the catabolism of branched amino acids such as isoleucine or generated through the de novo pathway and biosynthesis of amino acids [29]. From qualitative point of view, incorporation of free L. plantarum and encapsulated probiotics resulted in no significant differences, which could be due to the combination usage of probiotics that do not affect the profile of major aroma compounds, but had rather an effect on their concentration [56]. Exceptionally, limonene was not detected in WB while WBFLP and WBELP had about 1.02 and 1.08% w/w, respectively. The organic acids were well-represented class of volatile compounds in the WB beverages (Table 6). Accordingly, thioctic acid, octadecadienoic acid, butyric acid, hexanoic acid, octanoic acid, 4-methyl-3-Pentenoic acid, and benzoic acid were in high contents especially in WBELP. The addition of Lactobacillus probiotic has been well correlated with the formation of organic acids [56].
The common identified esters were butyl acetate, ethyl acetate, and butyl formate which were decreased especially for WBELP (especially two former volatiles). Possibly, WBELP had encapsulated probiotic cells and production of esters was limited. However, the presence of esters even in low concentration is essential for the formation of “fruity” and “floral” aromas and flavors, but is also associated with extended storage [56]. Butyl acetate, ethyl acetate, and butyl formate were the main esters found in the respective WB beverages. Two former esters were decreased especially for WBELP likely due to degradation into other volatile components (such as aldehydes and ketones) while they remained high in WBFLP due to non-entrapped form of probiotics. It seems that somehow encapsulation of probiotics may limit the production of volatile compounds. Albeit, it provides a stable generation of volatiles due to high survivability of probiotics.
Alcohols are common fermentation products of lactic acid bacteria, which some have an agreeable ethereal odor, and mild flavor note which contribute to the many dairy products [56]. According to Tables 1 and 6-Methyl-1-butanethiol, propylene glycol, 2-butoxyethoxy-2-Propanol, meso-3,4-Hexanediol, (2,5-dimethyloxan-2-yl) methanol, and Anethole were the alcohol products detected in WB, WBFLP, and WBELP (Fig. 2A-C). Some of the associated alcohols (for instance propylene glycol and anethole) were decreased for WBFLP and WBELP likely due to L. plantarum activity which fermented the alcohols to the carboxylic and organic acids (Fig. 2B-C) [56].
Aldehydes are also an important group of volatile compounds responsible for the formation of the characteristic aroma profile of dairy products [57]. The predominant detected aldehydes were acetaldehyde, furfural, benzaldehyde, and 4-methylbenzaldehyde (Table 1).
Regarding the ketones, acetone, 2,3-pentanedione, octalactone, 2-heptanone, acetoin (3-hydroxy-2-butanone), and 2-nonanone were the main contributing ketones in the obtained WBs. The octalactone and 2-nonanone were higher in WBELP than especially WB (Table 1 and Fig. AC). The concentration of acetoin was higher in WBFLP and WBELP, as constant metabolic products of citrate metabolism that affect the creamy and butter-like flavor, as well as δ- dodecalactone with a positive effect on final flavor formation [56] (Table 1) (Fig. 2B-C).
Aldehydes and ketones were the highest concentrations of volatiles found in the tested samples which is in consistent with other reports [56].
Total count
Total count of WBs was determined during a period of 7 days storage and are presented in Table 7. For WB, the number of cells was decreased from 6.58 log CFU/g at day 1 to 5.21 log CFU/g at day 7. The viable cells were high in WBFLP and WBELP due to incorporation of L. plantarum. Likewise, the number of probiotic cells of WBFLP was decreased from 8.77 log CFU/g at day 1 to 7.67 log CFU/g at day 7. The total count of WBELP was relatively higher than WBFLP but not significant (p > 0.05). It was decreased from 8.86 log CFU/g at day 1 to 8.35 log CFU/g at day 7. It was observed that encapsulation of probiotics in WBELP led to a high survivability when comparison was performed with WBFLP. The Alg-IL-DT system embedded the cells and higher viable cells were enumerated.
