Abstract
Rosemary is one of the well-known aromatic and therapeutic plants recognized for the interesting pharmacological properties of its essential oil. After hydrodistillation, a huge amount of solid residue-remains, which still contains non-volatile bioactive compounds. Our work aims to study in depth the effect of ethanol/water concentration on the extraction yield, total phenolic and flavonoid content, chemical profile, antioxidant and antimicrobial activities of Rosmarinus tournefortii de Noé solid residues. Phenolic and flavonoid content was estimated spectrophotometrically and for their identification, High-performance liquid chromatography-photodiode array detector (HPLC-DAD) analysis was adopted. The antioxidant activity was established using common methods such as DPPH, ABTS, and the Beta-carotene/linoleate model system. Furthermore, the antimicrobial capacity was investigated against Escherichia coli ATCC 25,922 and Listeria innocua ATCC 33,090, two well-known organisms representing gram-negative and gram-positive bacteria, respectively, as well as against the mold Geotrichum.s p and the yeast Rhodotorula glutinis. Based on the statistical analysis, a significant effect of ethanol/water concentration on the phenolic composition, antioxidant, and antifungal activity was revealed, while a slight difference was observed for the antibacterial activity. On the other hand, HPLC–DAD analysis endorsed the preferential extraction of gallocatechin and caffeic acid in 20% ethanol, homoplantaginin in 40%, cirsimaritin in 0% ethanol, and rosmarinic acid in 100% ethanol. Additionally, the 80% ethanol/water concentration indicated the highest extraction yield and flavonoid content (yield = 51.6%, TFC = 21.38 ± 0.23 mg QUE/g DW). On the contrary, 40% ethanol revealed both the highest phenolic content (TPC = 128.18 ± 0.56 mg GAE/g DW) and radical scavenging activities (IC50 = 0.051 ± 0.008 mg/mL, 0.061 ± 0.002 mg/mL, and 1.232 ± 0.013 mg/mL for DPPH, ABTS, and beta-carotene/linoleate model system, respectively). Besides, 20% was the highest concentration for the inhibition of the two bacteria Escherichia coli (7.35 ± 0.05%) and Listeria innocua (8 ± 0.1%) as well as the mold Geotrichum sp, (16.5 ± 0.3%) and for the yeast Rhodotorula (26 ± 1.2%), 50% ethanol was found to be the most appropriate concentration. These differences detected between the studied activities of rosemary solid residue extracts were strongly influenced by the target phenolic compounds extracted.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Annually, a huge number of different types of solid and liquid residues from hydrodistillation are generated by the agricultural industry based on aromatic and medicinal plants [1]. These residues are expected to be the most energy-rich resources on earth, and they can play an essential role in providing the raw materials for a sustainable bioeconomy pathway [2]. Nevertheless, the accretion of these residues leads to an environmental problem and the loss of potential elements, which can be a natural bioresource for the production of bioactive phenolic antioxidants [3, 4].
Rosmarinus includes two popular species of aromatic shrubs (Lamiaceae), Rosmarinus Tournefortii de Noé and Rosmarinus Officinalis L., which populates the forests, scrubland, and matorrals of the plains, low and medium mountains, on a limestone substratum [5]. Rosemary is one of the most popular therapeutic plants used worldwide for essential oil production (estimated at 150–200 tons/year) [6]. Tunisia (80 tons), Morocco (40 tons), Spain (28 tons), and France (5 to 10 tons) are the main producers of rosemary oil [6]. In this context, the yield of essential oil obtained by the two extraction methods (hydro distillation and steam distillation) only reaches a maximum of 2.5 percent of the essential oil [7, 8], which generates various kinds of solid residues (10–20 kilotons/year) that could create an environmental problem if not managed correctly [9, 10].
Furthermore, the solid residue of rosemary is rich with non-volatile bioactive compounds known as secondary metabolites, including phenolic acids and phenolic diterpenes such as rosmarinic acid, caffeic acid, carnosic acid, carnosol, as well as some flavonoids. These constituents are known to be potent antioxidants, antimicrobial, anti-proliferative, anticancer, anti-diabetic, anti-inflammatory, anti-viral, and anti-atherogenic [11,12,13,14,15,16,17,18]. Each feature makes these residues useful as health-promoting compounds, as ingredients for anti-aging agents in cosmetics products, and as antioxidant food additives, simultaneously increasing the crop's profit.
