Abstract
The main objective of this research effort was to study whether microencapsulation could be a viable alternative to obtain probiotic orange or peach juices. In order to be considered probiotic food, probiotic bacteria must be present in sufficient viable numbers to promote a benefit to the host. The survival and viability of Lactobacillus paracasei L26 in juices over 50 days of storage at 5°C was assessed, evaluating the potential use of encapsulated cells in alginate microcapsules. L. paracasei L26 demonstrated good viability in both orange and peach juices despite the low pH values of both juices. Microencapsulation in alginate, with or without double coating, revealed to be suitable to protect L. paracasei L26 since viable cells were approximately 9 log cfu/g after 50 days of storage at 5°C. In general, the probiotic fruit juices showed a decrease in pH during storage. Glucose and fructose contents as well as citric acid contents decreased during storage, whereas an increase in formic acid was observed. The outcome of this study points to L. paracasei L26 as having promising potential, especially in an encapsulated form, as functional supplements in fruit juices without dairy ingredients due to their tolerance in an acidic environment over 50 days of storage at 5°C. Further studies are warranted to prove the functionality of juices with encapsulated probiotic strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alternative functional foods containing probiotics, dairy products aside, are seen with potential interest, especially juices. According to several authors, fruit drinks can serve as good probiotic carriers if precautions are taken with regards to sensory characteristics and pH (Champagne and Gardner 2008; Luckow and Delahunty 2004; Tuorila and Cardello 2002). It is known that probiotics lose viability during storage in many fermented milks with pH values between 4.0 and 5.0 (Champagne et al. 2005). Studies performed by Champagne and Gardner (2008) in a commercial fruit drink (pH = 4.2) stored at 4°C up to 80 days demonstrated that viability was strain dependent, and strains of Lactobacillus acidophilus were less resistant to the juice matrix. Therefore, the intensive acid environment of some fruit juices is a technological challenge to overcome (Sheehan et al. 2007). Unless probiotics are delivered to the human host in adequate viable numbers, they will not confer a potential health benefit (Araya et al. 2002).
A possible way to raise pH in a fruit juice is blending it with milk ingredients which alter the sensory characteristics of the juice (Suomalainen et al. 2006). Consumer lactose intolerance and cholesterol content are also two major drawbacks related to dairy ingredients (Heenan et al. 2004; Yoon et al. 2006).
Some data on stability of probiotics in fruit juices have been obtained (Saarela et al. 2006; Sheehan et al. 2007), including attempts to improve storage stability of Bifidobacterium strains in low pH fruit juice by UV mutagenesis combined with a selection step in low pH to generate acid-resistant strains (Saarela et al. 2010; Saarela et al. 2011). Such endeavors demonstrate that other approaches could be of interest to try to improve the viability and stability of probiotics in low pH juice matrices. According to a review by Prado et al. (2008), there are only a few fruit juices with probiotics in the market, most from Northern European countries, with an increased demand from consumers for non-dairy probiotic products (Granato et al. 2010).
Microencapsulation of microorganisms has frequently been used for protection against stressing environmental factors (Sabikhi et al. 2010), and the delivery of active probiotic cells in microencapsulated form has recently received growing attention. Microencapsulation provides a particularly suitable micro-environment for the bacteria to survive processing and storage until their release at the appropriate location(s) in the gastrointestinal tract (Weinbreck et al. 2010). Experimental evidence has demonstrated that microencapsulation can protect probiotic strains against low pH values (Kailasapathy 2006; Anal and Singh 2007). According to Ding and Shah (2008) and Saarela et al. (2006), knowledge is limited as to the potential of encapsulation for protecting probiotic bacteria such as bifidobacteria against organic acids and low pH in fruit juices.
