Abstract
The object of this study was to produce bioactive hydrolysates from wheat gluten using ficin, a commercially important endopeptidase with plant origin, and evaluate antioxidant, antimicrobial and α-amylase inhibitory activities. Hydrolysate samples were collected at different time intervals (60, 120 and 180 min). Both samples obtained at 120 and 180 min were highly active against ABTS+ radical. The sample collected at 180 min was the most active hydrolysate in terms of DPPH radical scavenging activity, ferrous ion-chelating and α-amylase inhibition, and was further fractionated by ultrafiltration into three peptide fractions, T3-F1 (MW > 10 kDa), T3-F2 (3 < MW < 10 kDa) and T3-F3 (MW < 3 kDa). These fractions were compared in terms of antioxidant and α-amylase inhibitory abilities. The T3-F3 fraction, exhibited the strongest DPPH scavenging activity. The highest values of ABTS+ scavenging activity and α-amylase inhibition were detected in T3-F2 and T3-F3 and the lowest values in T3-F1, which were even lower than those of the parent hydrolysate. The fraction T3-F2 had the highest chelating ability and T3-F3 the lowest, even in comparison with the parent hydrolysate. The antibacterial properties of the hydrolysate sample collected at 180 min was evaluated against Staphylococcus aureus and Escherichia coli bacteria. The most significantly affected bacteria was S. aureus with the minimum inhibitory concentration value of 48 mg/mL, whereas the value obtained against E. coli was 52 mg/mL. The minimum bactericidal concentration was 60 mg/mL for both S. aureus and E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effect of natural bioactive peptides derived from food proteins on health is of great interest nowadays. These protein fragments are inactive in the parent protein molecules. Once released through enzymatic hydrolysis or during processing, other than nutritional properties, they exert various physiological, biological, and functional effects such as antioxidant, antidiabetic, and antibacterial, and several peptides have been shown to possess multifunctional properties [1,2,3]. Investigations have revealed that free radicals produced by oxidative reactions are likely to play a role in different disorders, such as cancer and coronary heart diseases. Natural antioxidants might be less effective than synthetic antioxidants, but they are regarded as safe, stable and health-promoting compounds [1, 4, 5]. Type 2 diabetes mellitus is a serious metabolic disease, characterized by high level of blood glucose. One of the key enzymes involved in the carbohydrate digestion process is α-amylase, and inhibitors of this enzyme, help decrease the rate of the final stages of carbohydrate digestion and as a result, the absorption of glucose. Synthetic and chemical α-amylase inhibitors have certain detrimental effects including gastrointestinal symptoms. In contrast, bioactive peptides from plants, as natural inhibitors have become very important for the treatment of diabetes as they are both effective and have less side effects, and yet, there is little data available on the α-amylase inhibitory activity of food-based hydrolysates [3, 6]. Long-term use and misuse of conventional antibiotics are issues that lead to bacterial drug resistance and subsequently, to a severe health concern worldwide. Bioactive peptides are also a novel alternative to classic antibiotics that could hinder the growth or destroy pathogenic bacteria through different mechanisms and therefore, present new possibilities for development of new pharmaceutical agents against resistant bacteria [7, 8].
Wheat gluten, a by-product of the wheat starch industry produced globally, is a promising and advantageous source of valuable functional ingredients [9, 10]. Gluten is a relatively complex protein composed of gliadins (soluble in 70% ethanol) and glutenins (insoluble in 70% ethanol), rich with proline- and glutamine-peptide sequences [11]. Wheat gluten peptides produced by hydrolysis have drawn scientific and industrial attention, and previous investigations have shown that enzymatic hydrolysis could be applied to improve the functional properties of gluten, contribute to the creation of hypoallergenic nutritional mixtures and release peptides with biological activity [9, 10, 12]. Protease enzymes have been long used for biocatalysis by mankind. However, the application of proteases from plant origin is still rather limited. Ficin (EC 3.4.22.3), is a proteolytic enzyme present in the latex, fruit and leaves of fig trees (F. glabrata and F. carica species) and ficin forms are recognized as sulfhydryl enzymes which contain cysteine residue in their active site. Generally, ficin has good stability, and its physical structure and hydrolysis mechanism are quite similar to that of papain [13]. This enzyme has been applied in different areas and modern applications include pharmaceutical industries, and the one that will be the main topic of this paper, the production of bioactive peptides [14]. The object of this study was to produce biologically active hydrolysate from wheat gluten using ficin and to evaluate the antioxidant, antimicrobial and α-amylase inhibitory activity of the resultant hydrolysates and selected fractions obtained by ultrafiltration. To our knowledge, little to no information has yet been available on this subject matter and therefore, it is of interest and potential practical value. The findings of this study might help provide new insights into the utilization of both ficin enzyme and wheat gluten in order to obtain bioactive food ingredients.
Materials and methods
Materials
Wheat gluten was purchased from Ardineh Esfahan. The protease ficin from fig tree latex, (EC 3.4.22.3, with the activity of ≥ 0.1 unit/mg solid) was used for enzymatic hydrolysis. The ficin protease and chemicals including 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt (ABTS+), ferrozine (3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4″-disulfonic acid sodium salt), trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), acarbose, starch (S9765), α-amylase (porcine pancreatic alpha amylase, A3176) were all purchased from Sigma-Aldrich company. The chemicals and reagents namely Ó-Phthaldialdehyde (OPA), ß-mercaptoethanol, sodium dodecyl sulfate (SDS), sodium tetra hydroborate, 3,5-dinitrosalicylic acid (DNS), serine were obtained from Merck, Germany. The ultrafiltration membranes (centrifugal) with 10 and 3 kDa molecular weight cut-off (MWCO) used for the fractionation of the most functional hydrolysate were from Sartorius, Germany. Cultures of Escherichia coli ATCC 8739, Staphylococcus aureus ATCC 6538 were obtained from Persian-type culture collection (PTCC) of the Iranian Research Organization for Science and Technology (IROST). Culture mediums including Mueller–Hinton broth (MHB) and Mueller–Hinton agar (MHA) were purchased from Sigma-Aldrich. All other chemicals and reagents used in this research were of analytical grade.
Wheat gluten chemical analysis
The moisture, ash, protein (based on dry matter), and fat content of commercial wheat gluten were determined using the American Association of Cereal Chemists (AACC) Approved Methods 44-15.02, 08-01.01, 46-12.01, and 30-10.01, respectively [15,16,17,18]. All measurements were performed in triplicates.