Solvent selection is critical in extraction processes as it directly affects selectivity, which in turn affects the extracted product's chemical profile and functional characteristics. The choice is often based on how easily the chosen solvent binds to the selected chemical. Since solubilization entails electrostatic repulsion and attraction forces between the solvent and solute, it has been generally recognized that polar solvents prefer solubilization and extraction of polar compounds. In contrast, less polar solvents would be suitable for less polar molecules [19].
Among the alternate solvents available for the extraction of natural products, hydro-alcoholic mixtures are more suitable competitors than a single solvent, leading to a higher yield of polyphenols [20,21,22,23]. Ethanol, methanol, and acetone in water are the most common solvents for extraction. Compared to these mixtures, ethanol/water mixtures seem to be the most suitable solvents for extraction, as they are not very selective and present a wide range of polarity of both solvents, the possibility of mixing them in any proportion, and their acceptability for human consumption [24]. In previous research, some studies [25, 26] suggested that carnosic acid, carnosol, and rosmarinic acid are recovered and claimed as the extract with the highest antioxidant content when the ethanol in water ratio is less than 70% v/v. On the contrary, Jacotet-Navarro et al. [19] studied the relationship between solubilization and antioxidant activities of the main rosemary compounds. The described works revealed that a high reducing activity is not necessary depending on the total solubilization of phenolic compounds but on the activity of reducing compounds, for which 30% ethanol showed high reducing activity and the highest TPC, whereas 90-100% ethanol provided the best solubilization of rosmarinic acid and carnosic acid. In addition, Irini Psarrou et al. [9], improved the production of the highest yield, total phenolic content (TPC), and antiradical activity by using 60% ethanol. On the other hand, Lefebvre et al. [27] studied the extraction selectivity of a particular compounds, and the highest fraction of carnosic acid, and carnosol attained with a 3% polar modifier (ethanol: water 50/50 v/v) and for rosmarinic acid, as well as genkwanin using 10% of the same modifier by applying supercritical fluid extraction. Moreover, regarding the antibacterial activity of rosemary, most studies examined mono-solvents such as ethanol and water, in which the ethanolic extract seems to be more active against both gram-positive and gram-negative bacterial than the aqueous extract [28] and vice versa for antifungal activity [29].
Therefore, these conflicting results suggest that most studies are only concentrated on determining the effect of ethanol/water mixture ratio on phenolic content and antioxidant properties of rosemary extracts. To the extent of our knowledge, there are no studies on the effect of ethanol/water mixtures on antimicrobial property, and we are aware of only a few studies that have examined the relationship between phenolic composition and their biological activity extracted with different ethanol/water ratios, especially for Rosmarinus tournefortii de Noé genus, which is undoubtedly rather unknown compared to Rosmarinus officinalis L.
In this context, the current study aims to investigate the effect of ethanol/water mixtures as a green solvent on phenolic contents, antioxidant and antimicrobial activities of the solid residues from Rosmarinus tournefortii de Noé hydrodistillation. The relationship between those activities and the chemical profile will be also the subject of this work. The different extracts of rosemary solid residues were detected and characterized using HPLC-DAD and to evaluate their antioxidant activities, three assays were selected: DPPH Free Radical Scavenging, ABTS Radical Scavenging, and beta-Carotene/linoleate model system. Furthermore, the antimicrobial performance of rosemary solid residue extracts was investigated by using the bacteria Listeria innocua ATCC 33,090 (gram-positive) and Escherichia coli ATCC 25,922 (gram-negative), as well as fungi (Rhodotorula glutinis and Geotrichum sp).
Materials and methods
Standards and reagents
Rosmarinic acid, carnosic acid, carnosol, ferulic acid, and caffeic acid are the main analysis standards (purity ≥ 96%). Ethanol and Methanol (HPLC grade) were used to extract rosemary extracts. Folin ciocalteu's phenol reagent, sodium carbonate, gallic acid, quercetin, Aluminum chloride anhydrous, sodium nitrate, and sodium hydroxide were used to quantify the phenolic and flavonoid tenor. Acetonitrile and formic acid are chromatographic grades and were used for HPLC analyses. All these reagents were acquired from Sigma Aldrich. Ascorbic acid, 2,2-diphenyl-1-picrylhdrazyl (DPPH), 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate, β-carotene, linoleic acid tween 20 and were purchased from oxford and used for the evaluation of the antioxidant capacity.
