Abstract
A sufficient amount of probiotics is essential to be consumed to improve the host’s health. In order to protect probiotics against the harsh gastrointestinal environment, probiotics were subjected to microencapsulation prior to product manufacturing or consumption. However, maintaining the viability of encapsulated probiotics remains a challenge. Hence, prebiotics were often incorporated with encapsulated probiotics with the intention to serve as a nutrient source or protectants for probiotics. In this paper, the effect of incorporating prebiotics to encapsulated probiotics on microencapsulation efficiency, microbeads size, and survivability under gastrointestinal conditions and storage were reviewed extensively. Besides, we also introduced potential emerging prebiotics that had been incorporated into encapsulated probiotics and compared them against common established prebiotics. Furthermore, this article also highlights the possible factors that may cause low viable cell count and small microbead size of encapsulated probiotics after the addition of prebiotics. Lastly, the importance of microencapsulating probiotics with synergistic prebiotics were emphasized in this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics that are often used in beverages or food products are the members of the genera Lactobacillus, Bifidobacterium, and Lactococcus [1]. These strains are recognized as Generally Recognized As Safe (GRAS) by the United States Food and Drug Administration (US FDA). There are various health properties associated with probiotics to improve human health conditions such as anti-microbial [2], anti-carcinogenic [3], anti-proliferative [4], anti-inflammatory [5], and immune-modulatory properties [6]. Hill et al. [7] recommended that a minimum amount of 9 log10 CFU/servings probiotics had to be consumed to confer health benefits. However, prolonged storage on the shelf and harsh gastrointestinal conditions could reduce the viability of probiotics [8]. Hence, microencapsulation of probiotics is explored to provide an adequate amount of probiotics to the host.
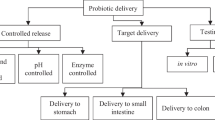
The microencapsulation technique is capable to shield the probiotics from a harsh outer environment, for example, oxygen, extreme pH, enzymes, and antimicrobial nutrients from either food, beverages, or the gastrointestinal tract [9,10,11,12]. Besides that, microencapsulation also allows the release of probiotics at controlled and targeted sites [13, 14]. Various microencapsulation processes are often adopted by the food industry for probiotics, for instance, extrusion, emulsification, and atomization (spray drying and freeze-drying) [15]. However, encapsulation efficiency, microbeads size, and survivability of the probiotics could vary with different encapsulation techniques. Hence, several criteria are considered by the food industry in selecting the encapsulation techniques which are the characteristics of the active ingredients and wall materials, desired microbeads size, food and beverage application, targeted release site, cost, and industrial applicability [16]. The materials involved in probiotics microencapsulation are commonly categorized into wall and core materials [17]. Wall materials are defined as the continuous polymers used to create a barrier between probiotics and the outer environment [18]. On the other hand, core materials in microencapsulation of probiotics refer to the probiotics or any other materials such as prebiotics mixed with probiotics (Fig. 1) [19].
There are two types of microbeads that can be formed with different types of microencapsulation techniques. Reservoir-type of microbeads are usually formed using techniques such as co-extrusion, coacervation, fluid bed coating, and emulsification or extrusion with coating. The wall or coating materials for reservoir-type of microbeads form an outer layer surrounding the active ingredients (probiotics and/or prebiotics) (Fig. 2a). In contrast, spray drying, freeze-drying, and emulsification or extrusion without coating would produce matrix-type microbeads. This type of microbead is produced by mixing wall and core materials prior to the encapsulation process, which resulted in the dispersing of the active ingredients within and on the surface of the wall material (Fig. 2b) [20].
The minimum amount of probiotics required to be in food or beverage products at their final shelf life is 6 log10 CFU/mL [7]. In order to improve the viability of probiotics in food products, microencapsulation technology had been adopted by the food industry to encapsulate probiotics. Encapsulated probiotics had been incorporated into various functional food products in the market such as yoghurts, chocolates, ice cream, dairy drinks, and nutrition bars [15]. Probiotics had been encapsulated prior to incorporating into food products to preserve the product’s sensory properties, improve the probiotics’ stability over long shelf life, and protect the probiotics against harsh gastrointestinal digestion upon consumption [21]. In addition to probiotics, prebiotics such as maltodextrin and inulin had also been incorporated into these commercial food products.
Prebiotics play a role in prolonging probiotics’ survivability throughout storage and consumption by providing nutrients to probiotics for growth [22,23,24]. Furthermore, prebiotics had been reported to improve probiotics’ survivability through the microencapsulation process and storage by acting as a protective matrix and nutrient source for probiotics [25]. However, the addition of prebiotics into encapsulated probiotics may also increase the size of the microbeads which will affect the texture of food products [18, 26]. Many reviews had been published on probiotics, prebiotics, and their impact on improving health [27,28,29]. On the other hand, there are limited reviews on the encapsulation efficiency, microbeads size, and survivability of encapsulated probiotics incorporated with prebiotics. Hence, this paper aimed to review the impact of prebiotics in microencapsulated probiotics in terms of microencapsulation efficiency, microbeads size, gastrointestinal digestion and storage.