Enzymatic hydrolysis of wheat gluten by ficin
In order to prepare wheat gluten suspension (5% w/v), wheat gluten was dissolved in 50 mM Sodium phosphate buffer, pH 7.0. The dispersion was heated at 85 °C for 10 min and then was cooled to the appropriate temperature. Ficin enzyme was added to the suspension based on the protein content of wheat gluten with an enzyme to substrate ratio of 1:20 (W/W). The reaction mixture was adjusted to pH 7.0 for optimal enzymatic activity, and incubated at 37 °C and 200 rpm for 3 h in order to produce bioactive peptides. In order to terminate the hydrolysis, the enzyme was inactivated by heating the mixture at 90 °C for 15 min. Insoluble portion was removed by centrifugation at 12,000×g for 10 min and the supernatant was collected, freeze-dried and stored at −20 °C as wheat gluten protein hydrolysate (WGPH) [3, 7].
Degree of hydrolysis
pH-stat assay
The progress of the reaction at alkaline pH was monitored using a pH-stat method to determine the degree of hydrolysis (DH). The DH was calculated based on the base volume consumed to maintain the constant pH of 7.0 using Eq. 1 [19, 20].
The h value represents the number of equivalents of peptide bonds hydrolyzed at the time expressed in meq/g and the htot is the theoretical number of peptide bonds per weight unit present in gluten protein (meq/g), the latter calculated to be 8.38 mM/g protein [21, 22].
Ó-Phthaldialdehyde spectrophotometric assay
In order to measure protein hydrolysis and free amino groups, the Ó-Phthaldialdehyde assay was used. By performing OPA assay, it is possible to accurately determine the number of peptide bonds released during hydrolysis of a protein substrate. A fresh OPA solution was prepared daily as followed: 25 mL of 100 mM sodium tetra hydroborate, 2.5 mL of 20% sodium dodecyl sulfate (SDS) solution (w/w), 40 mg of OPA (first dissolved in 1 mL of methanol) and 100 μL of ß-mercaptoethanol were mixed and adjusted to a final volume of 50 mL with distilled water. To assay proteolysis with wheat gluten protein as substrate, hydrolysate samples collected at different time intervals (0.25, 0.5, 1, 2, 3 h) were diluted using 50 mM Sodium phosphate buffer and then 50 μL aliquots of the diluted hydrolysate samples were added directly to 1.0 mL of OPA reagent in a 1 mL quartz cuvette. The solutions were mixed and incubated for 2 min at room temperature. The absorbance at 340 nm was measured using a spectrophotometer (Agilent, Cary 60 UV–Vis, USA). Serine was used as the standard amino acid [23].
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
Wheat gluten hydrolysate samples collected at different time intervals (0.25, 0.5, 1, 2, 3 h) were diluted with 50 mM sodium phosphate buffer to ensure that the protein concentration was 5 mg/mL and SDS-PAGE was performed to evaluate protein profiles during hydrolysis, and protein bands were detected following the standard protocol described by Schägger and von Jagow [24] using 15% acrylamide gels.
Fractionation of wheat gluten hydrolysates
Wheat gluten protein hydrolysate with highest functional activity was further fractionated using ultrafiltration membranes with the molecular weight cutoff of 10 and 3 kDa (Sartorius Centrifugal), centrifuged for 15 min at 6000×g (for 10 kDa) and 8000×g (for 3 kDa) at 4 °C. The obtained fractions, T3-F1 (> 10 kDa), T3-F2 (3–10 kDa) and T3-F3 (< 3 kDa) were collected, lyophilized and stored at −20 °C [7].
Antioxidant activity assays
DPPH radical scavenging assay
The DPPH˙ radical scavenging activities (RSA) of WGPHs were measured according to the method of Xia et al. [25]. Hydrolysates were dissolved in distilled water (500 μL) in different concentrations and then mixed 1:1 (v/v) with DPPH solution (0.1 mM) prepared in methanol. The mixtures were shaken and then were left at room temperature while protected from light for 30 min. The absorbances of the blank and sample solutions were determined by measuring the absorbance at 517 nm with the UV–visible spectrophotometer. The DPPH radical scavenging abilities of the hydrolysates were calculated by the following Eq. 2:
where Acontrol represents the control absorbance which contained everything except hydrolysates and Abackground is the absorbance of the hydrolysate dissolved in distilled water. Applying linear regression analysis, IC50 values were calculated and also, trolox (20–200 μmol/L) was used as standard.
ABTS+ radical scavenging assay
The ABTS+ radical scavenging assay was performed according to the method of Ngoh and Gan [26] and Karimi et al. [3]. The ABTS+ radical cation was produced by reacting ABTS+ (7 mM) with potassium persulfate in a 1:1 ratio, allowing the mixture to stand in darkness at room temperature for 12–16 h before use. The reagent was then diluted using distilled water to obtain an absorbance of 0.700 ± 0.01 at 734 nm. The ABTS+ solution (980 μL) was added to 20 μL of samples and mixed vigorously and then incubated in the dark at 25 °C for 10 min. The absorbance was taken at 734 nm using a UV spectrophotometer. The ABTS+ RSA was calculated in a manner similar to DPPH RSA using Eq. 2. The IC50 values were calculated and results were expressed as μmol trolox equivalents per g dry matter of samples using trolox (50 and 1100-μM) as standard.
Ferrous (Fe2+) chelating ability
The ferrous chelating abilities of hydrolysates were determined using the method of Karimi et al. [3]. The sample solutions of hydrolysates (500 μL) were mixed with 1850 μL distilled water and 50 μL FeCl2 (2 mM) and allowed to stand for 3 min. Then 100 μL of 5 mM ferrozine aqueous solution was added and the mixture was allowed to react for 20 min in the darkness and at room temperature. The absorbance was measured at 562 nm and IC50 values were obtained. Ferrous ion chelating ability (%) was calculated by applying Eq. 2. The control was prepared using distilled water. A standard curve was obtained by using EDTA (10–80 mg/L) to express results as μmol of EDTA equivalents/g dry matter of samples.
Alpha-amylase inhibitory assay
The α-amylase inhibition was determined according to the method described by Miller [27] with some adjustments. Starch solution [0.5% (w/v)] was prepared by mixing 50 mg starch (Sigma, S9765) in 10 mL of sodium phosphate-buffered saline (0.1 M, pH 6.9), boiled briefly and kept at 37 °C in a water bath until use. The α-amylase solution was prepared by mixing 1 mg of the α-amylase enzyme in 10 mL of saline phosphate buffer and was kept in ice. Next, 100 µl of sample solutions and 100 µl of α-amylase solution were incubated at 37 °C for 5 min. After pre-incubation, 100 µl of 0.5% (w/v) starch solution was added. Then, the reaction mixture was incubated for 20 min at 37 °C and afterwards centrifuged for 3 min at 13,000 rpm to separate the undigested starch. To terminate the reaction, 200 µL of DNS reagent was added to 100 µL of the collected supernatant. The solution was diluted by adding 500 µL of distilled water and the mixture was incubated for 5 min at 100 °C and then cooled to room temperature using cold water bath. The absorbance was recorded at 540 nm by UV-spectrophotometer. The α-amylase inhibitory activity was expressed as percentage inhibition rate and calculated using the following Eq. 3:
where Abscontrol was defined as the absorbance without any hydrolysates and Absbackground was the absorbance of the hydrolysates mixed with phosphate-buffered saline. The concentrations of hydrolysates resulting in 50% inhibition of enzyme activity (IC50) were determined. The IC50 value of acarbose was used as positive control.