Plant material and residue extracts
Fresh leaves of the wild plant Rosmarinus tournefortii de Noé were gathered at the flowering stage (13 March 2020) in the forest area of Megrez, Eastern region of Morocco (34° 43′ 52.6′′ N 2° 04′ 21.5′′ W) Fig. 1. The specimen was identified by the Forest Management Studies Service of Oriental.
The leaves of rosemary, Rosmarinus tournefortii de Noé, were dried at ordinary temperature for 10 days prior, and then about 100 g were hydro-distilled with a Clevenger for 3 h, as reported by Navarrete et al. [30]. The solid residue of hydrodistillation was dried for nearly 15 days. Additionally, 1 g of solid residue was powdered, mixed with 20 mL (solid: liquid ratio 1:20 w/v) of different extraction solvents: ethanol/water ratio (100:0, 80:20, 60:40, 50:50, 40:20, 20:60, 0:100; v/v). The mixtures were treated by the maceration technique for 48 h at room temperature to extract polyphenols, then filtered through Whatman (GF/A, 90 mm) and dried using a rotator evaporator Fig. 2. All samples were maintained in amber glass vials and refrigerated at 4 °C until analysis.
Total phenolic and flavonoid content
The total phenolic content (TPC) of rosemary solid residue extracts were derived by the Folin-Ciocalteu method as described by Cujic et al. [31]. In brief, 200 µL of each extract was mixed with 1000 µL of Folin-Ciocalteu reagent (diluted 10 folds). 800 µL of sodium carbonate solution (7.5 g/L) was added 4 min later. After incubation for 2 h at room temperature, the absorbance was measured at 760 nm using a UV–VIS Spectrophotometer (Shimadzu UV 1650-PC). The TPC was estimated using gallic acid as a reference, and the findings were represented in milligrams of gallic acid equivalents per gram of dry weight (mg GAE/g DW).
The flavonoids (TFC) was determined by a colorimetric approach using the aluminum chloride reagent according to Zhishen et al. [32] with slight modification. 1000 µL of each extract was added to 4000 µL of distilled water and 300 µL of sodium nitrate (5%). After waiting 5 min of incubation, 300 µL of aluminum chloride anhydrous was added. Following incubation of 6 min, 2000 µL of sodium hydroxide (1 M) was added. Finally, distilled water was added to achieve a final volume of 8 mL. After mixing the reagents well, a UV–VIS (Shimadzu UV 1650-PC) Spectrophotometer was used to measure the absorbance at 510 nm. The TFC was estimated using quercetin as a reference, and the results were represented in milligrams of quercetin equivalents per gram of dry extract (mg QUE/g DE).
HPLC-DAD analysis of the solid residue extracts of Rosmarinus tournefortii de Noé
Rosemary solid residue extracts were analyzed using high-performance liquid chromatography (Waters Alliance™ e2695 XC HPLC System) equipped with a 2998 Photodiode Array Detector, and a reversed-phase C18 column (5 µm, 250 mm × 4.6 mm). Extracts (10 mg/mL) were injected into the column at a flow rate of 1 mL/min using a gradient of binary solvents as described by Liu et al. [33]. The following gradient of mobile phase A (2% formic acid in water) and mobile phase B (acetonitrile) was used for polyphenols separation: 0–10 min, 30–70% B; 10–15 min, 70–30% B; 15–25 min, 70–30% B; 25–30 min, 30–70% B. The injection volume was 10 µL and the UV–VIS detection was performed in the 280–330 nm range. Individual polyphenols were identified by analyzing their holding time and maximum wavelength to standards and literature data.
Antioxidant activity
DPPH free radical scavenging assay
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical assay's scavenging capability was determined just as Zhang et al. [34] with modest modification.
