Abstract
The objective of the study was to determine chemical characteristics and antioxidant properties of wheat gluten hydrolysates (WGH) and utilize the WGH as ingredients in a beverage system. Single (2, 6, 12, and 24 h) and sequential enzymatic hydrolyses (8, 10, and 12 h) were conducted using commercial proteases (Alcalase, Flavourzyme, Protamex, and Neutrase). Yields of all the produced WGH were over 50%. Degree of hydrolysis of the WGH produced by Flavourzyme was the highest, while that of the WGH by Neutrase was the lowest. Sequential enzymatic hydrolysis remarkably decreased the fraction with more than 10 kDa and increased the fraction with less than 500 Da. Contents and composition of free amino acids were affected by the enzyme types and hydrolysis conditions. DPPH radical scavenging activity of the WGH significantly increased with hydrolysis time. The WGH produced by Protamex showed lower turbidity, better thermal stability, and higher solubility, suggesting they may be suitable for beverage development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrolysis of proteins has been widely used to produce ingredients with functional characteristics (foaming, solubility, emulsifying, etc.) [1] and various bioactivities (antioxidant, antimicrobial, anti-inflammatory, anti-hypertensive activities, etc.) [2]. Chemicals, including acidic/alkaline compounds, and enzymes have been used for hydrolysis. Chemical treatments are difficult to control specific cleavages in amino acid sequences of proteins and can destroy essential amino acids [3]. During neutralization of acidic and alkaline conditions, undesirable salts are readily formed. On the other hand, proteases have certain specificities for substrates, hydrolyzing proteins into peptides in a mild condition. The specificity and reaction conditions (pH, temperature, and time) during enzymatic hydrolysis can affect characteristics of protein hydrolysates such as peptide size, amino acid sequences, and amount of free amino acids [4]. Enzymatic hydrolysis has been utilized in a wide range of food materials including animal sources (milk, egg, beef, pork, chicken, etc.) and plant sources (rice, corn, soy, wheat, etc.) [5]. Wheat is one of the cereal crops widely consumed in the world. Wheat is available at a low cost because it is harvested over 700 million tons annually with technological advances. Wheat gluten (WG), a by-product of the wheat starch industry, is rarely water-soluble due to gluten forming a continuous network between gliadin and glutenin proteins [6]. Not only different functional characteristics [7, 8] but also biological activities such as antioxidant [9] and anti-hypertensive activities [10] have been reported on wheat gluten hydrolysates (WGH) produced by enzymatic hydrolysis.
Single enzymatic hydrolysis of WG has been investigated to determine the effect of enzyme types and hydrolysis time on the characteristics of hydrolysates [7]. Liu et al. [11] reported the effect of sequential enzymatic hydrolysis using endo- and exo-peptidases to reduce bitterness. Besides, sequential enzyme treatment was attempted to enhance the efficiency of hydrolysis in food proteins such as Nile tilapia proteins (Oreochromis niloticus) [12], duck egg white proteins [13], and muscle of brown stripe red snapper [14]. However, little information on the characteristics of WGH was available in terms of comparison between single and sequential enzymatic hydrolyses. Moreover, it is important to understand sensory properties of WGH because individual peptides have unique taste properties such as sweetness, sourness, umami, and bitterness [15]. However, the evaluation of physicochemical and sensory properties of WGH in a beverage system has not been studied.
The objective of this study was to determine chemical characteristics and antioxidant properties of WGH produced by single and sequential enzymatic hydrolyses using commercial proteases [Alcalase (A), Flavourzyme (F), Protamex (P), and Neutrase (N)] and also to determine turbidity, thermal stability, pH solubility, and sensory characteristics of a beverage system made with the WGH.