Determination of antimicrobial activity
The antibacterial activities of the selected hydrolysate treatment was evaluated against both Gram-negative (Escherichia coli ATCC 8739) and Gram-positive bacteria (Staphylococcus aureus ATCC 6538) using the broth micro-dilution technique with minor modifications. Mueller–Hinton broth (MHB) was used as growth medium and strains of bacteria from pure cultures were inoculated into sterile broth medium and incubated at 37 °C for 24 h to allow the optimum growth of the bacteria. Initial bacterial population was adjusted according to the 0.5 McFarland standard. The accurate optical density of the bacterial suspension was determined by spectrophotometry at 600 nm, with the approximate cell density of each bacterial strain being 1.5 × 108 cfu/mL [28].
Minimum inhibitory concentration assay
In order to determine the minimum inhibitory concentration (MIC), the lyophilized powder of the selected sample was dissolved in sterile water and serially diluted, providing final concentrations in the range of 60–10 mg/mL. A 100-μL aliquot of the standardized suspension of the test bacteria and 100 μL of different concentrations of the hydrolysate sample were dispensed into a sterile 96-well microplate containing MHB. The microplate was then incubated at 37 °C for 24 h. Growth controls and negative controls were included in each tray. The minimal inhibitory concentration value recorded was defined as the lowest concentration that inhibited the visible growth of the bacteria tested in micro-dilution wells as detected by the unaided eye [1, 9, 29].
Minimal bactericidal concentration
For the determination of minimum bactericidal concentration (MBC), MIC wells with no discernible bacterial growth were plated in MHA plates in triplicate and incubated at 37 °C for 24 h. The MBC was defined as the lowest concentration of the antimicrobial agent required to kill 99.9% of the final inoculum, revealing no bacterial growth after incubation at optimum [9, 29].
Statistical analysis
All experiments were carried out in triplicates and data was expressed as means with standard deviation. Statistical analysis was performed using one-way analysis of variance (ANOVA) and Student’s T-Test with JMP 10 statistical software. In order to compare means among different groups, p < 0.05 was considered as the level of significance.
Results and discussion
Chemical composition of the wheat gluten
The basic composition of the wheat gluten was analyzed as followed: 5.6% ± 0.02 moisture content, 1.4% ± 0.002 ash, 1.4% ± 0.003 fat, and 81% ± 0.009 protein content (based on dry matter). These values were consistent with the definition of vital gluten in the Codex Standard 163–1987.
Enzymatic hydrolysis
Preliminary experiments showed that the enzyme to substrate ratio (E:S) of 1:20 w/w was effective for gluten proteolysis (data not shown). The optimal pH and temperature for ficin enzyme activity on wheat gluten were 7.0 and 37 °C, respectively, which is within the range indicated by the producer (Sigma-Aldrich). Studies have shown that the rate and extent of gluten hydrolysis might vary according to the gluten concentration. Therefore, a 5% (w/v) concentration of gluten was used for hydrolysis [10]. The obtained hydrolysates were divided into three treatments collected at different time intervals (wheat gluten protein hydrolysate at 60 min or WGPHT1, wheat gluten protein hydrolysate at 120 min or WGPHT2 and wheat gluten protein hydrolysate at 180 min or WGPHT3).
Degree of hydrolysis
pH-stat assay
The reaction with the gluten concentration of 5% (w/v) proceeded at a rapid rate during the initial 60 min with a slow increase in hydrolysis rate for the next 120 min, and then entered a steady state. This was in agreement with hydrolysis curves observed for wheat gluten [10, 30, 31]. A rather sharp increase in the degree of hydrolysis was observed after the initial 15 min which might have been due to an increase in the solubility of gluten as the reaction was extended, as similar findings were reported by Wang et al. [32] and Elmalimadi et al. [10]. Using DH versus hydrolysis time, three significant DH values were identified for the thermally-treated wheat gluten protein hydrolysates. The highest DH value was 7 ± 0.1%, which was obtained for the hydrolysates collected at 180 min (WGPHT3), while those of the hydrolysates collected at 120 (WGPHT2) and 60 min (WGPHT1) were 6.35 ± 0.07% and 5.85 ± 0.06%, respectively. This medium value of DH could be attributed to the endoprotease activity of the ficin as a plant-derived enzyme [33]. Studies have found that using this protease, a wide variety of peptide bonds could be hydrolyzed, but peptide bonds following an aromatic residue appeared to be hydrolyzed more efficiently than the others. Therefore, this enzyme might have a preference for aromatic residues [34]. A high DH value in substrates could be associated with higher levels of essential amino acids and therefore linked with an increased nutritional value in produced food products and with a low DH, it is possible to take advantage of other functional properties such as foaming stability, since large peptides have a demonstrated tendency to be located at the oil/water interface and might be capable of forming stronger elastic films around gas bubbles than smaller peptides [20, 35, 36].