The main objective of this research effort was to study whether microencapsulation is a viable alternative for obtaining stable probiotic juices. Double coating of encapsulated cells was hypothesized as a protection from low pH or antimicrobial compounds, such as colorings and flavorings present in the juices. To the best of our knowledge, studies on juices with the addition of microencapsulated probiotics have not yet been fully attempted. As such, this study may provide added value to a possible technological alternative to produce non-dairy probiotic foods. In this study, we reported on the stability of Lactobacillus paracasei L26 in juices subject to storage at 5°C for 50 days, as fresh or encapsulated cells in alginate-based microcapsules.
Materials and Methods
Probiotic Strain and Culture Preparation
L. paracasei LAFTI® L26 was obtained from freeze-dried concentrated starter cultures (DELVO-PRO®, DSM, Sydney, Australia). This strain was selected for this study not only because of its viability but also for its probiotic features, e.g., high rate of survival in the human gastrointestinal tract (Welin and Henriksson 2005) and its antimicrobial effect against Escherichia coli (Pidcock et al. 2002).
L. paracasei LAFTI® L26 was reactivated using pre-culture in the Man-Rogosa-Sharpe (MRS) broth (from Biokar Diagnostics, Beauvais, France), incubated overnight at 37°C. The culture was propagated by inoculating fresh media at 10% (v/v) and incubated at 37°C. The resulting culture was centrifuged at 4,000 rpm for 20 min, at 4°C. The supernatant was then discarded, and the pellet was resuspended in one tenth of its original volume of aqueous 0.85% (w/v) NaCl (Panreac, Barcelona, Spain).
Microencapsulation Procedure
The probiotic suspension was added at 10% (v/v) to a 2% (w/v) sodium alginate solution (Fluka, Oslo, Norway). The alginate-culture mixtures (50 mL) were then extruded using a Nisco Var J30 (Nisco Engineering AG, Zurich, Switzerland) microencapsulation unit with a 0.5-mm orifice and a nitrogen pressure of 0.4 bar. The extrusion rate was 4.0 mL/min. The flow rate was controlled using a syringe pump (Genie Plus). The mixtures were extruded into 200 mL of CaCl2 solution 4% (w/v), stirred at 200 rpm.
The resulting microcapsules were left in contact with the CaCl2 solution for 30 min at room temperature to ensure complete solidification. Afterwards, the CaCl2 solution was removed through decantation, and the microcapsules were suspended in Ringer (Oxoid, Cambridge, UK) solution. The microcapsules were then recovered by gravity filtration, using a glass filter funnel, and divided into three groups. One group of alginate microcapsules was coated with 0.5% (w/v) low molecular weight (107 kDa) chitosan (Sigma-Aldrich, St. Louis, USA), with a degree of deacetylation (DD) of 75–85%. A second group was coated with 0.75% (w/v) of dextran sulphate (Sigma-Aldrich) and the third group of microcapsules, which was not coated, maintained their structure of alginate microcapsules.
The double coating with chitosan and dextran sulphate was achieved by leaving the microcapsules in contact with those solutions, stirred at 100 rpm for 30 min at room temperature. Subsequently, chitosan microcapsules were recovered by gravity filtration, using a glass filter funnel, after which they were suspended in Ringer solution until effective incorporation into the juices. The dextran sulphate microcapsules were recovered by centrifugation at 5,000 rpm for 15 min at 4°C and were then submitted to the same treatment as the chitosan double-coated microcapsules.
Incorporation in Juices and Storage at 5°C
Commercially available peach and orange juices were obtained from the Portuguese market (Companhia de Conservas Alimentares, SA, Portugal) and were evaluated as potential vectors for delivery of probiotic microcapsules. Peptone water (0.1%, w/v) with NaCl (0.85%, w/v) was also included in this study. For each liquid vector (juice or peptone water), 10 mL was transferred into 30 mL sterile capped tubes and 1 g of probiotic microcapsules (MC) was added followed by storage at 5°C; control samples composed only of 10 mL of liquid as well as samples with free cells were also a target of study. In Table 1, all studied combinations are displayed. For each combination (control, free cells or with MC), sampling was taken in duplicate at 0, 6, 13, 20, 30, and 50 days of storage.
