Abstract
The therapeutic use of food proteins and their derivatives is gaining more attention to replace synthetic compounds which has been linked to many side effects after prolonged use. This study aimed to determine the functional properties as well as the antioxidant and bactericidal potentials of Garcinia kola seed protein hydrolysates. Proteins were obtained with alkaline solubilization and acidic precipitation, and further hydrolyzed with specific proteases under optimum conditions. Functional properties were assessed using standard methods. 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging and Fe2+-chelating assays were used to estimate antioxidant potentials. Bacterial inhibition of Staphylococcus aureus, Escherichia coli and Proteus vulgaris was assayed by zone of inhibition. Protein hydrolysates obtained by pancreatin digestion exhibited the lowest hydrolysis degree. However, all protein hydrolysates showed excellent solubility across an extensive wide pH scale with the highest solubility at pH 11 and 12. All protein hydrolysates revealed significant (p < 0.05) antioxidant contents, with pepsin and trypsin hydrolysates generally showing the most DPPH radical scavenging (58.3%) and Fe2+-chelating activities (93.4%), respectively. Furthermore, hydrolysates obtained from pepsin digestion inhibited all test bacteria species and had the highest bactericidal activity. Thus, G. kola protein hydrolysates may be used as a natural source of antioxidants and antibiotics for bacterial infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein hydrolysates are essential ingredients for food and industrial applications because they contain bioactive peptides. They are a mixture of polypeptides, oligopeptides and amino acids essentially produced from protein sources by enzymatic hydrolysis using appropriate proteases under controlled conditions (Nasri 2017). Many therapeutic properties have been exhibited by different hydrolysates obtained from both dietary and non-dietary sources (Montalvo et al. 2020; Osukoya et al. 2021). In light of the associated physiological and bioactivities, protein hydrolysates have been used to substitute whole proteins in formulating food products and therapeutics for people with special needs, in weight-control diets and sports nutrition (Sharma 2014; Woolf and Adams 2020).
There is a growing interest in food proteins’ therapeutic applications and their derivatives/products, beyond their immediate nutritional effects as safer alternatives to synthetic compounds which are associated with adverse effects after prolonged use (Chakrabarti et al. 2014; Nasri 2017). These protein hydrolysates and their peptides are perceived to lack significant secondary effects because they are derived from food-based sources and are considered better alternatives to synthetic pharmaceuticals to prevent and treat chronic illness (Chakrabarti et al. 2014).
Protein hydrolysates obtained from various dietary sources have been shown to be of medicinal importance. For example, some protein hydrolysates have been shown to possess anticancer and immunomodulatory potentials and could be used in managing immune dysfunction (Chalamaiah et al. 2018; Kiewiet et al. 2018). Other medicinal uses of protein hydrolysates include antioxidant, antimicrobial (Osukoya et al. 2021), antiobesity, anihypertensive, hypocholesterolemic and anti-inflammatory effects (Ashaolu 2020b). Protein hydrolysates have also been implicated in the management of several chronic disesases. Aside their medicinal importance, protein hydrolysates have found importance in environmental management, such as plant biostimulants (Colla et al. 2015). Hence, finding more bioactive protein hydrolysates along with bioactive peptides from natural sources and exploring their various bioactivities is of great importance.
The most common way to produce protein hydrolysates is through enzymatic hydrolysis of whole protein molecules. Several proteases such as chymotrypsin, pancreatin, pepsin, thermolysin, alcalase and trypsin have been utilized to generate bioactive protein hydrolysates (Korhonen and Pihlanto 2003). The use of enzymatic hydrolysis is preferred to other forms of protein hydrolysis due to shorter reaction time, ease of scalability and predictability (Mashuri et al. 2019). Several factors, such as the type of enzymes used, temperature, time allowed for hydrolysis, affect the extent of hydrolysis and types of peptides produced (Ashaolu 2020a). The proteases used in this study included trypsin, pepsin and pancreatin.