Prebiotics
The concept of prebiotics were first introduced in 1995 and currently defined as a “substrate that is selectively utilized by host microorganisms conferring health benefits” [30]. Moreover, established criteria that were widely accepted are (i) resistance towards acid and bile; (ii) resists against hydrolysis by human digestive enzymes and intestinal absorption, (iii) allow the fermentation of selected intestinal microflora, (iv) stimulate the growth of selected bacteria that confer health benefits on the host, and (v) stable throughout processing conditions [31]. The presence of prebiotics in the diet may lead to numerous health benefits such as mineral absorption, lipid regulation, anti-inflammatory, and reducing the risk of colon cancer [32,33,34]. Furthermore, prebiotics are also used in the food industry to improve sensory attributes, nutritional content, and stability of the food after incorporation [15, 35].
Prebiotics have huge potential to serve as an agent in maintaining balanced intestinal bacteria as prebiotics may become an alternative to the probiotics bacteria [36]. However, the combination of probiotics and prebiotics are often used to ensure a superior effect, rather than using prebiotics or probiotics alone [31]. The concept of the incorporation between prebiotics and probiotics is also known as synbiotic [28]. This is to enhance the survivability of the probiotics present in the gastrointestinal tract, where the probiotics and prebiotics properties can overcome the adverse condition in order for the probiotics to remain viable in the gut [31]. Besides that, prebiotics can form three-dimensional networks of microcrystals that react and form small aggregation that gives better protection to the probiotics [37].
There is common established prebiotics that had been widely researched and available in the market such as fructo-oligosaccharide, mannitol, maltodextrin, inulin, and galacto-oligosaccharides [38,39,40]. On the other hand, researchers had also been exploring new prebiotics obtained from different sources. These emerging prebiotics such as tragacanth gum, Arabic gum, trehalose, isomalto-oligosaccharides, plant extracts (beetroot, ginger, Synsepalum dulcificum, Plantago psyllium), seeds (chia or flaxseed), fruit skin or peel extract, and different types of starches (potato, arrowroot, hi-maize, rice, wheat) were also used for microencapsulation by different studies (Table 1).
Microencapsulation efficiency and microbeads size of encapsulated probiotics with or without prebiotics
Different types of prebiotics or combination of prebiotics had been incorporated into various types of probiotics to increase their viability during the gastrointestinal tract and/ or storage (Table 1). From Table 1, it is observed that the inclusion of prebiotics had a different effect on the microencapsulation efficiency of probiotics. Most studies displayed higher microencapsulation efficiency with the inclusion of prebiotics [25, 41, 48, 54, 56,57,58,59,60], except for Kim et al. [54], Ng et al. [26], and Chan and Pui [51] who demonstrated lower microencapsulation efficiency with the addition of prebiotics. Furthermore, Shinde et al. [53], Siang et al. [52], Paim et al. [45], Savedboworn et al. [47], and Nami et al. [42] reported no significant difference in microencapsulation efficiency between prebiotics and without prebiotics. Moreover, microencapsulation efficiency for common prebiotics are ranged from 38.1 to 99.2%. Similarly, emerging prebiotics also showed a wide range of microencapsulation efficiency (22.5–99.3%) (Table 1).
According to Yee et al. [25] and Yong et al. [41], the presence of prebiotics during microencapsulation of probiotics could improve the probiotics’ survivability by acting as a nutrient source, hence demonstrating higher microencapsulation efficiency. Besides that, the increase in microencapsulation efficiency with the presence of prebiotics could be due to the protective effect exerted by prebiotics [41]. It is suggested that sugars, protein, or carbohydrates used as prebiotics, could also act as protectants to prevent cell damage [61]. The interaction between prebiotic and polymer (mannitol and calcium alginate) could be formed through hydrogen bonds during the microencapsulation process, which protected the probiotics [62].
On the other hand, Kim et al. [54], Ng et al. [26], and Chan and Pui [51] all displayed higher microencapsulation efficiency without prebiotics than with prebiotics (Table 1). This could be explained by the microbeads’ inner space were taken up the prebiotics, hence resulting in lower probiotics viable count encapsulated in the microbeads as suggested by Ng et al. [26]. Furthermore, Kim et al. [54] indicated that the low microencapsulation efficiency with the presence of prebiotics could also be due to the disruption in the chemical bond between wall and coating materials by the prebiotics during the encapsulation process. This shows the symbiotic relationship between prebiotics and wall materials is crucial to ensure high microencapsulation efficiency. Foroutan et al. [55] reported higher microencapsulation efficiency for prebiotics tragacanth than pectin and Arabic gum despite using the same probiotic strain, wall materials, and encapsulation technique. Hence, a preliminary study could be carried out by exploring different types of prebiotics against desired probiotics prior to the encapsulation process.