The assay is based on the measurement of the ability of rosemary solid residue extracts to reduce the stable free radical by receiving a hydrogen atom from antioxidants to the corresponding free radical, and the formation of the non-radical form DPPH-H resulting from the reaction. Briefly, 500 µL of various concentrations of rosemary extracts (0.004–0.2 mg/mL) were mixed with 2500 µL of DPPH in methanol (0.04 mg/mL). The resulting solution was incubated for 1 h at room temperature, and then its absorbance was measured at 517 nm using a UV–VIS (Shimadzu UV 1650-PC) Spectrophotometer. Ascorbic acid was employed as a negative control and as a standard antioxidant in the assay. The following Eq. (1) was used to determine the inhibitory activity (%) of DPPH radical.
where A0 is the absorbance of the negative control, and AE is the extracts' absorbance. DPPH radical-scavenging activity was expressed as IC50 (IC50 is the concentration necessary to inhibit 50% of the free radical, determined graphically on the curve I % = f (C) by extrapolating Y = 50% inhibition on the value of the abscissa axis).
ABTS radical scavenging assay
The radical scavenging activity of the ABTS was performed according to the technique described by Tran et al. [35] with some modifications.
The test is based on the capacity of free radical scavenging phenolic compounds to weaken the green–blue colored radical cation ABTS+ to an uncolored form [36]. The stock solutions of potassium persulfate (2.45 mM) and ABTS (7 mM) were prepared and mixed in equal parts. The mixture was then allowed to react for 12 to 16 h at room temperature, in the dark to allow the production of free radicals. Before analysis, the stock solution was diluted with anhydrous ethanol to obtain an absorbance of 0.7 at 734 nm. To determine the scavenging activity, 0.1 mL of rosemary solid residue extracts of various concentrations (0.004–0.5 mg/mL) were mixed with 2 mL of ABTS+ radical solution. The mixture was thoroughly sacked and then allowed to stand for 6 min. The absorbance was noted at 734 nm using the same volume of matching extraction solvents as the control sample. The standard curve used was ascorbic acid. The inhibition activity (%) of ABTS radicals was determined according to the following Eq. (2):
where A0 is the absorbance of the negative control, and AE is the extracts' absorbance. The linear regression analysis was used to determine the extract concentration (IC50) needed to inhibit 50% of ABTS radical.
Beta-carotene/linoleate model system
The beta-carotene bleaching test was established using the method of Tohma et al. [37] with minor modifications. The process is founded on the oxidative breakdown of beta-carotene in the presence of linoleic acid. As a result of the oxidation of beta-carotene, it loses its distinctive orange color. Therefore, the level of linoleic acid oxidation can be assessed indirectly by monitoring the decrease in absorbance of beta-carotene. The presence of antioxidants in the system prevents the bleaching of beta-carotene by opposing the formation of free radicals. The preparation of beta-carotene/ linoleic acid emulsion in distilled water was as follows: 1 mL beta-carotene solution (0.2 mg/mL chloroform), 20 mg linoleic acid, and 200 mg tween 20. After the elimination of chloroform, 30 mL of distilled water was added and kept in an ultrasonic bath for 5 min. 4 mL of the emulsion solution formed was mixed with 0.2 mL of rosemary solid residue extracts at different concentrations (0.1–5 mg/mL) and then incubated at 50 °C in a water bath for 120 min. The absorbance was determined at t = 0 for the monitoring solution (0.2 mL of water with 4 mL emulsion solution) and at t = 120 min for the monitoring solution and samples using a UV–VIS spectrophotometer at 470 nm. The percent of inhibition was calculated according to the following Eq. (3):
where AE (120) is the extracts` absorbance at 120 min; AC (120) is the control absorbance at 120 min, and AC (0) is the control absorbance at 0 min. The linear regression analysis was used to determine the concentration of the extracts (IC50) needed to inhibit 50% of oxidized beta-carotene.
Antimicrobial activity
The antimicrobial activity of Rosmarinus tournefortii de Noé solid residue extracts was investigated using the agar diffusion method on solid media, in accordance with some research [38, 39]. The in vitro antimicrobial activity was evaluated against four pathogenic microbes, including Escherichia coli ATCC 25,922 (gram-negative bacteria) and Listeria innocua ATCC 33,090 (gram-positive bacteria), the yeast Rhodotorula glutinis and the mold Geotrichum sp.