Ó-Phthaldialdehyde spectrophotometric assay
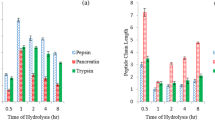
The extent of proteolysis based on OPA values was determined during the 3 h of proteolysis in the presence of ficin protease (Fig. 1).This assay is based upon OPA reaction with the amino terminal of protein and peptide chains, and allows real-time monitoring of hydrolysis, quantifying the amount of free amino groups released during the reaction [37]. In accordance with the absorbencies measured at 340 nm (Fig. 1A), protein (peptide) concentration, using serine standard and sample dilution factor of 20, was determined (Fig. 1B). Our findings revealed that the majority of wheat gluten protein degradation and cleavage of peptides occurred during the first hour of hydrolysis. It has been suggested that the peptides formed during the initial stages of hydrolysis might serve as substrate for the formation of smaller peptides [30] and studies have reported that this reduction in hydrolysis rate over time might be connected to a decrease in the availability of cleavable peptide bonds within the substrate [37]. The results acquired by the OPA method were in agreement with those obtained from the pH-stat assay.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
In order to investigate the molecular mass distribution of hydrolysates, untreated gluten and the hydrolysates obtained at different times of hydrolysis were analyzed by SDS-PAGE (Fig. 2). Studies have established that gluten proteins are comprised of gliadin and glutenin fractions with different relative molecular weights (MW). The glutenin subunits are categorized into two types, namely LMW-GS (30–50 k Da) and HMW-GS (65–90 kDa). The gliadins (28–55 kDa) are categorized into four classes, namely α, β, γ, and ω-gliadins. The relative molecular weights of the α, β-gliadins are about 30,000–40,000 g/mol. The γ-gliadins have higher molecular weight than α, β-gliadins and the ω-gliadins range from 44,000 to 80,000 (g/mol) [11, 12]. Overall, the unhydrolyzed gluten showed typical protein bands of different gluten protein types and major bands were observed at 25–85 kDa, approximately. In accordance with our results, researchers have reported that the MW of HMW-GS could be overestimated to 80–120 kDa in SDS-PAGE due to aggregation effects and this was visible in the untreated gluten sample, with three characteristic bands of HMW-GS in this range [38]. A protein band with MW ≤ 15 kDa was also present in the control sample, which might have been due to the residues of albumins and globulins [12]. SDS-PAGE profile shows that generally, as DH increased from about 5 to 7%, the number of protein bands was obviously reduced. After 30 min of hydrolysis by ficin (lane 1), a substantial reduction of bands corresponding to HMW-GS, LMW-GS and gliadins was observed and the major bands corresponding to HMW-GS and ω-gliadins were hardly visible in lane 1. Due to hydrolysis, clear bands could hardly be distinguished in the range of MW ≥ 63 kDa, and more bands could be observed in the low molecular weight region (lane 1–4, Fig. 2). The fractions of high molecular weight glutenins and gluten polymers (MW ≥ 75 kDa) appeared to be missing in lanes 2–4. Under ficin hydrolysis, proteins with Mr of about 35–63 kDa remained at every DH, whereas the proteins with MW ≥ 63 kDa were completely hydrolyzed. More specifically, lanes 1–4 showed one blurry band between 48 and 63 kDa and another between 35 and 48 kDa. The intensity of protein bands, especially at around 25 kDa and MW ≤ 15 kDa, was different among the hydrolysates obtained (lane 1–4) and with increasing time, they were somewhat enhanced.
SDS-PAGE profile of wheat gluten protein sample and its hydrolysates. In the presented SDS-PAGE profile, control (untreated wheat gluten protein) is indicated as WGP, lanes 1–4 show ficin-treated samples obtained after 30, 60, 120, 180 min of hydrolysis and M represents molecular mass marker. HMW-GS high molecular weight glutenin subunits; LMW-GS low molecular weight glutenin subunits
Antioxidant activity of wheat gluten protein hydrolysates and selected fractions
Antioxidant compounds might inhibit oxidation using different mechanisms, therefore three in vitro methods including scavenging of free radicals, DPPH and ABTS+, and ferrous ion-chelating activity were adopted to assess the antioxidant capacity of the gluten hydrolysates. DPPH and ABTS+ methods are broadly used to obtain meaningful data regarding the ability of compounds to act as free radical scavengers. Measuring the chelating ability of the compound is also quite significant. Transition metals such as iron and copper play a catalytic role in lipid peroxidation and particularly, ferrous ion is very influential. Chelation of ferrous ion hinders the peroxidation and as a result, protects cells and tissues from damage [39,40,41]. In our study, IC50 values (mg/mL) or half maximal inhibitory concentrations (concentration of samples required to scavenge or chelate 50% of free radicals and ferrous ions, respectively) were calculated by performing dose–response curves using different hydrolysate concentrations (1, 5, 7.5 mg/mL for DPPH and ferrous ion, 1, 3, 5 mg/mL for ABTS+) in order to evaluate the scavenging activity of the hydrolysates. The results are reported in Table 1 [40, 42, 43]. The antioxidant activities and metal chelating abilities of the whole protein hydrolysates and selected peptide fractions were also expressed using trolox and EDTA equivalent antioxidant capacity (Table 2).
As shown in Tables 1 and 2, all three hydrolysate samples had the ability to quench DPPH radical, and the IC50 values ranged from 5.63 ± 0.5 to 4.25 ± 0.09 mg/mL for the WGPHs collected at different time intervals (60, 120 amd 180 min). At the concentration of 5 mg/mL, the DPPH scavenging activity of the WGPHT1 was 8.41 ± 0.7 µmol trolox/g dry matter at 60 min for WGPHT1 and increased to 14.46 ± 0.7 µmol trolox/g dry matter for WGPHT3 (p < 0.05), with no significant difference between the trolox equivalent antioxidant capacity of the first two treatments (WGPHT1 and WGPHT2), and WGPHT3 was the most active hydrolysate against DPPH, leading to the conclusion that the DPPH free radical inhibitory activity was enhanced by increasing hydrolysis time to 180 min and achieving a higher degree of hydrolysis. Contrary to DPPH, both treatments WGPHT2 and WGPHT3 were highly active against ABTS+, which was also reflected in their IC50 values, with no significant differences among hydrolysates when the time of hydrolysis extended from 120 to 180 min. Overall, considering the results obtained from all three antioxidant assays, the ABTS+ free radical scavenging activities of WGPHs was exceptionally higher. For ferrous ion-chelating ability, significantly different values were obtained for all three hydrolysates and WGPHT3 demonstrated the lowest IC50 and the highest chelating ability.
In general, the antioxidant activities of WGPHs obtained by ficin enzyme were in agreement with the results obtained by other researchers who have reported that wheat gluten hydrolysates produced with papain [32] and alcalase [31] have demonstrated antioxidant abilities, indicating that all three hydrolysate treatments in our study contained amino acids or peptides that were electron or hydrogen donors and therefore, could react with free radicals in order to convert them to more stable products, terminating the radical chain reaction. The significant differences in the radical inhibiting activities of WGPHs might have been due to differences in amino acid compositions and sequences, and also peptide sizes, resulted from different degrees of hydrolysis [40, 44]. Studies have demonstrated that overall, a suitable degree of hydrolysis is desirable for achieving a high radical scavenging activity. According to our results, the treatment WGPHT3 was the most biologically active hydrolysate and SDS-PAGE results showed that this treatment also contained the greatest amount of peptides with medium to low molecular masses (Mr ≤ 15 kDa) and these findings were in agreement with other studies reporting that radical scavenging activity significantly increases with the progress of wheat gluten hydrolysis and achieving a suitably high DH since more hydrophobic and active peptides are released. This higher DH probably could also contribute to the Fe2+ ability of WGPHT3 by exposing more acidic and basic amino acids due to peptides cleavage since the carboxyl and amino groups in their side chains are capable of binding Fe2+ [3, 4, 10, 31, 41, 42, 45]. Differences observed between ABTS+ and DPPH assays were also reported for antioxidant protein fractions obtained from chia seeds [42] and Kluyveromyces marxianus [44] which could be explained by the difference of radicals’ solubility and diffusivity in the reaction medium. ABTS+ radicals are soluble in both organic and aqueous solutions, but DPPH radicals only dissolve in organic media. Antioxidant activity, cationic and water-soluble radical inhibitory ability of ABTS+, similar to other indices, are dependent on the type of protease enzyme, DH, and the amino acid composition of the peptides [42, 44].