Stability Studies and Parameters Evaluated Throughout Storage
Microbiological counts were performed for each sample (peptone, orange and peach juice). For the enumeration of free cells, in colony forming units (cfu) per milliliter, decimal dilutions—using peptone water 0.1% (w/v) (Sigma-Aldrich) and 0.85% (w/v) NaCl—were plated on MRS agar (Biokar Diagnostics) in duplicate, and the viable cells were enumerated according to Miles and Misra (1938), following incubation at 37°C over 48 h, under regular aerobic conditions. For the enumeration of the encapsulated bacteria, in colony forming units per gram of microcapsules, microcapsules were harvested by filtration with filter no. 1 (Whatman, Columbus, USA) and then suspended in a sodium citrate (Sigma-Aldrich) solution at 2% (w/v) in a 1:9 (g/mL) ratio and subjected to a stomacher at 260 rpm for 10 min, in order to rupture the microcapsules. The resulting solution was then treated similarly to free cells, according to the aforementioned protocol. In order to assess whether free viable cells were present in the filtered peptone or juices containing the different alginate capsules, the liquid medium was also evaluated and therefore the number of viable cells per milliliter was determined according to the same protocol.
Plate count agar (Biokar Diagnostics), incubated aerobically at 37°C for 48 h, was used in parallel to monitor putative cross-contamination arising from laboratory manipulation in all samples, but the viable counts found were always negligible (data not shown).
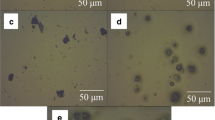
In order to monitor the morphological changes in microcapsules incorporated in peptone or juices throughout storage time, samples of MC were taken at various storage times and observed by optic microscope (Motic®, B1 Series, Hong Kong, China). Samples of filtered microcapsules were placed on glass microscope slides and then observed through ×40 objective lenses. Images were photographed using a digital camera.
The variation of pH was evaluated throughout storage time in all samples; the same is true for the variation of sugars and organic acids, which were evaluated by high performance liquid chromatography (HPLC) analysis in all samples after 0, 13, 30, and 50 days of storage.
Duplicate samples of each liquid vector were assessed for organic acids and sugars by HPLC in a single run, based on calibration curves previously prepared with appropriate chromatographic standards, using an apparatus from Merck (Whitehouse Station, USA). HPLC analysis was performed according to Zeppa et al. (2001), with some modifications. Two grams of each sample were diluted in 10 mL of sulphuric acid 13 mM (95–97% (p.a.), from Merck), homogenized with an Ultra-Turrax (T18 Basic, IKA Works Inc, USA) at 18,000 rpm for 3 min and centrifuged at 4,000 rpm for 10 min at 4°C (Universal 32R, Hettich, Germany). The resulting supernatant was then filtered with no. 1 filter (V. Reis, Portugal) and, immediately prior to injection, filtered with 0.22 μm filter (Orange Scientific, Braine-l’Alleud, Belgium).
The HPLC system consisted of a LaChrom L-7100 pump (Merck-Hitachi, Germany); an ion exchange Aminex HPX-87H Column (300 × 7.8 mm) (Bio-Rad, Philadelphia, USA), which was maintained at 65°C (L-7350 Column Oven; LaChrom, Merck-Hitachi); and two detectors in series, refractive index (L-7490 RI Detector; LaChrom, Merck-Hitachi) to determine sugars and spectrophotometry to analyze organic acids (220 nm) (L-7400 UV Detector; LaChrom, Merck-Hitachi). The mobile phase used was 13 mM sulphuric acid at a flow rate of 0.8 mL/min. The running time was 30 min, and the injection volume was 50 μL. Data was collected and analyzed by a D-7000 Interface (LaChrom, Merck-Hitachi) and HPLC System Manager 3.1.1 software (Merck-Hitachi).