Garcinia kola Heckel (common name: bitter kola), a medium-sized perennial dicotyledonous plant, belongs to the Clusiaceae family (WFO 2022). It is found throughout West and Central Africa. G. kola is highly valued for its medicinal use and is referred as a ‘wonder plant’ because every part of it is of therapeutic importance in West Africa folk medicine. Therapeutic/medicinal applications of different parts of the plant include antidiabetic (Iwu et al. 1990), antioxidant (Farombi et al. 2002), antimicrobial (Arekemase et al. 2012), cognition enhancing effect (Ishola et al. 2017), antinociceptive, anti-inflammatory and antihypertensive effects (Dogara et al. 2022). However, most of the research on G. kola seeds are majorly on the extracts and some isolated phytochemicals/secondary metabolites (Dogara et al. 2022). To the best of our knowledge, no protein hydrolysates have been produced from G. kola seeds. It is therefore important to hydrolyze the proteins from this beneficial seed, as many physiological properties of food proteins and their derivatives are given. Thus, this study aims to determine some functional properties as well as the antioxidant and bactericidal potentials of G. kola protein hydrolysates (GKPHs).
Methodology
Chemicals
Bovine serum albumin (BSA), 1,1-diphenyl-2-picrylhydrazyl (DPPH) and the enzymes used for hydrolysis (trypsin, pepsin and pancreatin) were obtained from Sigma Aldrich (St. Louis, MO, USA). All other reagents were procured from Loba Chemie (Mumbai, India).
Preparation of Garcinia kola seeds
Garcinia kola seeds were purchased from Odo-Oko Aro farm, Ijurin-Ekiti, Ekiti State, Nigeria (7.88702° N, 5.11339° E). The plant was identified by a taxonomist at IFE HERBARIUM, Department of Botany, Obafemi Awolowo University, Ile-Ife, Nigeria, where it was given the herbarium number: IFE-17,998, and a voucher copy deposited. The seeds were dehulled, air-dried, grounded and stored at -20 ℃.
Proximate analysis
Ash, crude protein, fat, crude fiber and carbohydrate contents were determined as described by AOAC (1995).
Preparation of protein isolate and enzymatic hydrolysis
Garcinia kola powder was homogenized in NaOH (0.1 M, pH 8, 20% w/v), stirred for four hours (4 °C) and centrifuged at 2,000 x g for 30 min and the supernatant consisting of soluble protein was precipitated with HCl to pH 4.5. This was further centrifuged and the residue was refrigerated as G. kola protein isolate (GKP). The enzymes trypsin (2,000 U/g), pancreatin (> 250 U/g) and pepsin (> 250 U/g) were used to hydrolyse GKP, separately, according to their optimal conditions (0.1 M phosphate buffer, pH 8 for trypsin and pancreatin; and 0.1 M glycine-HCl, pH 2 for pepsin) as follows: 1 part of enzyme (1 mg/mL) was used to digest 8 parts of GKP (4 mg/mL). The solution of protein and enzyme was incubated for 30 min, then was placed for 1, 2, 4 and 8 h at 37 ℃ on a magnetic stirrer (SB162 Stuart, UK). This was followed by enzyme inactivation by heating in a water bath at 100 ℃ for 10 min. The protein hydrolysates (GKPHs) were lyophilized and stored at -20 °C. Protein concentration was determined by taking absorption at 660 nm using UV-Visible spectrophotometer (752 N, Hinoteck, China) as described by Olson and Markwell (2007) using 1 mg/mL bovine serum albumin (BSA) as standard.
Determination of degree of hydrolysis and average peptide chain length
The degree of hydrolysis (DH) of hydrolysates was determined using the procedure of Osukoya et al. (2021). Briefly, 10 mL tricarboxylic acid (TCA, 20%) was mixed with 10 mL GKPHs, incubated at 4 ℃ for 30 min and centrifuged at 2,000 x g for 10 min. Protein content was assayed in the supernatant using the method described by Olson and Markwell (2007). DH (%) was estimated as a fraction of protein content in the supernatant against total protein content. The average peptide lengths (PCL) of GKPHs were deduced from the %DH (Osukoya et al. 2021).
Functional properties
Protein solubility and foaming analysis were done as described by Osukoya et al. (2021). For solubility, GKPHs (5 mL) were placed into separate tubes and distilled water (20 mL) was added. The mixtures were stirred, adjusted to pH 2–12 with HCl or NaOH, further stirred and centrifuged at 1500 x g for 10 min. Solubility (%) was estimated as a fraction of protein content in the supernatant to total protein content. For foaming properties, 2 mL of GKPHs was dissolved with distilled water (100 mL), stirred for 60 min, adjusted to pH 7 with HCl or NaOH and the volume (Vo) was taken. The mixture was homogenized for 30 s and volume (VT) was taken. The solution was incubated for 30 min, and volume Vt was taken. Foam expansion (%) and foam stability (%) were estimated as the percentage increase in volume after homogenization and incubation, respectively against the initial volume.