The strains were diluted and adjusted to 0.5 McFarland for the two types of microorganism: gram-negative and gram-positive bacteria and the yeast Rhodotorula glutinis, which correspond to 106 CFU/mL and 106 spores/mL for the mold Geotrichum sp. Furthermore, fresh cultures were diluted with Mueller–Hinton broth for bacteria and yeast, and with sterile physiological water for mold, and then inoculated onto the surface of the petri dish. This technique involves perforating the Mueller–Hinton agar (MHA) seeded with the bacteria/fungal to be tested to obtain wells (6 mm); these were filled with a 10 μL volume of samples (10 mg/mL). For bacterial and fungal growth, the agar plates were incubated for 18 h at 37 °C and 48 h at 25 °C, respectively. The antimicrobial activity of rosemary solid residue extracts was characterized by determining the diameter of the inhibition zone in the agar gel. All assays were achieved in triplicate. Cycloheximide and gentamicin were used as positive controls against fungi and bacteria, respectively.
Statistical analyses
The obtained results were subjected to descriptive statistical analysis, analysis of variance (ANOVA), and regression analysis using the software “SPSS for Windows version 20”, followed by Tukey's test with a post hoc multiple comparison threshold of 5%. Analyzes were performed in triplicate, and the results were given as mean values with standard error.
Results and discussion
Effect of ethanol/ water concentration on extraction yield and total phenolic and flavonoid content
Phenolic compounds are one of the most prevalent classes of secondary metabolites in plants with strong redox properties, which are involved in a wide range of biological processes, including antibacterial, antifungal, and antioxidant effects. In the present study, the recuperation of total phenolic compounds from the solid residues of Rosmarinus tournefortii de Noé has been achieved by the traditional maceration technique with different ratios of ethanol/water solvents. This mixture was chosen due to the greater possibility of solubilizing phenolic compounds and their safety effect on being introduced into food products [19, 40, 41].
As can be seen in Table 1, the highest extraction yield of rosemary was achieved with 80% ethanol as the best ratio of ethanol/water solvents used for the extraction of polyphenols, reaching a maximum of 51.6%, followed by the aqueous extract (26%) and significantly decreased with further increase in ethanol at 20%. The statistical analysis showed the existence of several subgroups and indicate a significant difference between the different ethanol/water concentrations regarding the extraction yield (P < 0.05). Our findings agreed with the previous studies. For example, Sun et al. [42] and Irini et al. [9] demonstrated an extraction solvent ratio close to our results, for which 75% and 60% ethanol were reported as the highest extraction yield. Also, a similar pattern was observed regarding the yield, where water was also recommended as one of the appropriate solvent to lead to a higher yield of bioactive compounds [43, 44].
This behavior can be explained by the fact that the presence of water (having a stronger dipole moment than the alcohols) destabilizes the cell walls. Consequently, penetrating deeper into the plant matrix, this phenomenon increases the contact surface between the solvent and the solute, which favors the extraction of phenolic compounds [15].
The total phenolic content (TPC) of rosemary solid residue extracts ranged from 32.41 to 128.18 mg GAE/g DW. Compared with the total phenolic content, total flavonoid content (TFC) varied from 6.58 to 21.38 mg QUE/g DW. In addition, the highest TPC and TFC were observed in a different range ratio, in which the TPC was clearly increased at the range of 20–50%, while the TFC was achieved from 60 to 100% ethanol concentration. Further, according to our results, the highest TPC was found to be in 40% (128.18 mg GAE/g DW) and for TFC, 80% was found to be the greatest flavonoid contents (21.38 mg QUE/g DW). The smallest phenolic and flavonoid contents seemed to be in ethanol absolute (32.41 mg GAE/g DW) and 40% ethanol (6.58 mg QUE/g DW), respectively. The statistical analysis demonstrated a significant difference between the tested sample concerning the TPC and TFC with P < 0.05. Comparing our finding with the literature, most studies of binary solvents extraction revealed that the extracted flavonoid content increased over 70–80% ethanol, while the amount of polyphenols was achieved from 50 to 0% ethanol [43, 45]. In light of our results, 40% ethanol is highly recommended for TPC, while 80% ethanol is suggested as the best extraction solvent in terms of yield and TFC.