Materials and methods
Materials
WG (78.92 ± 0.58% crude protein (N × 5.7), 4.83 ± 0.15% water, 4.36 ± 0.20% crude fat, and 0.60 ± 0.02% crude ash) was obtained from Anhui Ruifuxiang Company (Anhui, China). A (EC 3.4.21.62, from Bacillus licheniformis, 2.4 AU/g), F 1000 L (EC 3.4.11.1, from Aspergillus oryzae, 1000 AU/g), P (EC 3.4.24.28, from Bacillus subtilis, 1.5 AU/g), and N (EC 3.4.24.28, from Bacillus amyloliquefaciens, 1.5 AU/g) were purchased from Novozymes (Bagsvaerd, Denmark). o-Phthaldialdehyde (OPA), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), N,N-dimethyl-2-mercaptoethylammonium chloride (DMMAC), cytochrome C from equine heart, aprotinin from bovine lung, bacitracin, Gly-Gly-Tyr-Arg, Gly-Gly-Gly, and potassium persulfate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium tetraborate decahydrate was from Junsei Chemical Co., Ltd. (Tokyo, Japan). 9-Fluorenylmethyl chloroformate (FMOC) was from Agilent Technologies (Palo Alto, CA, USA). Mite hot chocolate powder was purchased from Dongsuh Food Co., Ltd. (Seoul, Korea). The other chemicals used in the present study were of analytical grade and purchased from Samchun Chemical Co. (Seoul, Korea).
Preparation of WGH
Single enzymatic hydrolysis of WG using the four commercial proteases (A, F, P, and N) was carried out under the conditions as follows: WG was suspended in water (20%, w/w). The suspension was continuously stirred using an overhead stirrer (Wisestir HS-30D, Daihan Scientific Co., Seoul, Korea) at 200 rpm under floating pH condition, and temperature was kept at 50 ± 1 °C in a water bath. WG was hydrolyzed with enzyme to WG ratio of 1:100 (w/w) for 2, 6, 12, and 24 h. After hydrolyzed, the mixture was heated for 10 min at 95 °C to inactivate the enzyme before centrifuging at 8000×g for 10 min at 20 °C. A and P were selected to conduct sequential enzymatic hydrolysis due to their high efficiency compared to the other two enzymes. Hydrolysis using the first enzyme was conducted for 6 h under the same hydrolysis conditions to the single enzymatic hydrolysis. After hydrolysis, the mixture was heated at 95 °C to inactivate the first enzyme and cooled down to 50 °C slowly. The second enzyme was added with enzyme to WG ratio of 1:100 (w/w), and hydrolysis was additionally conducted for 2, 4, and 6 h. After hydrolysis, the mixture was heated at 95 °C for 10 min to inactivate the enzyme before centrifuging at 8000×g for 10 min at 20 °C. WGH powder obtained after lyophilizing the supernatant at − 75 ± 5 °C below 20 mTorr for 1 week was stored at − 20 °C in darkness until further analysis.
Yield of the WGH
Yield of WGH was calculated using the following equation:
where W0 is weight of WG used (g, dry basis) and W1 is weight of freeze-dried WGH (g).
Degree of hydrolysis (DH)
DH was determined by the OPA method described by Wang et al. [8] and Frister et al. [16]. Each of the WGH was dissolved at 1.25 mg/mL in 12.5 mM sodium borate buffer (pH 8.5) containing 2% (w/v) sodium dodecyl sulfate (SDS). 50 µL of this solution was added to 1 mL of a reagent composed of 50 mL 0.1 M sodium borate buffer (pH 9.3), 1.25 mL 20% (w/v) SDS solution, 100 mg DMMAC, and 40 mg OPA dissolved in 1 mL methanol followed by incubation at room temperature for 2 min. Absorbance of the mixture was measured at 340 nm. The number of amino groups was determined with reference to an l-leucine standard curve (between 0.5 and 5 mM). DH was calculated using the following equation:
where nT is the total number of amino groups in the totally hydrolyzed gluten treated with 6 M HCl at 110 °C for 24 h, ni is the number of amino groups in native gluten, and α is the number of free amino groups of WGH.
Molecular weight (Mw) distribution
SDS polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was conducted according to Laemmli [17] using 15% acrylamide separating gel and 5% acrylamide stacking gel. Each sample of the WGH was prepared in 50 mM Tris–HCl buffer (pH 6.8) containing 0.1% bromophenol blue, 10% glycerol, 2.5% SDS, and 0.1 M 1,4-dithiothreitol, and loaded onto gel. After electrophoresis, gels were stained with Coomassie brilliant blue R-250 to detect proteins.