In vitro antioxidant activity assays
Determination of 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity
The DPPH scavenging ability of GKPHs was done according to the method described by Zhu et al. (2008). DPPH solution (0.25 mM, 0.1 mL) and 0.1 mL GKPHs were placed in tub, mixed well and further incubated for 20 min. Reduction in absorbance was monitored at 520 nm in a UV-Visible Spectrophotometer (752 N, Hinoteck, China).
Fe2+-chelating ability assay
This was done as described by Puntel et al. (2005). Briefly, 500 µM FeSO4 (900 µL) and GKPHs (150 µL) were mixed and allowed to stand for 5 min at 25 °C. This was followed by the addition of 78 µL 0.2% 1,10-phenanthroline (dissolved in ethanol). Absorbance was taken at 510 nm.
Bactericidal activities
Bactericidal activities was determined using the agar disk diffusion method described by Perez (1990) against both gram-positive (Staphylococcus aureus) and gram-negative bacteria (Escherichia coli and Proteus vulgaris). Bacterial cultures were kept on nutrient agar slants (at 4 ℃) and sub-cultured at 37 ℃ in nutrient agar broth for 24 h before assay. For bactericidal activity assay, 100 µL of GKPHs were placed into wells bored in Muller Hinton agar (MHA) media plates already swabbed with 24 h culture of bacterial strains and the diameter of clear inhibition zone around the wells were measured.
Statistical analysis
The experiments were conducted in triplicates of three independent assays in each case, and were presented as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad software (Prism 5; version 5.01). One-way analysis of variance followed by Tukey’s multiple comparison post hoc test was done to determine significant statistical differences. Significant differences were taken at p < 0.05.
Results and discussion
Proximate analysis of G. kola seeds
The proximate study of Garcinia kola seeds showed significant protein and carbohydrate along with minimal fiber, ash and lipids content. G. kola seeds contained 6.62% moisture, 4.89% crude fiber, 1.35% crude fat, 4.21% ash, 3.5% protein and 79.43% carbohydrate. This is in contrast to the report of Eleyinmi et al. (2006) (who reported a 3.9% protein, 4.3% lipid, 1.1% ash and crude fiber of 11.4%) and Adesuyi et al. (2012) (who reported a 1.86% protein, 0.19% crude fat, 0.47% ash, 1.23% crude fiber and 88.3% carbohydrate) for G. kola seeds. The difference in compositions could be due to difference in the climatic and soil conditions (which could influence the seeds’ chemical composition) of the regions where the seeds were grown and sourced from. After protein extraction, proximate analysis revealed 81.3% protein and 18.7% moisture content, revealing total elimination of carbohydrate and other constituents.
Degree of hydrolysis and peptide chain length of GKPHs
The total protein obtained for GKP was 172.8 mg, while the highest total protein obtained after hydrolysis with pepsin, trypsin and pancreatin were 19.86 mg (1 h), 21.56 mg (8 h) and 27.46 mg (4 h), respectively. The DH and average peptide chain length of each of GKPHs are depicted in Fig. 1 A and 1B, respectively. Pepsin hydrolysates had the highest DH of 98.98% (after 1 h). Generally, for all GKPHs, more peptide bonds were hydrolyzed at 1 h than other incubation/hydrolysis times. The degree of hydrolysis measures the extent of protein degradation during/after a hydrolytic process. Thus, it is used to improve the functional, bioactivity and sensory qualities of proteins (Morais et al. 2013). DH affects the size, amino acid content and biological activities of protein hydrolysates (Nasri 2017) and is in turn influenced by cleavage patterns, specificities of enzyme used, enzyme/substrate ratio and hydrolysis conditions (time, temperature and pH) (Nasri 2017; Noman et al. 2019; Osukoya et al. 2020). The high level of DH for pepsin, an aspartic protease, might be due to its preference in splitting peptide bonds at the C-terminus of hydrophobic and aromatic amino acids (Wang et al. 2020), which might be abundant in GKP. For GKPHs, more peptide bonds were hydrolyzed by the enzymes at one hour incubation period than other hydrolysis times (0.5, 2, 4 and 8 h) and a progressive decline in DH was observed as incubation time increased possibly because fewer peptide bonds were available to be hydrolyzed after the first hour.