Chemical profile of Rosmarinus tournefortii de Noé solid residue extracts
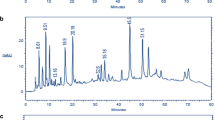
To evaluate the effect of ethanol/water concentration on the chemical profile of rosemary solid residues, an extensive analysis of phenolic compounds, including phenolic acids, phenolic diterpenes, and flavonoids, was performed based on HPLC-DAD data and data described in the literature. Rosmarinic acid, caffeic acid, ferulic acid, carnosol, and carnosic acid were easily identified by comparing their holding times and UV–Vis spectra with the reference standards. In addition, to identify flavonoids, data described in the literature was reviewed in depth with respect to the elution pattern of flavonoids [46,47,48,49,50].
As indicated in Table 2, three families were tentatively identified in the solid residue extracts of rosemary: phenolic acids (2, 3, and 5), phenolic diterpenes (9, 10, 11, 14, 15, 16, and 19) and flavonoids (1, 4, 6, 7, 8, 12, and 18). Rosemary extracted by pure ethanol recovered the widest range of phenolic compounds polarity as illustrated in the chromatogram (A) of Fig. 3. The elution at the beginning of chromatogram revealed the highest polar compounds with a mixture of phenolic acids and flavonoids (flavanol, flavones, and flavonoid glycoside). Gallocatechin could be a possible structure for the first peak, since the maximum band was detected between 284 and 320 nm which is consistent with previously published results [43]. Caffeic acid and ferulic acid were eluted approximately 1 min after peak 1. The flavonoid glycoside, homoplantaginin could possibly be the fourth peak's structure, since the absorbance band is found in the 275–340 nm range, values that are almost similar to the UV–Vis spectra detected by numerous studies (270–340 nm) [43, 46, 47], so the fourth peak may be assumed as homoplantaginin. Scutellarein and apigenin are two flavones eluted directly after rosmarinic acid with two distinctive absorbance bands: 267–342 nm and 340 nm, respectively, which are comparable to the λmax values and spectra pattern matching with the literature data [10, 43, 46, 49,50,51]. Therefore, the two UV spectra's patterns are identical, it is possible to identify scutellarein and apigenin as peaks 6 and 7, respectively
The second elution of the chromatogram contained a rich variety of phenolic diterpenes and flavones, peak 8 as dimethoxy flavone, with UV–vis maximum at 274 and 337 nm. The compound was candidate to be cirsimaritin, according to some previous studies [10, 43, 46, 50], almost the same spectral maxima (278–336 nm) were found, showing that the eighth peak could be identified as cirsimaritin. Moreover, rosmanol and its derivatives epirosmanol and epi isorosmanol could be assigned to peaks 9, 10 and 11, respectively, due to the spectrum maxima, matching with the literature [10, 43, 46,47,48, 52], which are equal to our results: respectively, 236–288 nm, 233–287 nm, and 235–287 nm. Another flavone family, genkwanin, has been dubbed "pic number 12" because of its UV–vis detection wavelengths maximum at 267 and 333 nm, which are comparable to the published reports [9, 10, 43, 48]. From the exhibit characteristic of the UV spectra and the elution pattern of flavone, we may concede that peak number 12 could be identified as genkwanin. Furthermore, epirosmanol methyl ether and rosmadial, two phenolic diterpenes with UV spectrum maxima of 233–288 nm and 235–288 nm, are projected to have peaks 15 and 16, which are consistent with prior research on rosemary extracts [46,47,48]. 4′-methoxytectochrysin is the only flavone eluted between the two phenolic diterpenes, carnosol and carnosic acid. As reviewed by several researchers [9, 10, 46, 47] working on rosemary extracts, the λmax values are 270, 278, and 332 nm, for which those values are almost equal to ours (λmax = 278, 332 nm). Thereby, 4′-methoxytectochrysin can be identified as peak number 18.
The chromatographic profile of rosemary extracted with 80% and 60% ethanol still harbored the same phenolic acids and flavonoids as the ethanolic extract at the beginning of elution with a different ratio of the peak areas of these compounds (Fig. 3B and C). Furthermore, in the second elution of the chromatogram, the decrease in ethanol concentration caused a significant decrease in some phenolic diterpenes and flavones. In the 80% ethanolic extract, there is an absence of rosmanol (peak 9) and its isomer epirosmanol (peak 10), while in the 60% ethanolic extract, there is a disappearance of other compounds including genkwanin, epirosmanol methyl ether, and rosmadial. Also, 4′-methoxytectochrysin (peak 18) was absent for both 80% and 60% ethanol concentrations.