Size exclusion chromatography (SEC)
Mw distribution of the WGH determined by SEC was performed using an HPLC system consisting of Waters 2695 and Waters 2996 PDA detector from Waters (Milford, MA, USA). A TSK gel 2000 SWXL column (300 × 7.8 mm) from Tosoh (Tokyo, Japan) was used with 70:30 (v/v %) acetonitrile/water containing 0.1% trifluoroacetic acid (TFA) as mobile phase. Each run was performed at 25 °C for 30 min. Injection volume was 20 µL. Flow rate was 0.5 mL/min. Detection wavelength was 214 nm. Data processing was performed using Empower software version 2 from Waters (Milford, MA, USA). A Mw calibration curve was prepared by following standards: cytochrome C from equine heart (12,500 Da), aprotinin from bovine lung (6500 Da), bacitracin (1450 Da), Gly-Gly-Tyr-Arg (451 Da), and Gly-Gly-Gly (189 Da). A relationship between the retention times and the log of Mw of the proteins used as standards was established. The samples were divided into the following classes: 0–500 Da, 500–1000 Da, 1000–3000 Da, 3000–5000 Da, 5000–10,000 Da, and above 10,000Da. The relative area of each fraction was given in percentage of the total area.
Free amino acids
Free amino acids in the WGH were determined by an HPLC system consisting of Dionex Ultimate 3000 and FL detector (Dionex, Idstein, Germany). A VDSspher 100 C18 column (150 × 4.6 mm) from VDS Optilab (Berlin, Germany) was used with mobile phase A (40 mM sodium phosphate dibasic buffer, pH 7) and mobile phase B (water/acetonitrile/methanol, 10:45:45, v/v). The gradient elution was as follows: 95% A for 3 min, in 24 min to 45% A, in 31 min to 20% A, and in 35 min to 95% A. Flow rate was 1.5 mL/min, and injection volume was 0.5 µL. Immediately after injection, an auto-sampler was used for the inline-derivatization by FMOC/OPA post column derivatization. OPA-derived amino acids were monitored at emission 450 nm and excitation 340 nm and FMOC-derived amino acids were monitored at emission 305 nm and excitation 266 nm. Data processing was performed using Chromeleon software 6.8 version from Dionex (Idstein, Germany). Individual free amino acids were expressed as mg/g of the lyophilized WGH.
Antioxidant properties
DPPH radical scavenging activity
DPPH radical scavenging activity was determined according to the method described by Kong et al. [18] with some modification. Each of the WGH was dissolved in water at 5 mg/mL. 200 µL of the solution was mixed with 200 µL 0.2 mM DPPH dissolved in 95% (v/v) ethanol. The mixture was shaken and then incubated in the dark for 20 min. The mixture was centrifuged at 8000×g for 10 min at room temperature after incubation. Absorbance of the supernatant was measured at 517 nm. DPPH radical scavenging activity was calculated using the following equation:
where A0 is absorbance of blank mixture (200 µL water mixed with 200 µL DPPH solution) and As is absorbance of the WGH.
ABTS radical scavenging activity
ABTS radical scavenging activity was determined as described by Re et al. [19]. ABTS radical solution was prepared by reacting 7 mM ABTS with 2.45 mM potassium persulfate at a ratio of 1:1 (v/v). The mixture was allowed to stand in the dark for 12–16 h before use. The ABTS radical solution was diluted with water to an absorbance of 0.7 ± 0.05 at 734 nm. Then 50 µL of the WGH was added to 950 µL diluted ABTS radical solution. The mixture was shaken and then incubated in the dark for 10 min. The mixture was centrifuged at 8000×g for 10 min. Absorbance of the supernatant was measured at 734 nm. ABTS radical scavenging activity was calculated using the following equation:
where A0 is absorbance of blank mixture (50 µL water mixed with 950 µL ABTS solution) and As is absorbance of the WGH.