The treatment WGPHT3 was further fractionated by ultrafiltration into three major peptide fractions, T3-F1 (MW > 10 kDa), T3-F2 (3 < MW < 10 kDa) and T3-F3 (MW < 3 kDa). Results revealed that using fractionation, antioxidant activity was enhanced. The DPPH radical scavenging activities of the fractions were distinctly different and peptide fraction T3-F3 (MW < 3 kDa), exhibited the strongest RSA, followed by T3-F2 with MW between 10 and 3 kDa and T3-F1 with MW > 10 kDa. The highest values of ABTS+ antioxidant activity were detected in peptide fractions T3-F2 and T3-F with no significant differences. The ABTS+ antioxidant activity of the fraction T3-F1 was significantly lower, even in comparison with the parent hydrolysate.
Studies have reported that low MW peptides interact more effectively with radicals and show high antioxidant activity. Chalamaiah et al. [40] and Karimi et al. [3] reported that the low MW peptides (< 10 kDa) present in hydrolysates from the common carp egg and corn germ protein respectively, were mainly responsible for the exhibited RSA. Similar results were reported by Zhu et al. [39] and Elmalimadi et al. [10] for wheat gluten, who also reported that peptides of medium to low size (3000–500 Da), (5.5 kDa) and (3–10 kDa) respectively, were primarily responsible for RSA. The type of amino acid also plays an important role in the antioxidant activity of the peptides. Aromatic amino acids such as tyrosine, histidine, tryptophan, and phenylalanine contribute to the radical-scavenging properties by donating protons, and hydrophobic amino acids such as leucine, isoleucine, valine, and proline have been described to be able to increase the solubility of peptides at the water–lipid interface, simplifying access to scavenge free radicals from the lipid phase [2, 3, 45].
The data obtained for ferrous ion-chelating ability showed distinct differences among the fractions. The fraction T3-F2 had the highest chelating ability, and the fraction T3-F1 demonstrated the second highest chelating value. In contrast with DPPH and ABTS+ RSA, the ferrous ion-chelating value obtained for the fraction T3-F3 was significantly lower than those of the other fractions and the unfractionated hydrolysates. Similar to RSA, studies have observed that the peptide chain length is crucial for the chelating activity of protein hydrolysates. There is evidence that low molecular weight peptides generally possess higher negative charge (carboxyl groups)-to-mass ratios than their larger counterparts, enabling them to form complexes with metal ions more effectively and decreasing their availability for chemical reactions, particularly lipid oxidation [46]. In contrast, some studies have reported that unfractionated hydrolysate and high MW fractions (5–10 kDa) have exhibited stronger chelating capacities in comparison with low MW fractions (< 3 kDa). The strong metal chelating properties of long-chain peptides might be due to synergistic effects of higher number of amino acid residues when compared to shorter peptides. This leads to the conclusion that a suitable peptide chain length might be necessary for a higher iron chelating effect [36]. It has also been speculated that high Fe2+ ion chelating activity might be related to the presence of histidine residues and an increased concentration of carboxylic groups and amino groups in branches of the acidic and basic amino acids that could enhance metal ion binding and remove metal ions from the system [3, 40].
Alpha-amylase inhibitory activity
The inhibitory activity of α-amylase was investigated as an in vitro indicator of the antidiabetic response. Acarbose, a safe prescribed drug for treating type 2 diabetes, was used as standard. The α-amylase inhibitory effects of the hydrolysates and selected fractions can be observed from Table 3. The unfractionated hydrolysates inhibited α-amylase activity in a concentration-dependent manner, with a rather drastic response to increase in hydrolysate concentration. The 50% α-amylase inhibitory concentrations (IC50) was calculated for these hydrolysates and significant differences were observed among these three treatments. The IC50 of WGPHT3 hydrolysate was significantly lower (p < 0.05) than those of the WGPHT1 and WGPHT2 treatments, allowing the conclusion that WGPHT3 had the highest α-amylase inhibitory activity among all hydrolysates. Among fractions obtained from WGPHT3 by ultrafiltration, the fine fractions of T3-F2 and T3-F3 showed the highest inhibition (%) at 0.5 mg/mL, and no significant difference (p ≥ 0.05) was observed between these two fractions. The lowest value for α-amylase inhibitory activity was found in T3-F1 fraction, significantly lower than those of the other fractions and even the parent hydrolysate. Similar studies on cereal proteins have observed that α-amylase inhibition activity was generally correlated with the degree of protein hydrolysis, and dependent on the proteolytic enzyme used, and that α-amylase could bind to peptides with cationic residues and certain amino acids found in peptides [3, 6, 26].
There has been speculation that bioactive peptides are capable of interacting or binding to the active site of the enzyme and subsequently, prevent it from binding to the substrate, and that α-amylase inhibition might also be achieved by binding to the enzyme allosteric sites (such as calcium ions and chloride ions), creating an unstable conformation as a result. Displacement of enzymes from substrates could be restricted by these conformational changes. Additionally, since calcium ions are necessary for many functions, structures, and also maintaining the stability of organisms, some α-amylases could become inactive if calcium ions are removed [3, 26]. According to recent reports, metal ion chelating and enzyme inhibition are strongly correlated, a theory suggesting that the interaction occurring between chelator peptides and calcium ions in the structure of the enzyme might be one of the reasons our data demonstrated that the enzyme was inhibited the most by the T3-F2 and T3-F3 fractions. The parent hydrolysate, WGPHT3, exhibited a higher α-amylase inhibition value, in comparison with the fraction T3-F1, and considering that WGPHT3 is also of higher MW, this might be due to the presence of amino acids mentioned above in the hydrolysate. Given that we obtained the highest α-amylase inhibitory activity data for fractions with Mr < 10 kDa, our results also support the hypothesis that MW of peptides and proteins might affect the α-amylase inhibition, which was also previously reported by other researchers [3, 26, 47].