Functional properties of G. kola protein hydrolysates
Only G. kola seed pepsin, pancreatin and trypsin hydrolysates obtained after two hours incubation time were used for functional studies.
Solubility
Figure 2 shows the results of GKPHs solubility. The protein hydrolysates have high solubility across all pHs (pH 2–12). However, they were most soluble at high alkaline pH range (pH 11 and 12), with pepsin hydrolysate having the highest solubility of 99.75% at pH 12, followed by the hydrolysates obtained with pancreatin (95.59%) and trypsin (82.3%). Functional properties of proteins depend on their effectiveness when mixed into solution initially. Thus, solubility is likely the most impactful factor in food system applications because of other functional properties’ dependence (Ashaolu 2020a). Generally, plant proteins have low solubility due to decreased entropy; however, solubility increases on protein hydrolysis as a result of reduction in size and production of more polar groups (Walstra 2003). Short peptides from hydrolysates with a high DH (as seen in GK pepsin hydrolysates) usually have more polar residues resulting in improved hydrogen bonding with water molecules and high solubility (Bao et al. 2017).
Foaming properties
The foaming properties of GKPHs are shown in Table 1. Pancreatin hydrolysates exhibited the highest foam expansion and stability (30.0% and 23.8%, respectively), while pepsin hydrolysates had the lowest. Foams play an essential role in food industries (most especially in manufacturing bread, cakes, coffee-milk mixes, beer and meringue) because of their textural and structural properties (Ashaolu 2020a). Hydrolysis could have reduced the foaming stability of pepsin and trypsin hydrolysates because small peptides cannot maintain stable foam (Karami and Akbari-adergani 2019; Yuan et al. 2017). The foaming capacity of proteins can however be enhanced by increasing its flexibility, reducing surface tension and exposure of more non-polar amino acid residues (Karami and Akbari-adergani 2019).
Antioxidant activities of G. kola protein hydrolysates
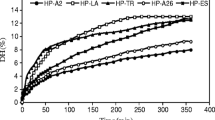
Table 2 shows the percentage DPPH scavenging ability of GKPHs. The hydrolysates obtained with pepsin after 8 h of hydrolysis had the highest DPPH scavenging activity (58.3%) than other hydrolysates. The high scavenging property observed could be because pepsin hydrolysates contained more substances acting as a proton donor, thereby scavenging free radicals by converting them into more stable products and terminating the radical chain reaction (Hassan et al. 2019). High bioactivities has been previously reported for Chlorella sorokiniana protein hydrolysates produced with pepsin (Tejano et al. 2019). The study revealed that pepsin-hydrolysed protein and peptide fractions showed high DPPH radical scavenging activity when compared to other hydrolysates.
The Fe2+-chelating ability of all GKPHs is also shown in Table 2. Trypsin hydrolysates after 1 h hydrolysis exhibited the highest Fe2+-chelating ability of 93.4% when compared to other hydrolysates for pancreatin (79.7%) and pepsin (47.15%) hydrolysates. Trypsin may have fragmented more Fe2+-chelating peptides than the other enzymes. Noman et al. (2019) observed that peptides with high concentrations of basic and acidic amino acids possess an increased ability to chelate Fe2+ and Cu2+. Pepsin hydrolysates (with the highest DH) had significantly lower (p < 0.05) metal-chelating properties than other hydrolysates (less than 50% chelating activity). Intarasirisawat et al. (2012) and Noman et al. (2019) noted that small peptides are generally incapable of forming a complex with metals and thus have lower chelating activity.