Functional properties
Turbidity and thermal stability
WGH were dissolved in water at 50 mg/mL. Turbidity of the samples was determined by measuring the optical density at 600 nm using a spectrophotometer (Optizen 2120UV; Mecasys, Daejeon, Korea). To determine thermal stability, the samples were heated at 90 °C for 10 min in a water bath and cooled down to room temperature. The turbidity of the samples was determined as mentioned above. Water was used as blank.
pH solubility
The WGH were dissolved at 50 mg/mL in a buffer solution using 0.2 M sodium phosphate and 0.1 M citric acid at pH 3, 5, and 7. The mixture was centrifuged at 15,000×g for 10 min. The supernatant was discarded, and the pellet was oven-dried at 105 °C for 1 h. Solubility (%) was calculated using the following equation:
where W0 is weight of dry WG used (g) and W1 is weight of dried WGH (g).
Sensory evaluation
Sensory evaluation was conducted two times. Firstly, the WGH produced by the single and sequential enzymatic hydrolyses for 12 h were selected for sensory evaluation on the basis of the results with relatively higher content of small peptides and lower turbidity of the WGH. Since the WGH produced by 24 h hydrolysis with much higher content of small peptides than those produced by 12 h hydrolysis and similar turbidity to those by 12 h hydrolysis were too bitter, those by 24 h hydrolysis were not selected for sensory evaluation. The WGH suspended at 1% concentration in water were presented for evaluation at room temperature in a randomized order. 36 participants (8 men and 28 women ranging from 19 to 30 years old) scored all the samples for flavor, appearance, taste, bitterness, and overall acceptability. Flavor, appearance, taste, and overall acceptability were scored on 15 cm line scales with anchors labeled ‘dislike very much’ (0) and ‘like very much’ (15). Bitterness was scored on a 15 cm line scale with anchors labeled ‘very weak’ (0) and ‘very strong’ (15). Secondly, based on the results of the first sensory evaluation, the WGH produced by 12 h hydrolyses using A and P were selected as ingredients of a chocolate beverage because of their higher overall acceptability than the others. The chocolate beverage was prepared using Mite hot chocolate powder at 18% (w/w) concentration in hot water. The WGH were added at two different concentrations of 2.5 and 5% (w/w). The beverages were served at room temperature in a randomized order and evaluated under the same procedure of the first sensory evaluation.
Statistical analysis
All the data were obtained by the tests conducted in triplicate. Results were subject to one-way analysis of variance (ANOVA) and Duncan’s new multiple range test at significant level of p < 0.05 using SPSS 23.0 software (SPSS Inc., Chicago, IL, USA).
Results and discussion
Yield and DH
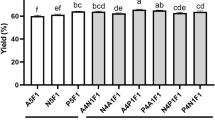
Yields of the WGH hydrolyzed by all the tested proteases were over 50% at any hydrolysis conditions (Table 1). Hydrolysis time little affected the yield of the WGH produced by A. On the other hand, the yield of the WGH using F increased from 59.2% at 2 h hydrolysis to 67.4% at 24 h. Yields of the WGH produced by P and N tended to decrease during the enzymatic hydrolysis. These results indicate that A and F are more efficient to produce water-soluble hydrolysates than P and N with hydrolysis time. The sequential enzymatic hydrolysis using A after 6 h hydrolysis with P produced more water-soluble hydrolysates than the hydrolysis using only P, implying that the sequential enzymatic hydrolysis may overcome low yield of single enzymatic hydrolysis using P.
As shown in Table 1, DH of the WGH was up to 52.9% depending on the enzyme types and hydrolysis conditions including hydrolysis time and the number of treated enzymes. The hydrolysis of WG with the proteases seemed to slow down after 6 h hydrolysis. DH of the WGH produced by F was the highest, indicating that F is the most efficient for WG hydrolysis. After 24 h hydrolysis, DH of the WGH produced by A was similar to that of the WGH produced by P, while that of the WGH produced by N was the lowest. Kechaou et al. [20] reported that A was the best to hydrolyze cuttlefish viscera (Sepia officinalis) and sardine viscera (Sardina pilchardus) among commercial proteases (A, F, and P).