Antimicrobial activity
Inner immunity system is largely dependent on antimicrobial peptides (AMPs) and these peptides are often the first line of defense against invading pathogens [48]. The antibacterial properties of the hydrolysate treatment, WGPHT3, was evaluated against Gram-positive (Staphylococcus aureus ATCC 6538) and Gram-negative (Escherichia coli ATCC 8739) bacteria, and showed distinctive antibacterial activity against the indicator organisms tested. Establishing MIC of an antibacterial compound is valuable in order to improve the efficacy of antibacterial compounds, and prevent further issues of bacterial resistance due to excessive use of high doses [49, 50]. Results showed that the most significantly affected bacteria was S. aureus with the MIC value of 48 mg/mL, and the MIC value obtained against E. coli was 52 mg/mL. The MBC of the AMPs within WGPHT3 was 60 mg/mL for both S. aureus and E. coli with no significant differences. Overall, the acquired data exhibited that WGPHT3 showed antibacterial efficiency versus both of the assessed bacteria, leading to the conclusion that WGPHT3 contained antimicrobial peptides (AMPs) capable of inhibiting both Gram-positive and Gram-negative bacteria. These results were in confirmation with previous reports of investigations on production of AMPs by enzymatic hydrolysis of wheat and other protein sources. Gottardi et al. [9] attempted to obtain AMPs by wheat hydrolysis using alcalase/flavourzyme and applied glucosamine to conjugate the resulting peptides and observed enhanced antimicrobial activity against E. coli with the MIC value of 40 mg/mL. The enzyme applied for hydrolysis could influence both the generation and the activities of AMPs [51], and a similar study on goat milk caseins [7] has reported that hydrolysates obtained by ficin enzyme demonstrate higher antimicrobial activity in comparison with those obtained by trypsin enzyme. Additionally, our results were in agreement with previous studies that observed stronger antimicrobial activities against Gram-positive bacteria (S. aureus) than against Gram-negative ones (E. coli), suggesting that Gram‐negative bacteria, such as E. coli, are more resistant to antimicrobial substances than Gram‐positive bacteria, such as S. aureus, since they have a thin cell wall with an additional structure, called the outer membrane which hinders the diffusion of antimicrobial compounds into the cell for later rupture. Also, the cell wall of Gram-positive bacteria contains peptide glucans and teichoic acids, while the cell wall of Gram-negative bacteria is composed of peptidoglucans, lipopolysaccharides, and lipoproteins. The inhibition against S. aureus is also quite significant as this particular bacteria is known to be resistant to several phytochemicals [52, 53].
Generally, antibacterial activities of AMPs are affected by several features e.g., size, the sequence of amino acid, composition, solubility, helical structure, net charge, isoelectric point, charge distribution, hydrophobicity and amphipathicity. Researchers have found that usually protein hydrolysates and peptides that are rich in cationic and hydrophobic amino acids and demonstrate cationic (positively charged) and amphiphilic (both hydrophilic and hydrophobic) properties, show higher antimicrobial activity [51, 53]. Additionally, most bioactive peptides have a high content of cysteine and/or glycine residues. Therefore, depending on these characteristics, AMPs affect bacteria using different mechanisms. The interaction of these peptides with bacterial membranes is both influenced by the peptide itself and by the lipid components of the membrane. The bactericidal activity of hydrolysates and peptides could be due to their net charge or hydrophobic properties. Most of the antibacterial peptides are positively charged, which enables them to electrostatically attach to negatively charged components of the bacterial cell wall, potentially causing the destruction of it. Furthermore, peptide hydrophobicity is an important factor contributing to the disruption of bacterial cell walls and membranes [7, 48].
It has been suggested that AMPs, usually with MW below 10 kDa, inhibit cell growth and kill several microorganisms and that in general, low MW peptides demonstrate higher antibacterial activity than high MW peptides, possibly because a low mass peptide might pass through the membrane of the bacteria with less effort [54]. Similar results have been reported by studies on camel whey protein hydrolysates [52] against E. coli and, on three common bean varieties against S. aureus [48].
Conclusion
Our findings revealed that hydrolysis with plant originated ficin protease was an effective method to obtain antioxidant, α-amylase-inhibitory and antimicrobial proteins and peptide fractions from wheat gluten, which then could be incorporated into functional food formulations and health-care products. Among different hydrolysates, the hydrolysate generated after 3 h, demonstrated significantly higher biological properties. Additionally, our experiment showed that this hydrolysate contained peptides with significant antibacterial activities against Gram-negative and Gram-positive bacteria. Fractionation enhanced bioactivities in terms of antioxidant and α-amylase-inhibitory activities and significant differences were observed among the peptide fractions, highlighting the importance of obtaining a suitable peptide chain length for different biological functionalities. For a deeper understanding of the relationship between peptide structure and in vitro biological activities, it is imperative to isolate, purify, and characterize the peptides responsible for the functional properties of WGPHs produced by ficin, and further studies are required to enhance the inhibitory activity of the obtained AMPs, and to investigate their antimicrobial activity in foods.
References
M. Memarpoor-Yazdi, A. Asoodeh, C.J. Khan, A novel antioxidant and antimicrobial peptide from hen egg white lysozyme hydrolysates. J. Funct. Foods 4, 278–286 (2012). https://doi.org/10.1016/j.jff.2011.12.004
F. Toldrá, M. Reig, M.C. Aristoy, L. Mora, Generation of bioactive peptides during food processing. Food Chem. 267, 395–404 (2018). https://doi.org/10.1016/j.foodchem.2017.06.119
A. Karimi, M.H. Azizi, G.H. Ahmadi, Fractionation of hydrolysate from corn germ protein by ultrafiltration: in vitro antidiabetic and antioxidant activity. Food Sci. Nutr. 8, 2395–2405 (2020). https://doi.org/10.1002/fsn3.1529