According to Table 1, Savedboworn et al. [47], Raddatz et al. [48], and Serrano-Casas et al. [49] used both common and emerging prebiotics for the same probiotic strain in their respective studies, however different microencapsulation efficiency was reported. Furthermore, common (inulin) and emerging prebiotics (Plantago psyllium fibre and potato starch) used by Peredo et al. [50] also displayed higher microencapsulation efficiency for L. plantarum Lp17 than L. casei Shirota (Table 1). This demonstrates that high microencapsulation efficiency is dependent on the compatibility between specific prebiotics and probiotics, regardless of common or emerging prebiotics.
In terms of the effect of prebiotics on microbeads size, all studies compiled in Table 1 shows larger microbeads size for probiotic microbeads with prebiotics than without prebiotics [26, 41,42,43, 51, 52, 54, 60], except for Paim et al. [45], Fritzen-Freire et al. [46], Bustamante et al. [59], and Raddatz et al. [48]. Besides that, the microbeads for all of the studies listed in Table 1 had microbeads size below < 1000 μm [25, 26, 41, 42, 44,45,46, 48, 49, 51, 52, 59, 60], except for Kumherová et al. [43], Peredo et al. [50], and Kim et al. [54]. Furthermore, the microbead size of encapsulated probiotics with common prebiotics ranged from 6.8 to 2300 μm; while encapsulated probiotics with emerging prebiotics had a range of microbeads size of 14.4–1830 μm (Table 1).
According to Haghshenas et al. [63], Lotfipour et al. [64], and Kim et al. [54], the increase of viscosity in the microbeads after the addition of prebiotics would increase the droplet size during microencapsulation, which further leads to the formation of large microbead size. Moreover, the low solubility compounds in prebiotics may also affect its interaction with other encapsulating materials such as wall and core materials during mixing or encapsulation, which resulted in poor gelation and larger bead size [49]. Furthermore, Nami et al. [42] also stated that the size of the large microbeads with prebiotics could be due to the thickness of prebiotics contributed to the larger microbead size. On the other hand, the smaller microbead size with the addition of prebiotics was not addressed by the studies [46, 52, 55, 56]. Nevertheless, the concentration and type of prebiotics used could have affected the microbead size [42, 65]. Besides that, Silva et al. [66] also stated that the symbiotic relationship between the prebiotics and wall materials may also reduce the microbead size. The prebiotics could occupy the free spaces between the wall materials through cross-linking bonds, hence taking up lesser space in the microbeads, resulting in smaller microbead size.
Small microbead size (< 1000 μm) is preferable in terms of the sensory aspect of food application [18, 43]. However, both microbeads with and without prebiotics demonstrated large microbeads size (> 1000 μm) for Kumherová et al. [43] and Kim et al. [54]. Furthermore, all the microbeads size for different prebiotics (common and emerging) used in the study by Peredo et al. [50] was more than 1000 μm. This shows that the cause of producing large microbeads size could be contributed by the encapsulation technique. The size of the microbeads produced through the emulsification technique could vary based on different factors such as the type of surfactants used, viscosity between water and oil phases, and flow rate of droplets [67]. Similarly, factors such as nozzle or syringe size, type and concentration of hardening solution, the distance between nozzle and hardening solution, and flow rate of droplets for extrusion technique could also affect the microbead sizes [68].
Probiotics encapsulated with either common or emerging prebiotics displayed a wide range of microbeads size. Samedi and Charles [44], Raddatz et al. [48], Serrano-Casas et al. [49], and Peredo et al. [50] used both common and emerging prebiotics for microencapsulation of probiotics. Samedi and Charles [44] reported larger microbeads size with emerging prebiotics (arrowroot starch) than common prebiotics (maltodextrin); in contrast, Raddatz et al. [48] demonstrated larger microbeads size using common prebiotics (inulin) as compared to emerging prebiotics (hi-maize starch and rice bran). Furthermore, common prebiotic inulin used in the microencapsulation of probiotics by Peredo et al. [50] had a larger microbead size than emerging prebiotic Plantago psyllium fibre but had a smaller microbead size as compared to emerging prebiotic potato starch. This shows that the microbead size is affected by the synbiotic between each prebiotics and probiotics, regardless of common or emerging prebiotics.
Survivability of encapsulated probiotics with or without prebiotics through simulated gastrointestinal conditions
Table 2 displayed the survivability of encapsulated probiotics with or without prebiotics under gastrointestinal digestion. According to Table 2, the addition of different prebiotics in most of the studies was able to protect the probiotics through both simulated gastric juice (SGJ) and simulated intestinal juice (SIJ) [25, 26, 42, 51,52,53,54, 56, 59, 69], except for Poletto et al. [69], Kumherová et al. [43], Savedboworn et al. [47], Raddatz et al. [48], Fazilah et al. [58], and Yong et al. [41]. Besides that, encapsulated probiotics incorporated with common established prebiotics had survivability of 68.8–97.8%; while encapsulated probiotics with emerging prebiotics had survivability of 0–104% after subjected to simulated gastric or intestinal conditions (Table 2).