The sequential enzymatic hydrolysis using A after 6 h hydrolysis with P was more efficient than the single enzymatic hydrolysis with P. On the other hand, the sequential enzymatic hydrolysis using P after 6 h hydrolysis with A was less efficient than the single enzymatic hydrolysis with A. The sequential enzymatic hydrolysis seemed to be more efficient on the production of WGH than the single enzymatic hydrolysis. However, Mw distribution determined by SEC should be considered to evaluate the hydrolysis efficiency, because DH was only calculated by the number of amino groups.
Mw distribution of the WGH
Mw of all the WGH in SDS-PAGE patterns dramatically decreased to below 15 kDa (data not shown), compared with raw WG, which consisted of high Mw subunit of glutenin (67–88 kDa), low Mw subunits of glutenin (32–35 kDa) and gliadin (28–55 kDa) [21].
As shown in Table 2, the SEC revealed that the fraction of the hydrolysates with more than 10 kDa decreased during hydrolysis, while the fraction with less than 1 kDa increased in all the WGH. After 2 h hydrolysis, A was the best to hydrolyze WG into peptides among the treated enzymes. The WGH produced by A contained the smallest amount of the fraction with more than 10 kDa and the largest amount of the fraction with less than 500 Da. The WGH produced after 6 h hydrolysis by F contained smaller amount of the fraction with more than 10 kDa and larger amount of the fraction with less than 500 Da than those produced by the other enzymes. However, it is hard to evaluate whether F is more efficient for the production of WGH with lower Mw because it has both endo- and exo-protease activities. Therefore, the amount of free amino acids should be considered. On the other hand, N showed the lowest efficiency to hydrolyze WG into peptides for all the hydrolysis times. The WGH produced by N contained the largest amount of the fraction with more than 10 kDa and the smallest amount of the fraction with < 500 Da at any hydrolysis times. Therefore, N may not be a suitable enzyme to hydrolyze WG effectively.
Mw distributions of the WGH produced by the single and sequential enzymatic hydrolyses are shown in Fig. 1. The sequential enzymatic hydrolysis decreased the fraction with more than 10 kDa and increased the fraction with less than 500 Da compared to the single enzymatic hydrolysis during the same hydrolysis time. The fraction with more than 10 kDa was much less in the WGH produced by 2 h hydrolysis with P after 6 h hydrolysis with A (3.0%) than by 8 h hydrolysis with A (7.8%). Moreover, the fraction with less than 500 Da was much more in the WGH produced by 2 h hydrolysis with P after 6 h hydrolysis with A (45.5%) than by 8 h hydrolysis with A (37.8%). The fraction with more than 10 kDa in the WGH produced by A after 6 h hydrolysis with P (2.3%) was also less than by 8 h hydrolysis with P (10.4%). Therefore, adding the second enzyme effectively hydrolyzed WG into smaller peptides.
Molecular weight distribution of wheat gluten hydrolysates determined by size exclusion chromatography. a Single enzymatic hydrolysis using Alcalase (A) and sequential enzymatic hydrolysis using Protamex (P) and A. b Single enzymatic hydrolysis with Protamex and sequential enzymatic hydrolysis using A and P. Numbers after A and P are hydrolysis times (h). All data represent the means and standard deviations (n = 3)
Free amino acids in the WGH
The enzyme types and the number of the treated enzymes significantly affected the amount of free amino acids and composition of the WGH (Table 3). F produced more free amino acids than the others. Although the amounts of the fractions with less than 500 Da in the WGH produced by 12 h hydrolyses with A, F, and P were not significantly different (p > 0.05), the amount of free amino acids in the WGH produced by F (24.5%) was much larger than in the WGH produced by A and P, indicating that the WGH produced by 12 h hydrolysis with F contained less amount of peptides with less than 500 Da than the others at the same hydrolysis time. Free amino acids except proline in the WGH produced by F were the largest. P produced significantly more free amino acids than A (p < 0.05). Free amino acids in the WGH produced by 12 h hydrolysis with P, except glutamic acid and threonine, were significantly more than in the WGH produced by 12 h hydrolysis with A. The WGH produced by 12 h hydrolysis with A had significantly more glutamic acid than by 12 h hydrolysis with P (p < 0.05), while the amount of threonine was not significantly different (p > 0.05). Although total free amino acids were not significantly different between the WGH produced by A and N, the compositions of free amino acids were remarkably different. Aaslyng et al. [22] reported that free amino acids contribute to the taste of hydrolyzed soy proteins, and especially the content of glutamic acids is important for umami taste.