Z. Shahi, S.Z. Sayyed-Alangi, L. Najafian, Effects of enzyme type and process time on hydrolysis degree, electrophoresis bands and antioxidant properties of hydrolyzed proteins derived from defatted Bunium persicum Bioss. press cake. Heliyon 6, e03365 (2020). https://doi.org/10.1016/j.heliyon.2020.e03365
A. Abbas, B. Sultana, A. Hussain, F. Anwar, N. Ahmad, Antioxidant potential, phenolics content and antimicrobial attributes of selected medicinal plants. Pak. J. Anal. Environ. Chem. 22(2), 307–319 (2021). https://doi.org/10.21743/pjaec/2021.12.10
L. Gong, D. Feng, T. Wang, Y. Ren, Y. Liu, J. Wang, Inhibitors of á-amylase and á-glucosidase: potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 8(12), 6320–6337 (2020). https://doi.org/10.1002/fsn3.1987
M. Esmaeilpour, M.R. Ehsani, M. Aminlari, Sh. Shekarforoush, E. Hoseini, Antimicrobial activity of peptides derived from enzymatic hydrolysis of goat milk caseins. Comp. Clin. Pathol. 25, 599–605 (2016). https://doi.org/10.1007/s00580-016-2237-x
A. Osman, G. Enan, A.R. Al-Mohammadi, S. Abdel-Shafi, S. Abdel-Hameid, M.Z. Sitohy, N. El-Gazzar, Antibacterial peptides produced by Alcalase from cowpea seed proteins. Antibiotics 10, 870 (2021). https://doi.org/10.3390/antibiotics10070870
D. Gottardi, PKh. Hong, M. Ndagijimana, B.M. Mirko, Conjugation of gluten hydrolysates with glucosamine at mild temperatures enhances antioxidant and antimicrobial properties. LWT 57, 181–187 (2014). https://doi.org/10.1016/j.lwt.2014.01.013
M.B. Elmalimadi, J.R. Jovanovića, A.B. Stefanovića, S.J. Tanaskovića, S.B. Djurovićb, B.M. Bugarskic, Z.D. Knežević-Jugovića, Controlled enzymatic hydrolysis for improved exploitation of the antioxidant potential of wheat gluten. Ind. Crops Prod. 109, 548–557 (2017). https://doi.org/10.1016/j.indcrop.2017.09.008
K. Pourmohammadi, E. Abedi, Hydrolytic enzymes and their directly and indirectly effects on gluten and dough properties: an extensive review. Food Sci. Nutr. 9(7), 3988–4006 (2021). https://doi.org/10.1002/fsn3.2344
A.M. Gabler, K.A. Scherf, Comparative characterization of gluten and hydrolyzed wheat proteins. Biomolecules 10(9), 1227 (2020). https://doi.org/10.3390/biom10091227
K.B. Devaraj, P.R. Kumar, P.V. Vishweshwaraiah, Purification, characterization, and solvent-induced thermal stabilization of ficin from Ficus carica. J. Agric. Food Chem. 56, 11417–11423 (2008). https://doi.org/10.1021/jf802205a
E.H. Siar, R. Morellon-Sterling, M.N. Zidoune, R. Fernandez-Lafuente, Use of glyoxyl-agarose immobilized ficin extract in milk coagulation: unexpected importance of the ficin loading on the biocatalysts. Int. J. Biol. Macromol. 144, 419–426 (2020). https://doi.org/10.1016/j.ijbiomac.2019.12.140
AACC Approved Methods of Analysis, 11th edn. Method 44-15.02. Moisture-Air-Oven Methods. Cereals & Grains Association, St. Paul. Accessed 3 Nov 1999
AACC Approved Methods of Analysis, 11th edn. Method 08-01.01. Ash-Basic Method. Cereals & Grains Association, St. Paul. Accessed 3 Nov 1999
AACC Approved Methods of Analysis, 11th edn. Method 46-12.01. Crude Protein-Kjeldahl Method, Boric Acid Modification. Cereals & Grains Association, St. Paul. Accessed 3 Nov 1999
AACC Approved Methods of Analysis, 11th edn. Method 30–10.01. Crude Fat in Flour, Bread, and Baked Cereal Products Not Containing Fruit. Cereals & Grains Association, St. Paul. Accessed 3 Nov 1999
J. Adler-Nissen, Enzymic Hydrolysis of Food Proteins (Elsevier Applied Science Publishers, New York, 1986), pp.11–12. https://doi.org/10.1016/0308-8146(87)90169-5
J.E. Zapata-Montoya, D.E. Giraldo-Rios, A.J. Baéz-Suarez, Kinetic modeling of the enzymatic hydrolysis of proteins of visceras from red tilapia (Oreochromis sp.): effect of substrate and enzyme concentration. Vitae 25(1), 17–25 (2018). https://doi.org/10.17533/udea.vitae.v25n1a03
P.M. Nielsen, D. Petersen, C. Dambmann, Improved method for determining food protein degree of hydrolysis. J. Food Sci. 66, 642–646 (2001). https://doi.org/10.1111/j.1365-2621.2001.tb04614.x
A.G.B. Wouters, I. Rombouts, E. Fierens, K. Brijs, Ch. Blecker, J.A. Delcour, B.S. Murray, Foaming and air-water interfacial characteristics of solutions containing both gluten hydrolysate and egg white protein. Food Hydrocoll. 77, 176–186 (2018). https://doi.org/10.1016/j.foodhyd.2017.09.033
F.C. Church, H.E. Swaisgood, D.H. Porter, G.L. Catignani, Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 66, 1219–1227 (1983). https://doi.org/10.3168/jds.S0022-0302(83)81926-2
H. Schägger, G. von Jagow, Tricine-sodium dodecyl sulfate polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166, 368–379 (1987). https://doi.org/10.1016/0003-2697(87)90587-2
Y. Xia, F. Bamdad, M. Gänzle, L. Chen, Fractionation and characterization of antioxidant peptides derived from barley glutelin by enzymatic hydrolysis. Food Chem. 134, 1509–1518 (2012). https://doi.org/10.1016/j.foodchem.2012.03.063
Y. Ngoh, C.H. Gan, Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 190, 331–337 (2016). https://doi.org/10.1016/j.foodchem.2015.05.120
G.L. Miller, Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3), 426–428 (1959). https://doi.org/10.1021/ac60147a030
A. Meillisa, E.A. Siahaan, J.N. Park, HCh. Woo, B.S. Chun, Effect of subcritical water hydrolysate in the brown seaweed Saccharina japonica as a potential antibacterial agent on food-borne pathogens. J. Appl. Phycol. 25, 763–769 (2013). https://doi.org/10.1007/s10811-013-9973-y
M. Balouiri, M. Sadiki, S. Koraichi Ibnsouda, Methods for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal. (2016). https://doi.org/10.1016/j.jpha.2015.11.005
X. Kong, H. Zhou, H. Qian, Enzymatic preparation and functional properties of wheat gluten hydrolysates. Food Chem. 101, 615–620 (2007). https://doi.org/10.1016/j.foodchem.2006.01.057
M.B. Elmalimadi, A.B. Stefanovića, N.Z. Sekuljica, M.G. Zuza, N.D. Luković, J.R. Jovanović, Z.D. Knezević-Jugović, The synergistic effect of heat treatment on alcalase-assisted hydrolysis of wheat gluten proteins: functional and antioxidant properties. J. Food Process. Preserv. 1(5), e13207 (2017). https://doi.org/10.1111/jfpp.13207
J. Wang, M.M. Zhao, Q.Z.H. Zhao, Y. Bao, Y.M. Jiang, Characterization of hydrolysates derived from enzymatic hydrolysis of wheat gluten. J. Food Sci. 72, C103–C107 (2007). https://doi.org/10.1111/j.1750-3841.2006.00247.x
M. Aider, Potential applications of ficin in the production of traditional cheeses and protein hydrolysates. JDS Commun. 2(5), 233–237 (2021). https://doi.org/10.3168/jdsc.2020-0073