Nami et al. [42] reported that L. lactis encapsulated with both fructo-oligosaccharides or inulin displayed lower reduction during sequential gastrointestinal digestion as compared to L. lactis without prebiotics. The study suggested that prebiotics could improve the viable cell count of encapsulated probiotics through harsh gastrointestinal conditions by serving as a nutrient source and protective matrix for the probiotics. Furthermore, prebiotics that is symbiotic with the wall or core materials could also form a better network via chemical bonds, hence reducing the space between core and wall material’s network and pore size [66]. This is demonstrated by Silva et al. [66] where L. acidophilus encapsulated with fructo-oligosaccharides had higher survivability and network resistance determined by scanning electron microscopy than L. acidophilus encapsulated without fructo-oligosaccharides throughout the gastrointestinal digestion. By improving the wall of the microbeads, the probiotics in the core will be shielded better from the outer harsh environment.
Despite its protective and prebiotics effect on probiotics, some studies also demonstrated that prebiotics could lower the survivability of probiotics through the simulated gastrointestinal environment [41, 47, 48, 58, 69]. Kumherová et al. [43] stated that the difference between encapsulated probiotics with and without prebiotics was not significant, indicating that prebiotics had no positive nor negative effect on the probiotics. Yong et al. [41] and Raddatz et al. [48] showed that encapsulated probiotics with prebiotics (mannitol, inulin, hi-maize starch) displayed lower survivability either through simulated gastric or intestinal conditions than encapsulated probiotics without prebiotics. This finding was not addressed by both studies. A possible explanation could be the network formed between the prebiotics and pH-sensitive wall materials degraded under the simulated gastrointestinal environment, resulting in the release of the cells [9, 70]. Nevertheless, this could be improved by using a combination of wall materials instead of single-wall material for the encapsulation of probiotics [71]. Besides that, double coating on encapsulated probiotics could also improve its survivability through gastrointestinal digestion [72].
Furthermore, Poletto et al. [69], Savedboworn et al. [47], and Fazilah et al. [58] showed that encapsulated probiotics with the incorporation of prebiotics rice bran, hi-maize starch, gum Arabic, and Synsepalum dulcificum seed demonstrated lower survivability than without prebiotics under gastrointestinal conditions. On the other hand, the addition of inulin, xylitol, fructo-oligosaccharide, polydextrose, Synsepalum dulcificum leaf and pulp to encapsulated probiotics displayed higher survivability than without prebiotics despite using the same wall materials and encapsulation technique in their respective studies. This showed the specificity in the symbiotic relationship between prebiotics, probiotics and wall materials. Similarly, Nami et al. [42], El-Abd et al. [56], Bustamante et al. [59], Serrano-Casas et al. [49], and Peredo et al. [50] also reported the different effects of prebiotics on encapsulated probiotics through the gastrointestinal condition. The negative effect of the prebiotics on the low probiotics’ survivability in the microbeads through the gastrointestinal environment could be due to the lack of utilization by the probiotics. According to Ann et al. [73], a large amount of prebiotics present in the microbeads might collide with the probiotics in the microbeads during the agitation, hence damaging the cell membrane and leads to cell death. Besides that, a large amount of unutilized prebiotics present in a medium may increase viscosity, which could also affect the survivability of probiotics [74, 75].
This demonstrates that common established prebiotics were more consistent in protecting different types of probiotics from different encapsulation techniques through the harsh gastrointestinal environment as compared to emerging prebiotics. However, emerging prebiotics beetroot extract, ginger extract, and xylitol were able to protect their respective probiotics with > 90% survivability under both gastric and intestinal conditions [47, 56]. Furthermore, Plantago psyllium fibre was able to increase the viable cell count of L. plantarum Lp17 after subjected to the simulated gastric condition [50]. These few emerging prebiotics demonstrates the promising effect that could be further explored by researchers to improve probiotics’ viability under gastrointestinal condition.
Survivability of encapsulated probiotics with or without prebiotics through storage
Most of the storage studies for the encapsulated probiotics with prebiotics displayed higher viability cell count at the end of their respective storage duration as compared to encapsulated probiotics without prebiotics [25, 26, 42, 46, 48, 50, 57, 59, 69, 76,77,78,79,80,81], except for Savedboworn et al. [47] as displayed in Table 3. Furthermore, the minimum amount of probiotics required to be in food or beverage products at their final shelf life is 6 log10 CFU/mL [7], which was fulfilled by the majority of the studies in Table 3 at the end of their storage studies (> 6 log10 CFU/mL).
Savedboworn et al. [47] encapsulated L. plantarum TISTR 2075 with rice protein as wall material and inulin or xylitol as prebiotics using the freeze-drying technique. The study showed lower viable cell count during the 90 days storage under 30 °C as compared to the absence of prebiotics. According to Savedboworn et al. [47], the incompatibility between polymer (rice protein) and prebiotic (xylitol or inulin) increases the glass transition temperature to be higher than the storage temperature (30 °C). The temperature difference between the storage and glass transition temperature may lead to non-enzymatic browning and cause an effect on the viability of the probiotics [82].