Although the enzyme and time for the hydrolysis were the same, sequence of enzyme treatments significantly affected the compositions and total amounts of free amino acids in the hydrolysates (Table 3). Total free amino acids in the WGH produced by 6 h hydrolysis with A after 6 h hydrolysis with P (32.3 mg/g) were significantly more than by 6 h hydrolysis with P after 6 h hydrolysis with A (22.9 mg/g) (p < 0.05). Most of the free amino acids in the WGH produced by 6 h hydrolysis with A after 6 h hydrolysis with P were significantly more than by 6 h hydrolysis with P after 6 h hydrolysis with A (p < 0.05). This might result from the changes in the cleavage sites available for the second enzymes due to the changes in the amino acid sequences of the WGH by the first enzymes.
Antioxidant properties of the WGH
DPPH radical scavenging activity of the WGH increased up to 68.1% depending on the enzyme type and hydrolysis time (Table 1). DPPH radical scavenging activity of the WGH generally increased with hydrolysis time. DPPH radical scavenging activity of the WGH produced by 24 h hydrolysis with A was the highest. The WGH produced by A and P showed higher DPPH radical scavenging activity than those by F. These results revealed that protein hydrolysates with higher contents of smaller peptides might have higher antioxidant properties than those with lower contents of smaller peptides. Alashi et al. [23] also reported similar results that the fraction with less than 1 kDa exhibited high antioxidant properties in Australian canola meal protein hydrolysates.
ABTS radical scavenging activity was over 60% regardless of the enzyme types and hydrolysis time (Table 1). This result seems to be different from the result of DPPH radical scavenging activity, which was influenced by the enzyme type and correlated with hydrolysis time. ABTS radical scavenging activity of the WGH was little changed by the enzyme type and hydrolysis time. Khantaphant et al. [14] reported the same results that DPPH radical scavenging activity in protein hydrolysates from muscle of brown stripe red snapper significantly increased with increasing DH, but ABTS radical scavenging activity was little changed with increasing DH.
Functional properties of the WGH
The treated enzymes, except P, and hydrolysis conditions including hydrolysis time and the number of treated enzymes significantly affected turbidity of the WGH solution (Fig. 2). All the WGH solutions had low turbidity after 12 h hydrolysis. The WGH solution prepared by P had lower turbidity regardless of hydrolysis time than the others. Turbidity of the WGH solution prepared by A decreased after 10 h hydrolysis. Adding A after 6 h hydrolysis with P was better to prepare a WGH solution with lower turbidity than adding P after 6 h hydrolysis with A. Thermal processing little influenced turbidity of the WGH regardless of the enzyme types and hydrolysis conditions including hydrolysis time and the number of treated enzymes (Fig. 2). This result is attributed to the fact that unpredictable aggregation is rarely formed during thermal processing in WGH with shorter peptides due to the lack of secondary structures.