Bactericidal activity of G. kola protein hydrolysates
Exploration of the bactericidal activity of protein hydrolysates can be of importance in therapeutic applications. Three bacteria were chosen in this study due to them being part of the human intestinal microflora and multi-drug resistant bacteria. Escherichia coli, despite being an important part of the normal human intestinal microflora, has many pathotypes that cause several diseases such as diarrhea, meningitis and urinary tract infections (Kaper et al. 2004). Staphylococcus aureus, a gram-positive bacterium, is a major and formidable pathogen that causes a number of clinical infections, such as bacteremia or sepsis, skin and soft tissue infections and infective endocarditis in humans (Tong et al. 2015). Proteus vulgaris, a gram-negative bacterium, is usually referred to as human opportunistic pathogen because of their ability to cause many infections, such as osteomyelitis, urinary tract infections, wound infections and meningitis, under favorable conditions (Buckle 2015; Kushwaha et al. 2014; Różalski et al. 2012). Pepsin hydrolysates at various hydrolysis times inhibited all the bacterial test strains (except at 8 h when the hydrolysates showed a clear inhibition zone against E. coli only). Trypsin hydrolysates at various incubation times inhibited only E. coli. Pancreatin hydrolysates (obtained after 0.5, 1 and 2 h of hydrolysis) showed a clear inhibition zone against E. coli, but only the ones hydrolyzed for two hours showed inhibition against S. aureus (Fig. 3). Zone of inhibition testing is an effective way of determining the qualitative activity of an antimicrobial agent. In this study, all hydrolysates demonstrated significant inhibition against at least one strain of bacteria, with pepsin hydrolysate exhibiting the highest bactericidal activity at various hydrolysis times. This is similar to the result obtained for Monodora myristica pepsin hydrolysates (Osukoya et al. 2021). Also, Chlorella sorokiniana pepsin hydrolysates had higher bacterial inhibition than other protein hydrolysates (Tejano et al. 2019). All G. kola hydrolysates were most potent against E. coli.
Conclusion
The present study reported the production of protein hydrolysates from Garcinia kola seeds for the first time, using the proteases trypsin, pepsin and pancreatin. The protein hydrolysates produced with pepsin had the highest degree of hydrolysis generally, and therefore produced the shortest peptides (in terms of the calculated average peptide chain length). Pepsin hydrolysates produced after 1 h of digestion showed the highest degree of hydrolysis. Hydrolysis/incubation time and degree of hydrolysis affected bioactivities, even though a longer hydrolysis time and a higher degree of hydrolysis did not necessarily correspond to higher activity. All G. kola protein hydrolysates exhibited significant antioxidant (DPPH radical scavenging and iron-chelating activities) as well as bactericidal activities against E. coli, S. aureus and P. vulgaris. However, G. kola seed pepsin hydrolysate was the most effective in respect to its bactericidal activity and radical scavenging properties. Also, all Garcinia kola protein hydrolysates showed good functional properties in respect to their solubility and foaming abilities. All the protein hydrolysates were most soluble at pH 12; however, pancreatin produced the hydrolysates with highest foaming abilities. Thus, Garcinia kola protein hydrolysates may be applied in food systems and as potential therapeutics for oxidative stress and bacterial infections.
References
Adesuyi A, Elumm I, Adaramola F, Nwokocha A (2012) Nutritional and Phytochemical Screening of Garcinia kola. Adv J Food Sci Technol 4:9–14
AOAC (1995) Official Methods of Analysis of AOAC. AOAC Arlington, Virginia
Arekemase MO, Babandoko AM, Ojo KRM, Elizabeth AA, Kamoldeen AA (2012) Antimicrobial Effects of Garcinia Kola (Bitter Kola) on Some Selected Pathogens from University of Ilorin Teaching Hospital Ilorin, Nigeria. J Asian Sci Res 2:159–169