The encapsulated probiotics with prebiotics with viable cell count less than 6 log10 CFU/mL reported by Yee et al. [25] and Sulabo et al. [57] was stored under room temperature. According to Ferdousi et al. [83], probiotics stored at room temperature have higher cell metabolism which leads to the occurrence of biochemical reactions that may harm the probiotics. Nevertheless, the higher viable cell count in prebiotic mannitol encapsulated with L. acidophilus in mulberry tea by Yee et al. [25] as compared to encapsulated L. acidophilus without prebiotics indicates its protective capacity. In contrast, Poletto et al. [69] reported that inulin was not able to exert a prebiotic effect towards encapsulated L. acidophilus. On the contrary, rice bran and hi-maize starch used in the study were able to preserve encapsulated L. acidophilus for 75 days under 7 °C. Similarly, Peredo et al. [50] also displayed lower viability (< 6 log10 CFU/mL) for L. casei Shirota with all prebiotics (inulin, potato starch, and Plantago psyllium fibre) than L. plantarum Lp17. Both of these studies indicate the importance of symbiotics between prebiotics and probiotics under prolonged storage to maintain high viability.
Phoem et al. [76], Poletto et al. [69], Savedboworn et al. [47], Raddatz et al. [48], and Poletto et al. [69] used both common established and emerging prebiotics in their studies. Most of the emerging prebiotics (oligosaccharides extract, rice bran, hi-maize starch, and potato starch) were able to preserve encapsulated probiotics better than common established prebiotics [48, 50, 69, 76], except for inulin and fructo-oligosaccharides used in respective studies by Raddatz et al. [48] and Savedboworn et al. [47]. Furthermore, probiotics encapsulated with flaxseed, chia seed, cashew flower, or oligosaccharides extract displayed a high viable cell count (> 8 log10 CFU/mL) after their respective storage period. This showed the potential of emerging prebiotics in protecting the viability of probiotics throughout the storage period.
Different prebiotics used by Savedboworn et al. [47] and Raddatz et al. [48] had different effects on their respective encapsulated probiotics that are stored at different temperatures. According to Scott et al. [84], storage temperature and moisture could have an impact on the prebiotics’ composition, which may affect the prebiotics’ efficiency on probiotics survival under different storage temperatures. Nevertheless, both emerging and common established prebiotics could preserve probiotics at different storage temperatures as reported by Raddatz et al. [48]. This emphasizes the importance of evaluating specific prebiotics and their effect on encapsulated probiotics under different storage temperatures. Different food or beverages products require different storage temperatures. Hence, the findings of the optimum storage temperature for respective encapsulated probiotics with prebiotics would benefit researchers in exploring its potential to develop functional foods products.
Conclusion and future prospective
In this paper, both common and emerging prebiotics displayed a similar range on microencapsulation efficiency and microbeads size. Emerging prebiotics incorporated with encapsulated probiotics such as beetroot extract, ginger extract, and xylitol displayed high survivability after gastrointestinal simulation (> 90%); while flaxseed, chia seed, cashew flower, and oligosaccharides extract showed high viable count after storage (> 8 log10 CFU/mL). These potential emerging prebiotics with encapsulated probiotics could be further explored by researchers to be used to develop functional foods. Most of the studies reported high microencapsulation efficiency, large microbead size, and high survivability under gastrointestinal conditions and storage for encapsulated probiotics with the addition of prebiotics. On the other hand, factors that may cause small microbeads size or low viable cell count after encapsulation, gastrointestinal simulation and storage by prebiotics were also discussed in this review. Nevertheless, this review emphasizes the importance of selecting prebiotics that are symbiotic with probiotics and wall materials to achieve high microencapsulation efficiency, small microbeads size, high survivability in gastrointestinal conditions and storage.
This review introduced different emerging prebiotics that were encapsulated with probiotics. These new prebiotics that were able to protect probiotics through the encapsulation process, storage, and gastrointestinal digestion should be further explored on their potential to improve human health. Hence, future studies could examine the health effects of the encapsulated synbiotic (probiotics with emerging prebiotics) through in vivo studies. Besides that, more studies could incorporate encapsulated probiotics with emerging prebiotics into various food or beverage products as value-added ingredients and evaluate their sensory characteristics.
The authors declare no conflict of interest.