Turbidity and thermal stability of wheat gluten hydrolysates produced by single and sequential enzymatic hydrolyses using Alcalase (A), Flavourzyme, Protamex (P), and Neutrase. A6P, wheat gluten hydrolysates produced by P after 6 h hydrolysis with A; and P6A, wheat gluten hydrolysates produced by A after 6 h hydrolysis with P. All data represent the means and standard deviations (n = 3). Different small letters indicate significant differences among wheat gluten hydrolysates produced by the same enzymes (p < 0.05; one-way ANOVA and Duncan’s multiple range test)
Most of the beverage industry considers food processing to avoid undesirable turbidity and precipitation in the final products [24]. Moreover, a maintenance of clarity has been a concern in beverage products, and thermal treatments are required for safety and shelf stability in the beverage industry [25, 26]. As a result, WGH with shorter peptides, having lower turbidity and higher thermal stability, are suitable to be ingredients for the beverages. Enzymatic hydrolysis could be also utilized to improve functional properties such as turbidity and thermal stability of proteins in food manufacturing.
Solubility of WG was < 10% at pH 3, 5, and 7, while most of the WGH had high solubility over 96% at pH 3, 5, and 7 except the WGH produced by 2 and 6 h hydrolyses with A (data not shown). This result might be attributed to the fact that smaller and more hydrophilic peptides are produced via enzymatic hydrolysis. Kong et al. [7] also reported that solubility of WGH increased over 60% by enzymatic hydrolysis using commercial proteases.
Sensory characteristics of WGH beverages
Sensory profiles of WGH solutions and chocolate beverages with the WGH are shown in Fig. 3. The WGH solutions produced by single and sequential enzymatic hydrolyses for 12 h differed mainly in flavor, taste, bitterness, and overall acceptability (Fig. 3a). The WGH produced by 12 h hydrolysis with A and 12 h hydrolysis with P had higher overall acceptability. On the other hand, overall acceptability of the WGH produced by N was the lowest. The WGH produced by the sequential enzymatic hydrolysis had stronger bitterness than by the singe enzymatic hydrolysis. Bitterness of the WGH produced by 12 h hydrolysis with A was the lowest among the tested six hydrolysates. The WGH with lower bitterness tasted better as expected.
Sensory profile of wheat gluten hydrolysates produced by Alcalase (A), Flavourzyme (F), Protamex (P), and Neutrase (N). a Wheat gluten hydrolysates suspended in water (1%, w/w) and b chocolate beverages prepared with wheat gluten hydrolysates (2.5 and 5%, w/w) produced by 12 h hydrolyses with A and P. Each value represents the mean scored on 15 cm line scale by 36 panelists. C control; numbers after the letters are hydrolysis times (h); and numbers before the letters are concentrations of wheat gluten hydrolysates
The chocolate beverage without the WGH had the highest overall acceptability, taste, appearance and the lowest bitterness (Fig. 3b). Bitterness of the chocolate beverage prepared with the WGH produced by 12 h hydrolyses with A and P significantly increased with WGH concentration. Chocolate beverage prepared at 5% (w/w) with the WGH produced by 12 h hydrolysis with P had lower bitterness, better taste, and higher overall acceptability than that with the WGH produced by 12 h hydrolysis with A. Although there was little difference in Mw distribution of the WGH produced by 12 h hydrolyses with A and P, the differences of peptide sequence and the content of free amino acids in the WGH might contribute to sensory properties. Liu et al. [27] reported that smaller peptides with larger amounts of hydrophobic amino acids had stronger bitterness in soy protein hydrolysates and high content of free amino acids, especially glutamic acid, had umami tastes. Moreover, bitterness of protein hydrolysates has been concerned for application in food system [28]. Therefore, agents inhibiting bitter taste in WGH need to be studied to develop a better beverage.
In conclusion, enzyme types and hydrolysis conditions including hydrolysis time and the number of treated enzymes significantly affected chemical characteristics and antioxidant properties of the WGH. Sequential enzymatic hydrolysis could be more efficient in hydrolyzing proteins into peptide products than singe enzymatic hydrolysis. Hydrolysis conditions (enzyme types, enzyme to substrate ratio, pH, temperature, the number of treated enzymes, etc.) could be controlled to produce protein hydrolysates with diverse kinds of functional properties. Besides, masking unique taste of WGH needs to be studied to apply WGH in food systems.