Statistical Analysis
To evaluate whether each liquid vector (peptone water, orange or peach juices) and storage time were a significant source of variation for L. paracasei L26 viability (as free cells), a two-way analysis of variance (ANOVA) was performed. In turn, a three-way ANOVA was performed to evaluate if the liquid vector (peptone water, orange or peach juices), encapsulation type (MC composition: alginate with or without coating with chitosan or dextran sulphate), and storage time were a significant source of variation for L. paracasei L26 viability. For pH, sugars, and organic acids, a two-way ANOVA analysis was performed to evaluate, in each liquid vector, whether the L. paracasei L26 form (free or encapsulated) and storage time were significant sources of variation. All ANOVAs were performed with SigmaStat™ (Systat Software, Chicago, USA) at a significance level of 0.05.
Results and Discussion
Viability and Stability of L. paracasei L26 Over Storage Time
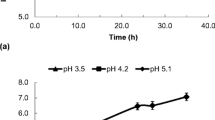
In order to observe if free cells of L. paracasei L26 were able to survive in pure juices as well as in peptone water throughout storage at 5°C, viable cells were determined and are displayed in Fig. 1. L. paracasei L26 demonstrated good viability in both orange and peach juices despite the low pH values registered in both juices (3.7–2.9, respectively). This fact contrasts with the recognized trend for probiotics to lose viability during storage in harsh acid environments (Dave and Shah 1997; Sheehan et al. 2007). According to Ding and Shah (2008), several lactobacilli strains including L. paracasei decreased two or more logarithmic cycles (from 8 to 6 log cfu/mL) after 3 weeks storage in orange or in apple juices. In peptone water, a minimum medium with a pH value of 7.0–7.2, an accentuated decrease in viable cells of L. paracasei L26 was observed, reaching values of 7 log cfu/mL after 30 days of storage. Statistically significant variations were obtained between both juices and peptone water (p < 0.05) but not between orange or peach juices (p = 0.05) throughout storage time.
Viable cell numbers above 7 log cfu/mL (around 9 log cfu/mL in the juices) are promising since two probiotic-carrying fruit juice blends currently on the Canadian market contain 1–3 billion probiotic cells per 250 ml portion (Champagne and Gardner 2008), and therefore levels of 7 log cfu/mL are of interest for juice supplementation. It should be emphasized that most of the juice blends in the market contain dairy ingredients (ex. whey butter cheese with acerola juice Cruz et al. (2009)) which naturally confer a more suitable matrix for lactic bacteria survival. Food formulation strongly affects the viability of probiotics during storage (Mattila-Sandholm et al. 2002; Saarela et al. 2006), and juices may contain natural microbial growth additives or inhibitors (Vinderola et al. 2002). According to Champagne and Gardner (2008), strains of Lactobacillus such as Lactobacillus rhamnosus, Lactobacillus fermentum, Lactobacillus reuteri, and Lactobacillus plantarum were able to survive in a commercial fruit drink (with dairy ingredients) with pH of 4.2 at 4°C for up to 80 days. However, according to findings reported by Shah et al. (2010), strains of L. rhamnosus, Bifidobacterium lactis, and L. paracasei L26 did not survive well in the harsh environment of model fruit juices.
Peptone water as well as both juices were supplemented with 10% (w/v) alginate capsules and alginate capsules coated with chitosan or dextran sulphate containing L. paracasei L26. The average size of these capsules varied between 20 and 120 μm, which is in accordance with results published by Sousa et al. (unpublished data); such small dimensions were responsible for not being detectable in the mouth (data not shown), especially in both juices under assessment. In fact, the extrusion technique developed (Sousa et al. unpublished data) has the advantage of producing very small size microcapsules. Information on capsule sizes is not readily available in published studies—Krasaekoopt and Kitsawad 2010; Ding and Shah 2008—yet it is of major importance for sensorial acceptance. In Fig. 2, the variation of viable cell numbers in the capsules throughout storage is displayed, whereas in Table 2, the number of cells (as free cells) present in the filtered liquids after removal of the capsules is displayed. The viability of encapsulated L. paracasei L26 in both juices was very good since no decrease was observed over the 50 days of storage in any type of capsule except in alginate capsules coated with dextran sulphate at 50 days of storage. An increase in the viable cells of L. paracasei L26 in the first 20 days of storage was observed in orange juices, especially in those encapsulated in alginate. These results contrast with the tendency reported by (Ding and Shah 2008) on survival of encapsulates (alginate microcapsules) of several lactobacilli strains including L. paracasei in orange or apple juices at 4°C over 6 weeks; reductions around three logarithmic cycles (from 8 to 5 log cfu/mL) were observed in both juices.