Numeration of probiotics
The number of probiotics was determined for WBs and are presented in Table 7. WB did not show L. plantarum while WBFLP and WBELP indicated viable probiotic cells as 7.05 and 7.54 log CFU/g on day 1 which were decreased to 5.75 and 7.42 log CFU/g on day 7, respectively. The number of L. plantarum was significantly decreased for WBFLP after 7 days of storage (p < 0.05). When the probiotic cells were incorporated in the encapsulated form, the number of cells was not significantly altered (p > 0.05). It showed that encapsulation of sensitive cells can increase their survivability [13, 34, 58]. Possibly, using IL and DT led to remaining high number of the probiotic cells which was in accordance with those reported [23, 34].
pH and acidity determination
The pH and acidity of WBs were determined and are presented in Table 7. The pH was reduced for all samples during 7 days of storage due to the fermentation and lactic acid formation. The lower pH was obtained for WBFLP and WBELP due to the inoculation of L. plantarum in free and encapsulated types. Especially, WBELP had high survivability of probiotic cells, thus, relatively higher lactic acid was formed [56].
Regarding the acidity, higher values were obtained for WBFLP and WBELP (Table 7). It could be related to L. plantarum presence which fermented the lactose into lactic acid [56].
Sensory analysis
The sensory properties of WBs were assessed and presented in Table 8. WBFLP and WBELP indicated higher taste scores possibly due to development of some volatile compounds explained in Sect. 3.5.1 [56, 59]. Within the scores obtained for color, it was not observed significant difference (p > 0.05). Moreover, WBFLP and especially WBELP indicated higher odor scores which can be associated with the development of volatile compounds. Regarding the concentration parameter, the WBs which had L. plantarum cells either in free or entrapped types, indicated higher scores which could be related to the Alg and prebiotics used to reach higher viability (Alg-IL-DT system). These biopolymers provide a high viscosity media which can increase the mouthfulness. Overall, the highest acceptability scores were obtained for WBs which were incorporated with free and encapsulated probiotic cells. Especially, WBELP exhibited relatively higher scores compared to WBFLP.
According to the reports, sensory evaluation demonstrated that judges did not perceive the presence of capsules in blackberry jam set-style yogurt, but were sensed in milk and peach nectar [23]. Based on the results obtained in present study, WB which was inoculated with encapsulated L. plantarum, was more palatable which can due to the activity of high viable cells remained in the Alg-IL-DT microspheres.
Conclusion
Using IL and DT as carrier agents increased the EE obtained for Alg-IL-DT system. IL and DT supported the cell survivability at intensive gastric and biliary conditions. pH was decreased through the inoculation of free and encapsulated probiotic cells. The WBs with microencapsulated L. plantarum had higher palatability which could be attributed to the generation of volatile compounds such as alcohols, esters, organic acids, and aldehydes.
Therefore, microencapsulation of L. plantarum within the Alg-IL-DT system can be a promising approach to increase the cell viability. Also, high pH and acidity fluctuations do not occur during the storage.