Ashaolu TJ (2020a) Applications of soy protein hydrolysates in the emerging functional foods: a review. Int J Food Sci Technol 55:421–428. https://doi.org/10.1111/ijfs.14380
Ashaolu TJ (2020b) Health Applications of Soy Protein Hydrolysates. Int J Pept Res Ther 26:2333–2343. https://doi.org/10.1007/s10989-020-10018-6
Bao Z-j, Zhao Y, Wang X-y, Chi Y-J (2017) Effects of degree of hydrolysis (DH) on the functional properties of egg yolk hydrolysate with alcalase. J Food Sci Technol 54:669–678. https://doi.org/10.1007/s13197-017-2504-0
Buckle J (2015) Chap. 7 - Infection. In: Buckle J (ed) Clinical Aromatherapy (Third Edition). Churchill Livingstone, St. Louis, pp 130–167. https://doi.org/10.1016/B978-0-7020-5440-2.00007-3
Chakrabarti S, Jahandideh F, Wu J (2014) Food-derived bioactive peptides on inflammation and oxidative stress. Biomed Res Int 2014:608979. https://doi.org/10.1155/2014/608979
Chalamaiah M, Yu W, Wu J (2018) Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem 245:205–222. https://doi.org/10.1016/j.foodchem.2017.10.087
Colla G, Nardi S, Cardarelli M, Ertani A, Lucini L, Canaguier R, Rouphael Y (2015) Protein hydrolysates as biostimulants in horticulture. Sci Hort 196:28–38. https://doi.org/10.1016/j.scienta.2015.08.037
Dogara AM, Hamad SW, Hama HA, Bradosty SW, Kayfi S, Al-Rawi SS, Lema AA (2022) Biological Evaluation of Garcinia kola Heckel. Advances in Pharmacological and Pharmaceutical Sciences 2022:3837965. https://doi.org/10.1155/2022/3837965
Eleyinmi AF, Bressler DC, Amoo IA, Sporns P, Oshodi AA (2006) Chemical composition of bitter cola (Garcinia kola) seed and hulls. Pol J Food Nutr Sci 56:395–400
Farombi EO, Akanni OO, Emerole GO (2002) Antioxidant and Scavenging Activities of Flavonoid Extract (Kolaviron) of Garcinia kola Seeds. Pharm Biol 40:107–116. https://doi.org/10.1076/phbi.40.2.107.5838
Hassan MA, Xavier M, Gupta S, Nayak BB, Balange AK (2019) Antioxidant properties and instrumental quality characteristics of spray dried Pangasiusvisceral protein hydrolysate prepared by chemical and enzymatic methods. Environ Sci Pollut Res 26:8875–8884. https://doi.org/10.1007/s11356-019-04144-y
Intarasirisawat R, Benjakul S, Visessanguan W, Wu J (2012) Antioxidative and functional properties of protein hydrolysate from defatted skipjack (Katsuwonous pelamis) roe. Food Chem 135:3039–3048. https://doi.org/10.1016/j.foodchem.2012.06.076
Ishola IO, Adamson FM, Adeyemi OO (2017) Ameliorative effect of kolaviron, a biflavonoid complex from Garcinia kola seeds against scopolamine-induced memory impairment in rats: role of antioxidant defense system. Metab Brain Dis 32:235–245. https://doi.org/10.1007/s11011-016-9902-2
Iwu MM, Igboko OA, Okunji CO, Tempesta MS (1990) Antidiabetic and aldose reductase activities of biflavanones of Garcinia kola. J Pharm Pharmacol 42:290–292. https://doi.org/10.1111/j.2042-7158.1990.tb05412.x
Kaper JB, Nataro JP, Mobley HLT (2004) Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. https://doi.org/10.1038/nrmicro818
Karami Z, Akbari-adergani B (2019) Bioactive food derived peptides: a review on correlation between structure of bioactive peptides and their functional properties. J Food Sci Technol 56:535–547. https://doi.org/10.1007/s13197-018-3549-4
Kiewiet MBG, Faas MM, De Vos P (2018) Immunomodulatory protein hydrolysates and their application. Nutrients 10:904. https://doi.org/10.3390/nu10070904
Korhonen H, Pihlanto A (2003) Food-derived bioactive peptides-opportunities for designing future foods. Curr Pharm Des 9:1297–1308
Kushwaha K, Babu D, Juneja VK (2014) Proteus. In: Batt CA, Tortorello ML (eds) Encyclopedia of Food Microbiology (Second Edition). Academic Press, Oxford, pp 238–243. https://doi.org/10.1016/B978-0-12-384730-0.00281-0
Mashuri N, Tan HL, Lim YP, Maqsood-Ul-Haque SNS (2019) Isolation of antimicrobial peptide from food protein hydrolysates: an overview. Key Eng Mater 797:168–176. https://www.scientific.net/KEM.797.168
Montalvo GEB, Vandenberghe LPdS, Soccol VT, de Carvalho JC, Soccol CR (2020) The antihypertensive, antimicrobial and anticancer peptides from Arthrospira with therapeutic potential: A mini review. Curr Mol Med 20:593–606. https://doi.org/10.2174/1566524020666200319113006