Variation of viable cells of L. paracasei L26 incorporated in MC of alginate (empty triangle), MC of alginate coated with chitosan (empty square) and in MC of alginate coated with dextran sulphate (empty circle) in peptone water (i), orange juice (ii), and peach juice (iii) over 50 days of storage at 5°C
The composition of the microcapsule as well as the liquid vector and time were statistically significant factors for L. paracasei L26 viability (p < 0.001). According to our results, both orange and peach juices used in this study were revealed as suitable for carrying L. paracasei L26. Despite the significant differences observed as a function of composition of MC which are naturally responsible for the variation that occurred in peptone water and in orange juice, it is not clear whether coating is in fact beneficial for the alginate microcapsule. While the double coating could be more protective for the encapsulated strain, it could also act as a barrier to nutrients.
As mentioned, in peptone water, some variability was observed and the numbers of viable cells of L. paracasei L26 encapsulated in alginate coated with chitosan diminished over time, decreasing approximately 3 log cycles after 50 days of storage. However, for plain alginate capsules and capsules coated with dextran sulphate, the number of viable cells of L. paracasei L26 contained in those capsules were of 9 log cfu/g after 50 days of storage at 5°C. Lower values were registered by Sousa et al. (unpublished data) in storage at 4°C of alginate capsules with L. paracasei L26 in Ringer solution.
Values between 3 and 6 log cfu/mL in the filtered peptone water and juices were registered throughout the storage period (Table 2). The presence of free cells observed even at 0 days suggests two possible origins: (1) free cells mixed with capsules or (2) cells which were at the surface of the capsules and somewhat decoupled from the capsules. Since some disintegration of the capsules could occur throughout storage, visualization of the capsules was performed at each sampling point. No evidence of rupture was ever observed. In Fig. 3, microphotographs from optical microscopy showing perfect alginate capsules with our without coating are depicted. The variability of viable cells of L. paracasei L26 in the various sampling points could be related to the replicas per se.
Chemical Stability of Liquid Delivery Systems During Storage
The variation of pH, displayed in Table 3, demonstrates that L. paracasei L26 (as free or encapsulated cells) was not metabolically inactive throughout the storage period. As expected, only slight variations were observed in control samples, especially between 0 and 6 days of storage, which could probably be related to some degree of oxidation due to manipulation since no contamination was found over 50 days of storage; the juices were packed in an airtight and light proof package.
The variation of pH in peptone water and in the two juices was different. In peptone water, no statistical significant variation of pH (p > 0.05) was observed over the 50 days of storage, which is naturally related to the absence of sugars, since peptone is the only organic source for L. paracasei L26 in free or encapsulated form. No significant variation in organic acids, namely citric, lactic, formic, or acetic acids, was observed in peptone water except for peptone water with free cells of L. paracasei L26 where lactic acid was produced attaining values 5.34 ± 0.07 g/L after 50 days of storage. L. paracasei is well known for its capacity to produce lactic acid and, besides being a carbon source, there is a very specific need for nitrogenous compounds such as peptone; Vodnar et al. (2010) observed that peptone was essential to achieve good productivity levels of lactic acid from L. paracasei 168 in discontinuous fermentation using lucerne green juice.