Data Availability
Data will be made available on request.
References
A. Rezvankhah, Z. Emam-Djomeh, G. Askari, Dry. Technol. 38, 235 (2020)
K.T. Magalhães, G. Dragone, G.V. de Melo, J.M. Pereira, L. Oliveira, J.A. Domingues, Teixeira, J. B. A. e Silva, and R.F. Schwan, Food Chem. 126, 249 (2011)
N. Obradović, M. Volić, V. Nedović, M. Rakin, B. Bugarski, J. Food Eng. 110948 (2022)
O.-A. Praepanitchai, A. Noomhorm, A.K. Anal, Biomed Res. Int. 2019, (2019)
K. Smilkov, T. Petreska Ivanovska, L. Petrushevska Tozi, R. Petkovska, J. Hadjieva, E. Popovski, T. Stafilov, A. Grozdanov, K. Mladenovska, https://doi.org/10.3109/02652048.2013.824511 31, 166 (2014)
B. Speranza, D. Campaniello, L. Petruzzi, C. Altieri, M. Sinigaglia, A. Bevilacqua, M.R. Corbo, Beverages 2020, Vol. 6, Page 20 6, 20 (2020)
K. Ebrahimi Monfared, M. Gharachorloo, A. Jafarpour, J. Varvani, J. Food Meas. Charact. 1 (2021)
N. Sabokbar, M. Moosavi-Nasab, F. Khodaiyan, Food Sci. Biotechnol. 24, 2095 (2015)
C. Xu, Q. Ban, W. Wang, J. Hou, Z. Jiang, J. Control Release 349, 184 (2022)
A. Rezvankhah, Z. Emam-Djomeh, M. Safari, M. Salami, G. Askari, J. Food Process. Preserv. e16554 (2022)
A.R. Donthidi, R.F. Tester, K.E. Aidoo, https://doi.org/10.3109/02652040902982183 27, 67 (2010)
Z. Emam-Djomeh, A. Rezvankhah, Release Bioavailab. Nanoencapsulated Food Ingredients (Elsevier, 2020), pp. 79–120
H. Gandomi, S. Abbaszadeh, A. Misaghi, S. Bokaie, N. Noori, LWT-Food Sci. Technol. 69, 365 (2016)
Q. Guo, S. Li, J. Tang, S. Chang, L. Qiang, G. Du, T. Yue, Y. Yuan, Int. J. Biol. Macromol. 194, 539 (2022)
G.B. Brinques, M.A.Z. Ayub, J. Food Eng. 103, 123 (2011)
W. Krasaekoopt, S. Watcharapoka, LWT-Food Sci. Technol. 57, 761 (2014)
S. Nualkaekul, D. Lenton, M.T. Cook, V.V. Khutoryanskiy, D. Charalampopoulos, Carbohydr. Polym. 90, 1281 (2012)
G. Poletto, G.C. Raddatz, A.J. Cichoski, L.Q. Zepka, E.J. Lopes, J.S. Barin, R. Wagner, C.R. de Menezes, Food Hydrocoll. 95, 238 (2019)
A. Nakkarach, U. Withayagiat, Agric. Nat. Resour. 52, 467 (2018)
Y. Nami, G. Lornezhad, A. Kiani, N. Abdullah, B. Haghshenas, LWT 124, 109190 (2020)
G.C. Raddatz, B. de Souza da Fonseca, G. Poletto, E. Jacob-Lopes, A.J. Cichoski, E.I. Muller, E.M.M. Flores, C. de Bona da Silva, and C. Ragagnin de Menezes, Powder Technol. 362, 409 (2020)
M. Jouki, N. Khazaei, S. Rashidi-Alavijeh, S. Ahmadi, Food Hydrocoll. 120, 106895 (2021)
A. García-Ceja, E. Mani-López, E. Palou, A. López-Malo, LWT - Food Sci. Technol 63, 482 (2015)
M. Afzaal, F. Saeed, M. Saeed, A. Ahmed, H. Ateeq, M.T. Nadeem, T. Tufail, J. Food Process. Preserv 44, e14346 (2020)
F.J. Rodrigues, M.H. Omura, M.F. Cedran, R.F.H. Dekker, A.M. Barbosa-Dekker, S. Garcia, J. Microencapsul. 34, 431 (2017)
M. Mahmoud, N.A. Abdallah, K. El-Shafei, N.F. Tawfik, and H. S. El-Sayed, Heliyon 6, e03541 (2020)
R. Li, Y. Zhang, D.B. Polk, P.M. Tomasula, F. Yan, L. Liu, J. Control Release 230, 79 (2016)
D. Dimitrellou, Y. Kourkoutas, A.A. Koutinas, M. Kanellaki, Food Microbiol. 26, 809 (2009)
K.T. Magalhães, G. Dragone, G.V. De Melo Pereira, J.M. Oliveira, L. Domingues, J.A. Teixeira, J. B. A. E Silva, and R.F. Schwan, Food Chem. 126, 249 (2011)
A. Rezvankhah, M.S. Yarmand, B. Ghanbarzadeh, H. Mirzaee, J. Food Process. Preserv 45, e15932 (2021)
A. Rezvankhah, M.S. Yarmand, B. Ghanbarzadeh, H. Mirzaee, J. Food Meas. Charact. 15, 5021 (2021)