References
A.D. Neklyudov, A.N. Ivankin, A.V. Berdutina, Appl. Biochem. Microbiol. 36, 452–459 (2000)
R.J.S. Castro, H.H. Sato, Food Res. Int. 74, 185–198 (2015)
J.W. Finley, E.L. Wheeler Jr., H.G. Walker, A.J. Finlayson, J. Agric. Food Chem. 30, 818–820 (1982)
B.H. Sarmadi, A. Ismail, Peptides. 31, 1949–1956 (2010)
D. Agyei, M.K. Danquah, Trends Food Sci. Technol. 23, 62–69 (2012)
P.R. Shewry, J. Exp. Bot. 60, 1537–1553 (2009)
X. Kong, H. Zhou, H. Qian, Food Chem. 102, 759–763 (2007)
J. Wang, M. Zhao, X. Yang, Y. Jiang, J. Cereal Sci. 44, 93–100 (2006)
C. Qiu, W. Sun, C. Cui, M. Zhao, Food Chem. 141, 2772–2778 (2013)
R.E. Cian, J. Vioque, S.R. Drago, Food Res. Int. 69, 216–223 (2015)
B.Y. Liu, K.X. Zhu, W. Peng, X.N. Guo, H.M. Zhou, RSC Adv. 6, 27659–27668 (2016)
S. Yarnpakdee, S. Benjakul, H.G. Kristinsson, H. Kishimura, J. Food Sci. Technol. 52, 3336–3349 (2015)
Y. Ren, H. Wu, X. Li, F. Lai, G. Zhao, X. Xiao, Appl. Biochem. Biotechnol. 172, 1227–1240 (2014)
S. Khantaphant, S. Benjakul, H. Kishimura, Process Biochem. 46, 318–327 (2011)
F. Shahidi, Flavor of Meat and Meat Products (Springer, New York, 2012), pp. 98–115
H. Frister, H. Meisel, E. Schlimme, Fresenius J. Anal. Chem. 330, 631–633 (1988)
U.K. Laemmli, Nature 227, 680–685 (1970)
X. Kong, H. Zhou, Y. Hua, J. Sci. Food Agric. 88, 920–926 (2008)
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Free Radic. Biol. Med. 26, 1231–1237 (1999)
E.S. Kechaou, J. Dumay, C. Donnay-Moreno, P. Jaouen, J.P. Gouygou, J.P. Bergé, R.B. Amar, J. Biosci. Bioeng. 107, 158–164 (2009)
H. Wieser, Food Microbiol. 24, 115–119 (2007)
M.D. Aaslyng, M. Martens, L. Poll, P.M. Nielsen, H. Flyge, L.M. Larsen, J. Agric. Food Chem. 46, 481–489 (1998)
A.M. Alashi, C.L. Blanchard, R.J. Mailer, S.O. Agboola, A.J. Mawson, R. He, A. Girgih, R.E. Aluko, Food Chem. 146, 500–506 (2014)
M. Pinelo, B. Zeuner, A.S. Meyer, Food Bioprod. Process. 88, 259–265 (2010)
J.W. Beecher, M.A. Drake, P.J. Luck, E.A. Foegeding, J. Dairy Sci. 91, 2553–2560 (2008)
C.E. LaClair, M.R. Etzel, J. Food Sci. 75, 21–27 (2010)
P. Liu, M. Huang, S. Song, K. Hayat, X. Zhang, S. Xia, C. Jia, Food Bioprocess Technol. 5, 1775–1789 (2012)
S. Yang, X.Y. Mao, F.F. Li, D. Zhang, X.J. Leng, F.Z. Ren, G.X. Teng, Eur. Food Res. Technol. 235, 91–97 (2012)
Acknowledgements
This work was supported by Anhui Ruifuxiang Company (Anhui, China) and Anhui International Science and Technology Cooperation Project (Grant No. 1604b0602002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, Y., Lim, T., He, Y. et al. Chemical characteristics and antioxidant properties of wheat gluten hydrolysates produced by single and sequential enzymatic hydrolyses using commercial proteases and their application in beverage system. Food Measure 13, 745–754 (2019). https://doi.org/10.1007/s11694-018-9987-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9987-x