In both juices, pH variation was found to be statistically significant (p < 0.05) over storage time naturally due to the presence of sugars in both juices which could be fermented by L. paracasei L26 as both free or encapsulated cells. The total content in fructose and glucose was 145 ± 9 and 97 ± 9 g/L in orange and peach juice, respectively. The highest pH decrease between 0 and 50 days was observed for free cells of L. paracasei L26 and was higher in peach juice (∆pH = 0.89) than in orange juice (∆pH = 0.73). A similar tendency was observed in juices with encapsulated L. paracasei L26; independent of the type of microcapsule, a ∆pH change between 0.44 and 0.48 in peach juice vs. lower variation of 0.29 and 0.39 in orange juice was observed. The lower magnitude of pH variation in juices with encapsulated cells was expected since the capsule membrane probably limited the diffusion of sugars into the capsule, especially in those with double coating, which is in accordance to data reported by Ding and Shah (2008).
Although the pH values did not differ substantially between both juices (3.8–4.0) and the higher sugar content in orange juice, the average content in organic acids was higher in orange juice (112 ± 6 g/L) than in peach juice (81 ± 3 g/L). The variation of sugars (glucose and fructose) and organic acids (citric and formic) over storage time is displayed in Table 4; acetic acid was never found in any of the analyzed samples, malic and lactic acids eluted together, and no adequate separation was possible so that the effect of the presence of L. paracasei L26 in free or encapsulated form in the fruit juices merits further discussion.
Organic acids give fruit products their characteristic tartness and vary in combination and in concentrations among different juices. On the other hand, intense fermentation processes may lead to important product losses (Loredana et al. 2006). The main acids of orange juice identified by previous researchers are citric acid followed by lactic acid, with trace amounts of tartaric, benzoic, oxalic, and succinic acids (Cunha et al. 2002; Nour et al. 2010); peach juice reveals a similar trend, especially for citric and malic acids. Our study corroborates orders of magnitude reported in the literature for citric acid in both orange and peach juice as well as for free glucose and fructose contents. These concentrations were generally higher in orange juice than in peach juice, independent of the sampling time.
In general, concentration of glucose decreased due to L. paracasei L26 fermentation both in free or encapsulated cells in comparison with control juices. It is well known that L. paracasei L26 is a homofermentative strain that is able to ferment glucose to lactic acid. Nevertheless, it has been shown before that L. paracasei subsp. paracasei 8700:2 may shift between homolactic and heterolactic fermentation as a function of monosaccharide availability (Makras et al. 2005). Inspection of Table 4 also indicates a possible citric acid metabolism since citric acid concentrations decrease over the 50 days of storage, although to different extents in orange and peach juices and in free or encapsulated form. On the other hand, formic acid steadily increases over the 50-day storage period, an observation that supports previously mentioned citric acid mobilization; overall, microencapsulated L. paracasei L26 produced less formic acid than free cells. Such metabolic activity, in the case of encapsulated cells, may be explained either by the free cells (Table 2) present in the liquid media or by eventual exchange of nutrients between microcapsule and environment.
Conclusions
Viable cell increments between 0 and 50 days of storage at 5°C were slightly negative for free cells in both juices (−0.1) and more detrimental in peptone water (−2.4). All increments were positive for encapsulated cells in both juices (0.1–0.3), especially for those encapsulated in alginate in orange juice (0.9); an exception was accounted for by cells encapsulated in alginate capsules with dextran sulphate in orange juice. However, according to our results, apparently no beneficial evidence resulted from the coating of the capsules with chitosan or dextran sulphate. Nevertheless, this study demonstrated the potential use of juices, without dairy ingredients, to deliver L. paracasei L26 alginate microcapsules. More research is warranted to explore storage at room temperature, where the double coating could play some protective role, as well as to extend these studies to other probiotic strains; the strain specific nature of these responses are very important. Additionally, sensory evaluation including consumer acceptance should be performed together with gastrointestinal resistance studies to truly evaluate the fruit juice as an effective vehicle to deliver probiotics to consumers in adequate amounts and hence be considered a functional food.
References
Anal, A.-K., & Singh, H. (2007). Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends in Food Science and Technology, 18, 240–251.