B. Haghshenas, Y. Nami, M. Haghshenas, A. Barzegari, S. Sharifi, D. Radiah, R. Rosli, N. Abdullah, Asian J. Pharm. Sci. 10, 350 (2015)
R. Rajam, C. Anandharamakrishnan, LWT-Food Sci. Technol. 60, 773 (2015)
Y. How, L. Pui, J. Food Meas. Charact. 15, 4899 (2021)
Y. Nami, A. Kiani, D. Elieh-Ali‐Komi, M. Jafari, B. Haghshenas, Int. J. Dairy Technol. (2022)
A.G. Peredo, C.I. Beristain, L.A. Pascual, E. Azuara, M. Jimenez, LWT - Food Sci. Technol 73, 191 (2016)
X. Yan, X. Gong, Z. Zeng, M. Ma, J. Zhao, J. Xia, M. Li, Y. Yang, P. Yu, D. Gong, D. Wan, Foods 11, 3066 (2022)
M. Sampath, A. Pichaimani, P. Kumpati, B. Sengottuvelan, Int. J. Biol. Macromol. 162, 748 (2020)
Z. Cheng, A. Al Zaki, I.W. Jones, H.K. Hall, C.A. Aspinwall, A. Tsourkas, Chem. Commun. 50, 2502 (2014)
S. Tantratian, M. Pradeamchai, LWT 123, 109075 (2020)
N. Liao, B. Luo, J. Gao, X. Li, Z. Zhao, Y. Zhang, Y. Ni, F. Tian, Biotechnol. Lett. 41, 263 (2019)
A. Shah, A. Gani, M. Ahmad, B.A. Ashwar, F.A. Masoodi, Int. J. Biol. Macromol. 82, 217 (2016)
S. Razavi, S. Janfaza, N. Tasnim, D.L. Gibson, M. Hoorfar, Food Hydrocoll. 120, 106882 (2021)
S. Sharafi, L. Nateghi, Iran. J Chem Chem Eng 41, 1812 (2022)
L. Luca, M. Oroian, Foods 2021, Vol. 10, Page 710 10, 710 (2021)
R. Gheorghita, L. Anchidin-Norocel, R. Filip, M. Dimian, M. Covasa, Polym. 2021, Vol. 13, Page 2729 13, 2729 (2021)
A. Atia, A. Gomaa, I. Fliss, E. Beyssac, G. Garrait, M. Subirade, J. Microencapsul. 33, 89 (2016)
P. Barajas-Álvarez, M. González-Ávila, H. Espinosa-Andrews, Food Rev. Int. 1 (2021)
H.A. Albadran, A. Monteagudo-Mera, V.V. Khutoryanskiy, D. Charalampopoulos, Appl. Microbiol. Biotechnol. 104, 5749 (2020)
A. Rashidinejad, A. Bahrami, A. Rehman, A. Rezaei, A. Babazadeh, H. Singh, S.M. Jafari, https://doi.org/10.1080/10408398.2020.1854169 62, 2470 (2020)
S. Sathyabama, M. Ranjith kumar, P. Bruntha devi, R. Vijayabharathi, V. Brindha priyadharisini, LWT - Food Sci. Technol 57, 419 (2014)
B. Haghshenas, N. Abdullah, Y. Nami, D. Radiah, R. Rosli, A. Yari Khosroushahi, J. Appl. Microbiol. 118, 1048 (2015)
L.E. Shi, Z.H. Li, D.T. Li, M. Xu, H.Y. Chen, Z.L. Zhang, Z.X. Tang, J. Food Eng. 117, 99 (2013)
F. Zarei, L. Nateghi, Human, Heal. Halal Metrics 2, 1 (2021)
C. Gebara, K.S. Chaves, M.C.E. Ribeiro, F.N. Souza, C.R.F. Grosso, M.L. Gigante, Food Res. Int. 51, 872 (2013)
D. Dimitrellou, P. Kandylis, S. Lević, T. Petrović, S. Ivanović, V. Nedović, Y. Kourkoutas, LWT 116, 108501 (2019)
K. Skryplonek, I. Dmytrów, A. Mituniewicz-Małek, J. Dairy. Sci. 102, 7773 (2019)
D. Rodrigues, S. Sousa, A.M. Gomes, M.M. Pintado, J.P. Silva, P. Costa, M.H. Amaral, T. Rocha-Santos, A.C. Freitas, Food Bioprocess. Technol. 5, 2748 (2012)
W.F. Castro, A.G. Cruz, D. Rodrigues, G. Ghiselli, C.A.F. Oliveira, J.A.F. Faria, H.T. Godoy, J. Dairy. Sci. 96, 96 (2013)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare no conflict of interest in all parts of work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saniani, M., Nateghi, L. & Hshemiravan, M. Investigation of microencapsulated lactobacillus plantarum survival in alginate microsphere incorporated with inulin and dextran in order to produce a novel probiotic whey beverage. Food Measure 17, 3683–3694 (2023). https://doi.org/10.1007/s11694-023-01902-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-01902-y