As can be seen in Fig. 4, at the beginning of the chromatogram, the 50% and 40% ethanol extracts kept retaining the same compounds as the other ethanolic extracts (100%, 80%, and 60% ethanol), except for rosmarinic acid, one of the most known phenolic acids of rosemary, which disappered in 40% ethanol. On the contrary, in the second elution of the chromatogram, the decrease in ethanol concentration to 40% affected the chemical profile of the extracts, since all phenolic diterpenes and flavonoids significantly disappeared, except for rosmanol in the solid residue of rosemary extracted with 40% ethanol (Fig. 4A and B).
The chemical profile of the two extracts extracted with 20% and 0% ethanol in water was significantly dependent on the ethanol concentration. At the beginning of the chromatograms F and G (Fig. 4), it was worthwhile to reveal the absence of the two phenolic acids, ferulic acid and rosmarinic acid as well as the absence of the flavone apigenin (peak 7). The flavone glycoside homoplantaginin (peak 4) was also absent in the aqueous extract. Further, new phenolic diterpenes appeared in the second elution: carnosol and its isomer carnosic acid, as well as the reappearance of cirsimaritin (peak 8) in both concentrations. Rosmanol retained its position in 20% ethanol but disappeared in 0% ethanol, with the advent of genkwanin in the latter concentration (Table 2).
In general, pure ethanol and 80% highlight the total phenolics in the solid residue of Rosmarinus tournefortii de Noé for the reason of its suitable extraction of bioactive compounds with a wide range of polarity. These findings are in the same line with Chunli sun et al. [42]. Moreover, to recover a specific compound, an exact concentration of ethanol in water is required to extract its maximum. For example, rosmarinic acid, genkwanin, epirosmanol methyl ether, rosmadial, and 4′-methoxytectochrysin reached their maximum with 100% ethanol, while rosmanol and its isomers; epi rosmanol and epi isorosmanol bring their extraction with 60% ethanol. Indeed, 50% ethanol had the perfect concentration for ferulic acid recovery, and for the three flavonoids, 40% ethanol proved to be the highest efficiency to extract all of homoplantaginin, scutellarein, and apigenin expected as peaks 4, 6, and 7, respectively. Caffeic acid, carnosic acid, and gallocatechin increased with 20% ethanol, and cirsimaritin and carnosol reached their best ratio in the aqueous extract.
Compared our results with the literature, Psarrou et al. [9] reported almost the same extraction solvent for genkwanin and 4′-methoxytectochrysin, while disagreed on rosmarinic acid recovery, which they found to be highly recovered on the aqueous extract than on the ethanolic extract, which is contradictory to our results. Our finding can be supported by the fact that rosmarinic acid is highly soluble in an aqueous solvent, so it can be found directly in the aqueous residue extract rather than in the aqueous solid residue of rosemary. Also for ferulic acid, Raphaela G et al. [53] reported that its solubility in ethanol presented higher values than the solubility in water. Furthermore, the greater solubility of caffeic acid in water has been previously improved and confirmed. The glycosylated flavonols have significantly increased water solubility compared to the corresponding aglycone [54]. The high presence of carnosol and carnosic acid in the aqueous extracts had already detected by Tzima et al. [53], who evaluated the ethanolic degradation of carnosol, carnosic acid, and their mixture using high-performance liquid chromatography, and subsequently confirmed that their recovery could be robustly in an aqueous solvent.
Thus, from our results, we can demonstrate that the chemical profile of the solid residue extracts of Rosmarinus tournefortii de Noé varies significantly with the concentration of ethanol in water.
Effect of ethanol/water concentration on the antioxidant activity of Rosmarinus tournefortii de Noé solid residue extracts
Antioxidants, whether from natural or synthetic sources, have proven to be highly effective in controlling the amount of free radical production, preventing their unwanted effects, and supporting the body's antioxidant and detoxifying mechanisms [55]. Phenolic compounds are among the most important groups of natural antioxidants, responsible for reducing oxidative stress and the resulting cell damage [56]. Their likelihood is directly correlated with the type of extraction solvent used.