Araya, M., Morelli, L., Reid, G., Sanders, M.E., Stanton, C., Pineiro, & Ben Embarek, P. (2002). Guidelines for the evaluation of probiotics in food. Joint FAO/WHO working group report on drafting guidelines for the evaluation of probiotics in food, London, (ON, Canada) April 30 and May 1, (pp. 1–11).
Champagne, C., & Gardner, N.-J. (2008). Effect of storage in a fruit drink on subsequent survival of probiotic lactobacilli to gastro-intestinal stresses. Food Research International, 41, 539–543.
Champagne, C.-P., Roy, D., & Gardner, N. (2005). Challenges in the addition of probiotic cultures to foods. Critical Reviews in Food Science and Nutrition, 45, 61–84.
Cruz, A.-G., Sant’Ana, A.-S., Macchione, M.-M., Teixeira, A.-M., & Schmidt, F.-L. (2009). Milk drinks using whey butter cheese (queijo manteiga) and acerola juice as a potential source of vitamin C. Food and Bioprocess Technology, 2, 368–373.
Cunha, S.-C., Fernandes, J.-O., & Ferreira, I. (2002). HPLC/UV determination of organic acids in fruit juices and nectars. European Food Research and Technology, 214, 67–71.
Dave R-I & Shah N-P. (1997). Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures. International Dairy Journal, 7, 31–41.
Ding W-K & Shah N-P. (2008). Survival of free and microencapsulated probiotic in orange and apple juices. International Food Research Journal, 15, 219–232.
Granato, D., Branco, G.-F., Nazzaro, F., Cruz, A.-G., & Faria, J.-A.-F. (2010). Functional foods and nondairy probiotic food development: trends, concepts, and products. Comprehensive Reviews in Food Science and Food Safety, 9, 291–302.
Heenan, C.-N., Adams, M.-C., Hosken, R.-W., & Fleet, G.-H. (2004). Survival and sensory acceptability of probiotic microorganisms in a nonfermented frozen vegetarian dessert. Lebensmittel-Wissenschaft und-Technology, 37, 461–466.
Kailasapathy, K. (2006). Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT Food Science and Technology, 39, 1221–1227.
Krasaekoopt, W., & Kitsawad, K. (2010). Sensory characteristics and consumer acceptance of fruit juice containing probiotics beads in Thailand. Australian Journal of Technology, 14, 33–38.
Loredana, L., Diehl, H., & Socaciu, C. (2006). HPLC fingerprint of organic acids in fruit juices. Bulletin USAMV-CN, 62, 288–292.
Luckow, T., & Delahunty, C. (2004). Which juice is healthier? A consumer study of probiotic non-dairy juice drinks. Food Quality and Preference, 15, 751–759.
Makras, L., Van Acker, G., & De Vuyst, L. (2005). Lactobacillus paracasei subsp. paracasei 8700:2 degrades inulin-type fructans exhibiting different degrees of polymerization. Applied and Environmental Microbiology, 71, 6531–6537.
Mattila-Sandholm, T., Myllarinen, P., Crittenden, R., Mogensen, G., Fonden, R., & Saarela, M. (2002). Technological challenges for future probiotic foods. International Dairy Journal, 12, 173–182.
Miles, O., & Misra, S. (1938). The estimation of the bactericidal power of the blood. The Journal of Hygiene, 38, 732–749.
Nour, V., Trandafir, I., & Ionica, M.-E. (2010). HPLC organic acid analysis in different citrus juices under reversed phase conditions. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 38, 44–48.
Pidcock, K., Heard, G.-M., & Henriksson, A. (2002). Application of non-traditional meat starter cultures in production of Hungarian salami. International Journal of Food Microbiology, 76, 75–81.
Prado, F.-C., Parada, J.-L., Oandey, A., & Soccol, C. (2008). Trends in non-dairy probiotic beverages. Food Research International, 41, 111–123.
Saarela, M., Virkajari, I., Alakomi, H.-L., Sigvard-Mattila, P., & Matto, J. (2006). Stability and functionality of freeze-dried probiotic Bifidobacterium cells during storage in juice and milk. International Dairy Journal, 16, 1477–1482.