Morais HA, Silvestre MP, Silva VD, Silva MR, e Silva ACS, Silveira JN (2013) Correlation between the degree of hydrolysis and the peptide profile of whey protein concentrate hydrolysates: effect of the enzyme type and reaction time. Am J Food Technol 8:1–16. https://doi.org/10.3923/ajft.2013.1.16
Nasri M (2017) Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Advances in Food and Nutrition Research, vol 81. Elsevier, pp 109–159. https://doi.org/10.1016/bs.afnr.2016.10.003
Noman A, Qixing J, Xu Y, Ali AH, Al-Bukhaiti WQ, Abed SM, Xia W (2019) Influence of degree of hydrolysis on chemical composition, functional properties, and antioxidant activities of chinese sturgeon (Acipenser sinensis) hydrolysates obtained by using alcalase 2.4 L. J Aquat Food Prod Technol 28:583–597. https://doi.org/10.1080/10498850.2019.1626523
Olson BJSC, Markwell J (2007) Assays for determination of protein concentration. Curr Protocols Protein Sci 48. 3.4.1–3.4.29. https://doi.org/10.1002/0471140864.ps0304s48
Osukoya O, Nwoye-Ossy M, Olayide I, Ojo O, Adewale O, Kuku A (2020) Antioxidant activities of peptide hydrolysates obtained from the seeds of Treculia africana Decne (African breadfruit). Prep Biochem Biotechnol 50:504–510. https://doi.org/10.1080/10826068.2019.1709980
Osukoya OA, Adewale OB, Falade AE, Afolabi OB, Awe JO, Obafemi TO, Kuku A (2021) Antioxidant and antibacterial properties of Monodora myristica (Calabash nutmeg) seed protein hydrolysates. J Food Meas Charact 15:2854–2864. https://doi.org/10.1007/s11694-021-00871-4
Perez C (1990) Antibiotic assay by agar-well diffusion method. Acta Biologiae et Medicine Experimentalis 15:113–115
Puntel RL, Nogueira CW, Rocha JBT (2005) Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem Res 30:225–235. https://doi.org/10.1007/s11064-004-2445-7
Różalski A et al (2012) Proteus sp. – an opportunistic bacterial pathogen – classification, swarming growth, clinical significance and virulence factors. Acta Universitatis Lodziensis Folia Biologica et Oecologica 8:1–17. https://doi.org/10.2478/fobio-2013-0001
Sharma R (2014) Functional foods roundup. Food Australia 66:36
Tejano LA, Peralta JP, Yap EES, Chang Y-W (2019) Bioactivities of enzymatic protein hydrolysates derived from Chlorella sorokiniana. Food Sci Nutr 7:2381–2390. https://doi.org/10.1002/fsn3.1097
Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr (2015) Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. https://doi.org/10.1128/cmr.00134-14
Walstra P (2003) Physico-chemical properties of proteins and basic aspects of the various functional properties. In: Walstra P (ed) Physical Chemistry of Foods. Marcel Dekker Inc., New York
Wang R et al (2020) Presence of small resistant peptides from new in vitro digestion assays detected by liquid chromatography tandem mass spectrometry: An implication of allergenicity prediction of novel proteins? PLoS ONE 15:e0233745. https://doi.org/10.1371/journal.pone.0233745
WFO (2022) Garcinia kola Heckel. CC BY 4.0. http://www.worldfloraonline.org/taxon/wfo-0000694394. Accessed 12 Oct 2022
Woolf LI, Adams J (2020) The early history of PKU. Int J Neonatal Screen 6:59
Yuan H, Wang H, Wang L, Chai L, Tian C (2017) Nutritional evaluation and functional properties of the antioxidant polypeptide from Zanthoxylum bungeanum Maxim seeds kernel protein hydrolysate. CyTA-Journal of Food 15:425–432. https://doi.org/10.1080/19476337.2017.1288171
Zhu L, Chen J, Tang X, Xiong YL (2008) Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate. J Agric Food Chem 56:2714–2721. https://doi.org/10.1021/jf703697e
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Osukoya, O., Gbabo, F., Fadugba, A. et al. Some functional properties, antioxidant and bactericidal activities of Garcinia kola Heckel protein hydrolysates: in vitro. Vegetos 36, 1231–1238 (2023). https://doi.org/10.1007/s42535-022-00526-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-022-00526-9