References
C.R. Soccol, L.P. de Souza Vandenberghe, M.R. Spier, A.B.P. Medeiros, C.T. Yamaguishi, J.D.D. Lindner, A. Pandey, V. Thomaz-Soccol, Food Technol. Biotechnol. 48, 413 (2010)
A. Akbar, M.B. Sadiq, I. Ali, M. Anwar, N. Muhammad, J. Muhammad, M. Shafee, S. Ullah, Z. Gul, S. Qasim, S. Ahmad, A.K. Anal, CYTA - J. Food 17, 214 (2019)
E. Jacouton, F. Chain, H. Sokol, P. Langella, L.G. Bermúdez-Humarán, Front. Immunol. 8, 1 (2017)
A. Tiptiri-Kourpeti, K. Spyridopoulou, V. Santarmaki, G. Aindelis, E. Tompoulidou, E.E. Lamprianidou, G. Saxami, P. Ypsilantis, E.S. Lampri, C. Simopoulos, I. Kotsianidis, A. Galanis, Y. Kourkoutas, D. Dimitrellou, K. Chlichlia, PLoS ONE 11, e014790 (2016)
T.D. Luerce, A.C. Gomes-Santos, C.S. Rocha, T.G. Moreira, D.N. Cruz, L. Lemos, A.L. Sousa, V.B. Pereira, M. De Azevedo, K. Moraes, D.C. Cara, J.G. Leblanc, V. Azevedo, A.M.C. Faria, A. Miyoshi, Gut Pathog. 6, 1 (2014)
V. Garcia-Castillo, R. Komatsu, P. Clua, Y. Indo, M. Takagi, S. Salva, M.A. Islam, S. Alvarez, H. Takahashi, A. Garcia-Cancino, H. Kitazawa, J. Villena, Front. Immunol. 10, 1 (2019)
C. Hill, F. Guarner, G. Reid, G.R. Gibson, D.J. Merenstein, B. Pot, L. Morelli, R.B. Canani, H.J. Flint, S. Salminen, P.C. Calder, M.E. Sanders, Nat. Rev. Gastroenterol. Hepatol. 11, 506 (2014)
P. Fernandez-Pacheco, M. Arévalo-Villena, A. Bevilacqua, M.R. Corbo, A. Briones Pérez, LWT - Food Sci. Technol. 97, 332 (2018)
A.B. Shori, HAYATI J. Biosci. 24, 1 (2017)
T. Heidebach, P. Först, U. Kulozik, Crit. Rev. Food Sci. Nutr. 52, 291 (2012)
H. Liu, S.W. Cui, M. Chen, Y. Li, R. Liang, F. Xu, F. Zhong, Crit. Rev. Food Sci. Nutr. 59, 2863 (2019)
M. Aspri, P. Papademas, D. Tsaltas, Fermentation 6, 1 (2020)
S. Basu, D. Banerjee, R. Chowdhury, P. Bhattacharya, J. Food Eng. 238, 61 (2018)
A. Jeyakumari, A. Zynudheen, U. Parvathy, MOJ Food Process. Technol. 2, 214 (2016)
J. Burgain, C. Gaiani, M. Linder, J. Scher, J. Food Eng. 104, 467 (2011)
A.E. Abd El Kader, H.M. Abu Hashish, Egypt. J. Chem. 63, 1881 (2020)
L. Serna-Cock, V. Vallejo-Castillo, African J. Microbiol. Res. 7, 4743 (2013)
A. Nag, K.S. Han, H. Singh, Int. Dairy J. 21, 247 (2011)
A. Feucht, H.S. Kwak, Korean J. Food Sci. Anim. Resour. 33, 229 (2013)
N.J. Zuidam, E. Shimoni, in Encapsulation Technol. Act. Food Ingredients Food Process, ed. by V. Nedovic, N.J. Zuidam (Springer, New York, 2010), pp. 3–29
F. Nazzaro, F. Fratianni, R. Coppola, A. Sada, P. Orlando, J. Funct. Foods 1, 319 (2009)
M. Chávarri, I. Marañón, R. Ares, F.C. Ibáñez, F. Marzo, M. del Carmen Villarán, Int. J. Food Microbiol. 142, 185 (2010)
S.H. Al-Sheraji, A. Ismail, M.Y. Manap, S. Mustafa, R.M. Yusof, F.A. Hassan, J. Funct. Foods 5, 1542 (2013)
H. Gandomi, S. Abbaszadeh, A. Misaghi, S. Bokaie, N. Noori, LWT - Food Sci. Technol. 69, 365 (2016)
W.L. Yee, C.L. Yee, N.K. Lin, P.L. Phing, Ciência e Agrotecnologia 43, 1 (2019)
S.L. Ng, K.W. Lai, K.L. Nyam, L.P. Pui, Malays. J. Microbiol. 15, 408 (2019)
K.R. Pandey, S.R. Naik, B.V. Vakil, J. Food Sci. Technol. 52, 7577 (2015)
A.T. Vieira, M.M. Teixeira, F.S. Martins, Front. Immunol. 4, 1 (2013)
H.L. Lauzon, A. Dimitroglou, D.L. Merrifield, E. Ringø, S.J. Davies, Aquac. Nutr. GutHealth, Probiotics Prebiotics, 1st edn. (John Wiley & Sons, Ltd, New Jersey, 2014), pp. 169–184