Saarela, M., Alakomi, H.-L., Mättö, J., Ahonen, A.-M., Puhakka, A., & Tynkkynen, S. (2010). Acid tolerant mutants of Bifidobacterium animalis subsp. lactis with improved stability in fruit juice. LWT–Food Science and Technology, 44, 1012–1018.
Saarela, M., Alakomi, H.-L., Mättö, J., Ahonen, A.-M., Puhakka, A., & Tynkkynen, S. (2011). Improving the storage stability of Bifidobacterium breve in low pH fruit juice. International Journal of Food Microbiology. doi:10-1016/j.ijfoodmicro.2010.12.002.
Sabikhi, L., Babu, R., Thompkinson, D.-K., & Kapila, S. (2010). Resistance of microencapsulated Lactobacillus acidophilus LA1 to processing treatments and simulated gut conditions. Food and Bioprocess Technology, 3, 586–593.
Shah, N.-P., Ding, W.-K., Fallourd, M.-J., & Leyer, G. (2010). Improving the stability of probiotic bacteria in model fruit juices using vitamins and antioxidants. Journal of Food Science, 75, M278–M282.
Sheehan, V.-M., Ross, P., & Fitzgerald, G.-F. (2007). Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innovative Food Science & Emerging Technologies, 8, 279–284.
Suomalainen, T., Lagstrom, H., Matto, J., Saarela, M., Arvilommi, H., Laitinen, I., et al. (2006). Influence of whey-based fruit juice containing Lactobacillus rhamnosus on intestinal well-being and humeral immune response in healthy adults. LWT Food Science and Technology, 39, 788–795.
Tuorila, H., & Cardello, A.-V. (2002). Consumer responses to an off-flavor in juice in the presence of specific healh claims. Food Quality and Preference, 13, 561–569.
Vinderola, C.-G., Costa, G.-A., Regenhardt, S., & Reinheimer, J. A. (2002). Influence of compounds associated with fermented dairy products on the growth of lactic acid starter and probiotic bacteria. International Dairy Journal, 12, 579–589.
Vodnar, D.-C., Venus, J., Schneider, R., & Socaciu, C. (2010). Lactic acid production green juice as nutrient substitute. Chemical Engineering and Technology, 33, 468–474.
Weinbreck, F., Bodnár, I., & Marco, M. L. (2010). Can encapsulation lengthen the shelf-life of probiotic bacteria in dry products? International Journal of Food Microbiology, 136, 364–367.
Welin, A., & Henriksson, A. (2005). Survival of Lactobacillus acidophilus LAFTI L10 and L. paracasei LAFTI L26 in the human gastrointestinal tract and perceived effects on health, UNSW Nutrafoods, 4, 9–14.
Yoon, K.-Y., Woodams, E.-E., & Hang, Y.-D. (2006). Production of probiotic cabbage juice by lactic acid bacteria. Bioresource Technology, 97, 1427–1430.
Zeppa, G., Conterno, L., & Gerbi, V. (2001). Determination of organic acids, sugars, diacetyl, and acetoin in cheese by high-performance liquid chromatography. Journal of Agricultural and Food Chemistry, 49, 2722–2726.
Acknowledgments
The authors acknowledge FMC BioPolymer (Ireland) for having provided the necessary alginate for microencapsulation and DSM for the probiotic strain. Financial support was provided by a fellowship within the framework of PROBIOCAPS—references PTDC/AGR-ALI/71051/2006 and FCOMP-01-0124-FEDER-008792, funded by the Fundação para a Ciência e a Tecnologia (Portugal).
The authors wish to thank Maria C. Arau for the English revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, D., Sousa, S., Gomes, A.M. et al. Storage Stability of Lactobacillus paracasei as Free Cells or Encapsulated in Alginate-Based Microcapsules in Low pH Fruit Juices. Food Bioprocess Technol 5, 2748–2757 (2012). https://doi.org/10.1007/s11947-011-0581-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0581-z