R. Morellon-Sterling, H. El-Siar, L.O. Tavano, Á. Berenguer-Murcia, R. Fernández-Lafuente, Ficin: a protease extract with relevance in biotechnology and biocatalysis. Int. J. Biol. Macromol. 162, 394–404 (2020). https://doi.org/10.1016/j.ijbiomac.2020.06.144
A.G. Wouters, I. Rombouts, E. Fierens, K. Brijs, J.A. Delcour, Relevance of the functional properties of enzymatic plant protein hydrolysates in food systems. Compr. Rev. Food Sci. Food Saf. 15(4), 786–800 (2016). https://doi.org/10.1111/1541-4337.12209
W. He, R. Yang, W. Zhao, Effect of acid deamidation-alcalase hydrolysis induced modification on functional and bitter-masking properties of wheat gluten hydrolysates. Food Chem. 277, 655–663 (2019). https://doi.org/10.1016/j.foodchem.2018.11.004
R. Jahanbani, S.M. Ghaffari, M. Salami, K. Vahdati, H. Sepehri, N. Namazi Sarvestani, N. Sheibani, A.A. Moosavi-Movahedi, Antioxidant and anticancer activities of walnut (Juglans regia L.) protein hydrolysates using different proteases. Plant Foods Hum. Nutr. 71(4), 402–409 (2016). https://doi.org/10.1007/s11130-016-0576-z
B. Lagrain, I. Rombouts, H. Wieser, J.A. Delcour, P. Koehler, A reassessment of the electrophoretic mobility of high molecular weight glutenin subunits of wheat. J. Cereal Sci. 56, 726–732 (2012). https://doi.org/10.1016/j.jcs.2012.08.003
K.X. Zhu, C.Y. Su, X.N. Guo, W. Peng, H.M. Zhou, Influence of ultrasound during wheat gluten hydrolysis on the antioxidant activities of the resulting hydrolysate. Int. J. Food Sci. Technol. 46, 1053–1059 (2011). https://doi.org/10.1111/j.1365-2621.2011.02585.x
M. Chalamaiah, T. Jyothirmayi, P.V. Diwan, K.B. Dinesh, Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg). J. Food Sci. Technol. 52(9), 5817–5825 (2015). https://doi.org/10.1007/s13197-015-1714-6
H. Agrawal, R. Joshi, M. Gupta, Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 204, 365–372 (2016). https://doi.org/10.1016/j.foodchem.2016.02.127
J. Cotabarren, A. Rosso, M. Tellechea, J. Garcia Pardo, J. Lorenzo, W. Obregón, M. Parisi, Adding value to the chia (Salvia hispanica L.) expeller: production of bioactive peptides with antioxidant properties by enzymatic hydrolysis with Papain. Food Chem. 274, 848–856 (2019). https://doi.org/10.1016/j.foodchem.2018.09.061
A. Abbas, F. Anwar, S.M. Alqahtani, N. Ahmad, S.H. Al-Mijalli, M. Shahid, M. Iqbal, Hydro-distilled and supercritical fluid extraction of Eucalyptus camaldulensis essential oil: characterization of bioactives along with antioxidant, antimicrobial and antibiofilm activities. Dose-Response (2022). https://doi.org/10.1177/15593258221125477
M. Mirzaei, S. Mirdamadi, M.R. Ehsani, M. Aminlari, Production of antioxidant and ACE-inhibitory peptides from Kluyveromyces marxianus protein hydrolysates: purification and molecular docking. J. Food Drug Anal. 26(2), 696–705 (2018). https://doi.org/10.1016/j.jfda.2017.07.008
M. Sbroggio, M. Montilha, V. Figueiredo, S. Georgetti, L. Kurozawa, Influence of the degree of hydrolysis and type of enzyme on antioxidant activity of okara protein hydrolysates. Food Sci. Technol. (Campinas) (2016). https://doi.org/10.1590/1678-457X.000216
M. Nikoo, S. Benjakul, M. Yasemi, H. Ahmadi Gavlighi, X. Xu, Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing by-product with different pretreatments: antioxidant activity and their effect on lipid and protein oxidation of raw fish emulsion. LWT 108, 120–128 (2019). https://doi.org/10.1016/j.fbio.2019.100418
R.A. Sarteshnizi, M.A. Sahari, H. Ahmadi Gavlighi, J.M. Regenstein, M. Nikoo, C.H.C. Udenigwe, Influence of fish protein hydrolysate-pistachio green hull extract interactions on antioxidant activity and inhibition of α-glucosidase, α-amylase, and DPP-IV enzymes. LWT 142, 111019 (2021). https://doi.org/10.1016/j.lwt.2021.111019
C.E. Salas, J.A. Badillo-Corona, G. Ramírez-Sotelo, C. Oliver-Salvador, Biologically active and antimicrobial peptides from plants. Biomed. Res. Int. (2015). https://doi.org/10.1155/2015/102129
P. Lestari, Suyata, Antibacterial activity of hydrolysate protein from Etawa goat milk hydrolysed by crude extract bromelain. Mater. Sci. Eng. 509, 012111 (2019). https://doi.org/10.1088/1757-899X/509/1/012111
A. Abbas, F. Anwar, N. Ahmad, Variation in physico-chemical composition and biological attributes of common basil essential oils produced by hydro-distillation and super critical fluid extraction. J. Essent. Oil Bear. Plants 20(1), 95–109 (2017). https://doi.org/10.1080/0972060X.2017.1280418
M. Roy, A. Sarker, M.A.K. Azad, M.R. Shaheb, M.M. Hoque, Evaluation of antioxidant and antimicrobial properties of dark red kidney bean (Phaseolus vulgaris) protein hydrolysates. Food Meas. 14, 303–313 (2020). https://doi.org/10.1007/s11694-019-00292-4
M. Salami, A.A. Moosavi-Movahedi, M.R. Ehsani, R. Yousefi, T. Haertle, J.M. Chobert, Improvement of the antimicrobial and antioxidant activities of camel and bovine whey proteins by limited proteolysis. J. Agric. Food Chem. 58, 3297–3302 (2010). https://doi.org/10.1021/jf9033283
L.A. Tejano, J.P. Peralta, E.E.S. Yap, Y.W. Chang, Bioactivities of enzymatic protein hydrolysates derived from Chlorella sorokiniana. Food Sci. Nutr. 7, 2381–2390 (2019). https://doi.org/10.1002/fsn3.1097
E.C.N. Rathnapala, D.U. Ahn, E.D.N.S. Abeyrathne, Enzymatic hydrolysis of ovotransferrin and the functional properties of its hydrolysates. Food Sci. Anim. Resour. 41(4), 608–622 (2021). https://doi.org/10.5851/kosfa.2021.e19
Acknowledgements
The authors would like to acknowledge Tarbiat Modares University for their support.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MS-A: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Visualization; Writing. M-HA: Conceptualization; Formal analysis; Funding acquisition; Methodology; Project administration; Resources; Supervision; Validation. MS: Conceptualization; Formal analysis; Funding acquisition; Methodology; Resources; Supervision; Validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that they have no conflicts of interest with respect to the work described in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seyedain-Ardabili, M., Azizi, MH. & Salami, M. Evaluation of antioxidant, α-amylase-inhibitory and antimicrobial activities of wheat gluten hydrolysates produced by ficin protease. Food Measure 17, 2892–2903 (2023). https://doi.org/10.1007/s11694-023-01829-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-01829-4