G.R. Gibson, R. Hutkins, M.E. Sanders, S.L. Prescott, R.A. Reimer, S.J. Salminen, K. Scott, C. Stanton, K.S. Swanson, P.D. Cani, K. Verbeke, G. Reid, Nat. Rev. Gastroenterol. Hepatol. 14, 491 (2017)
P. Markowiak, K. Ślizewska, Nutrients 9, 1 (2017)
K.E. Scholz-Ahrens, B. Adolphi, F. Rochat, D.V. Barclay, M. de Vrese, Y. Açil, J. Schrezenmeir, J. Soc. Nutr. Food Sci. 3, 41 (2016)
C.C. Alves, D.L. Waitzberg, L.S. de Andrade, L. dos Santos Aguiar, M.B. Reis, C.C. Guanabara, O.A. Júnior, D.A. Ribeiro, P. Sala, Front. Microbiol. 8, 1 (2017)
M. Rivera-Huerta, V.L. Lizárraga-Grimes, I.G. Castro-Torres, M. Tinoco-Méndez, L. Macías-Rosales, F. Sánchez-Bartéz, G.G. Tapia-Pérez, L. Romero-Romero, M.I. Gracia-Mora, Biomed Res. Int. 2017, 9758982 (2017)
S. Padma Ishwarya, P. Prabhasankar, Crit. Rev. Food Sci. Nutr. 54, 511 (2014)
D. Mohanty, S. Misra, S. Mohapatra, P.S. Sahu, Food Biosci. 26, 152 (2018)
A. Tárrega, A. Rocafull, E. Costell, LWT - Food Sci. Technol. 43, 556 (2010)
D. Davani-Davari, M. Negahdaripour, I. Karimzadeh, M. Seifan, M. Mohkam, S.J. Masoumi, A. Berenjian, Y. Ghasemi, Foods 8, 1 (2019)
J.L. Carlson, J.M. Erickson, B.B. Lloyd, J.L. Slavin, Curr. Dev. Nutr. 2, 1 (2018)
S. Patel, A. Goyal, 3 Biotech 2, 115 (2012)
A.K.L. Yong, K.W. Lai, H.M. Ghazali, L.S. Chang, L.P. Pui, Asia-Pacific J. Mol. Biol. Biotechnol. 28, 32 (2020)
Y. Nami, G. Lornezhad, A. Kiani, N. Abdullah, B. Haghshenas, LWT - Food Sci. Technol. 124, 1 (2020)
M. Kumherová, K. Veselá, K. Jokešová, I. Klojdová, Š Horáčková, Czech J. Food Sci. 38, 57 (2020)
L. Samedi, A.L. Charles, Foods 8, 1 (2019)
D.R.S.F. Paim, S.D.O. Costa, E.H.M. Walter, R.V. Tonon, LWT - Food Sci. Technol. 74, 21 (2016)
C.B. Fritzen-Freire, E.S. Prudêncio, R.D.M.C. Amboni, S.S. Pinto, A.N. Negrão-Murakami, F.S. Murakami, Food Res. Int. 45, 306 (2012)
W. Savedboworn, K. Teawsomboonkit, S. Surichay, W. Riansa-ngawong, S. Rittisak, R. Charoen, K. Phattayakorn, Food Sci. Biotechnol. 28, 795 (2019)
G.C. Raddatz, G. Poletto, C. de Deus, C.F. Codevilla, A.J. Cichoski, E. Jacob-Lopes, E.I. Muller, E.M.M. Flores, E.A. Esmerino, C.R. de Menezes, Food Res. Int. 130, 108902 (2020)
V. Serrano-Casas, M.L. Pérez-Chabela, E. Cortés-Barberena, A. Totosaus, J. Funct. Foods 38, 293 (2017)
A.G. Peredo, C.I. Beristain, L.A. Pascual, E. Azuara, M. Jimenez, LWT - Food Sci. Technol. 73, 191 (2016)
L.Y. Chan, L.P. Pui, Carpathian J. Food Sci. Technol. 12, 26 (2020)
S.C. Siang, L.K. Wai, N.K. Lin, P.L. Phing, Food Sci. Technol. 39, 601 (2019)
T. Shinde, D. Sun-Waterhouse, J. Brooks, Food Bioprocess Technol. 7, 1581 (2014)
J.U. Kim, B. Kim, H.M. Shahbaz, S.H. Lee, D. Park, J. Park, Int. J. Food Sci. Technol. 52, 519 (2017)
N.S. Foroutan, F. Tabandeh, M. Khodabandeh, N. Mojgani, A. Maghsoudi, M. Moradi, Appl. Food Biotechnol. 4, 133 (2017)
M.M. El-Abd, M. Abdelhamid, H. El-Sayed, H.A. El-Metwaly, M.E. El-Demerdash, Z. Mohamed, Middle East J. Appl. Sci. 08, 837 (2018)
A.S.L. Sulabo, M.E.L. Villasanta, K.G. Hermo, R.A. Lascano, L.S. Collado, G.M.O. Babaran, A. Julian, Food Res. 4, 964 (2020)
N.F. Fazilah, N.H. Hamidon, A.B. Ariff, M.E. Khayat, H. Wasoh, M. Halim, Molecules 24, 1 (2019)
M. Bustamante, B.D. Oomah, M. Rubilar, C. Shene, Food Chem. 216, 97 (2017)
M.A.K. Zanjani, M.R. Ehsani, B. Ghiassi, Tarzi, A. Sharifan, J. Food Process. Preserv. 42, 1 (2018)
W. Savedboworn, N. Kerdwan, A. Sakorn, R. Charoen, S. Tipkanon, K. Pattayakorn, Int. Food Res. J. 24, 787 (2017)
D. Dianawati, V. Mishra, N.P. Shaha, Appl. Environ. Microbiol. 78, 6914 (2012)
B. Haghshenas, Y. Nami, M. Haghshenas, A. Barzegari, S. Sharifi, D. Radiah, R. Rosli, N. Abdullah, Asian J. Pharm. Sci. 10, 350 (2015)
F. Lotfipour, S. Mirzaeei, M. Maghsoodi, Sci. World J. 2012, 680108 (2012)
K. Lai, Y. How, L. Pui, J. Microencapsul. 38, 134 (2020)
K.C.G. Silva, E.C. Cezarino, M. Michelon, A.C.K. Sato, LWT-Food Sci. Technol. 89, 503 (2018)
O. Alliod, L. Messager, H. Fessi, D. Dupin, C. Charcosset, Chem. Eng. Res. Des. 142, 87 (2019)
G.K. Gbassi, T. Vandamme, Pharmaceutics 4, 149 (2012)
G. Poletto, B. de S. Fonseca, G.C. Raddatz, R. Wagner, E.J. Lopes, J.S. Barin, E.M. de M. Flores, C.R. de Menezes, Ciência Rural 49, 1 (2019)
E. Capuano, Crit. Rev. Food Sci. Nutr. 57, 3543 (2017)
O. Sandoval-Castilla, C. Lobato-Calleros, H.S. García-Galindo, J. Alvarez-Ramírez, E.J. Vernon-Carter, Food Res. Int. 43, 111 (2010)
H. Chen, D. Ma, Y. Li, Y. Liu, Y. Wang, Acta Univ. Cibiniensis. Ser. E Food Technol. 21, 1 (2017)
E.Y. Ann, Y. Kim, S. Oh, J.Y. Imm, D.J. Park, K.S. Han, S.H. Kim, Int. J. Food Sci. Technol. 42, 411 (2007)
N. Larsen, T.B. Cahú, S.M. Isay Saad, A. Blennow, L. Jespersen, Food Microbiol. 74, 11 (2018)
A. Shafizadeh, L. Golestan, M. Ahmadi, P. Darjani, A. Ghorbani-HasanSaraei, J. Food Meas. Charact. 14, 1901 (2020)
A.N. Phoem, S. Chanthachum, S.P. Voravuthikunchai, Nutrients 7, 2469 (2015)
K.W. Lai, Y.H. How, L.P. Pui, J. Food Process. Preserv. 44, 1 (2020)
A.C. Khorasani, S.A. Shojaosadati, Appl. Food Biotechnol. 4, 179 (2017)
P. Chaikham, Food Biosci. 12, 61 (2015)
M.G. Shehata, H.S. Abd-Rabou, S.A. El-Sohaimy, J. Pure Appl. Microbiol. 13, 609 (2019)
W. Krasaekoopt, S. Watcharapoka, LWT - Food Sci. Technol. 57, 761 (2014)
S. Passot, S. Cenard, I. Douania, I.C. Tréléa, F. Fonseca, Food Chem. 132, 1699 (2012)
R. Ferdousi, M. Rouhi, R. Mohammadi, A.M. Mortazavian, K. Khosravi-Darani, A.H. Rad, Iran. J. Pharm. Res. 12, 137 (2013)
K.P. Scott, R. Grimaldi, M. Cunningham, S.R. Sarbini, A. Wijeyesekera, M.L.K. Tang, J.C.Y. Lee, Y.F. Yau, J. Ansell, S. Theis, K. Yang, R. Menon, J. Arfsten, S. Manurung, V. Gourineni, G.R. Gibson, J. Appl. Microbiol. 128, 934 (2020)
Funding
The work was supported by the UCSI University through UCSI Research Excellence & Innovative Grant (REIG) under REIG-FAS-2020-003.
Author information
Authors and Affiliations
Contributions
Conceptualization: Liew Phing Pui; Literature search and data analysis: How Yu Hsuan; Draft: How Yu Hsuan; Revised: Liew Phing Pui & How Yu Hsuan.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Consent for publication
All authors consented for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
How, Y., Pui, L. Effect of prebiotics encapsulated with probiotics on encapsulation efficiency, microbead size, and survivability: a review. Food Measure 15, 4899–4916 (2021). https://doi.org/10.1007/s11694-021-01059-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-01059-6