For this propose, the effect of ethanol/water solvent mixture on the antioxidant activity of rosemary solid residue extracts was evaluated using three commonly method, namely: DPPH, ABTS, and Beta-carotene/linoleate model system. The IC50 values of rosemary solid residue extracts ranged from 0.051 to 0.18 mg/mL for both the stable free radical DPPH and the ABTS assays. Whereas, the IC50 values of rosemary extracts varied from 1.22 to 2.65 mg/ml for Beta-carotene/linoleate model system, on the other hand, the ANOVA test show that there is a significant difference between the different ethanol/water concentrations used for the extraction process regarding their antioxidant activity. The post-hoc test showed the existence of several sub-group (P < 0.05), as highlighted in Table 3.
The IC50 values of rosemary extracts for the three antioxidant methods decrease from 60 to 40% of the ethanol concentration, resulting in a significant increase in the radical scavenging activity of the extracts, which is clearly correlated with TPC. The results indicate that the rosemary extracted with 40% ethanol had the lowest IC50 value (0.051 ± 0.008 mg/mL, 0.061 ± 0.002 mg/mL, and 1.232 ± 0.013 mg/mL for DPPH, ABTS, and beta-carotene/linoleate model system, respectively), meaning higher antioxidant activity than the other extracts. The lowest antioxidant activity was observed with 100% ethanol for both methods; DPPH and ABTS, while 20 = 0% ethanol for the Beta-carotene/linoleate model system. Based on our chemical profile data, 40% ethanol has the highest content of the three flavones; homoplantaginin (27.04%), scutellarein (4.93%), and apigenin (2.74%) accompanied by a significant ratio of caffeic acid (50.70%). The reducing capabilities of the solid residue extract of rosemary extracted with 40% ethanol are related to the high presence of these redeeming compounds, which have been proven to perform antioxidant activities by breaking the chain of radicals by donation a hydrogen atom [57, 58], thus, we may speculate that the highest antioxidant of rosemary solid residue extracted by 40% ethanol in water results from the ratio of each individual phenolic compound exist in this extract.
Moreover, despite the presence of the different families of phenolic compounds including phenolic acids, phenolic diterpenes, and flavonoids on 100–80% ethanol extracts, they exhibited the lowest antioxidant activity to scavenge DPPH and ABTS+ free radicals. From these results, we can conclude that the combination of all these phenolic compounds does not necessarily increase the antioxidant activity. The same results were founded by some researchers [59, 60], reporting that the crude extract was less inhibitory than the other fractions. On the other hand, compared to DPPH and ABTS methods, the 20–0% ethanol showed the lowest antioxidant activity for the Beta-carotene/linoleate model system. These differences could be related to the different mechanisms of oxidation. YURTTAS et al. [61], reported significant differences (p < 0.05) between the antioxidant activity for the hydrolyzed and the nonhydrolyzed extracts, for which they found that the polar fractions had better antioxidant activity than the less polar compounds. Depending on the chemical profile provided by 20–0% ethanol extract, these extracts had fewer polar compounds compared to the other extracts. This naturally make sense because the most polar compounds were assigned to be in the water residue extract rather than the aqueous solid residue extract of rosemary.
The effect of binary solvents on the antioxidant activity of rosemary extracts has been studied by several researchers. For example, Jacotet-Navarro et al. [19], reported that 30% ethanol was the great concentration to extract the most antioxidant compounds, while other researchers [9, 24] evaluated that 60% ethanol owned the strongest antioxidant activity. Therefore, in the present work, 40% ethanol in water was the best solvent mixture for antioxidant activity, which is highly correlated with TPC.
Effect of ethanol/water concentration on antimicrobial activity of Rosmarinus tournefortii de Noé solid residue extracts
Food safety authorities, the food industry, and consumers are all very concerned about foodborne illnesses. In order to increase food quality and shelf life, a lot of work has been put into finding natural antimicrobials that can prevent bacterial and fungal growth. Similar to this, consumers now ask about the safety of artificial preservatives used in food. Because of this, there is a rising need for national resources that can replace food preservatives [62]. In view of the above, the examination of the antibacterial and antifungal properties of rosemary solid residue extracts was evaluated by investigating the efficacity of extracts obtained using various solvent extraction systems [28, 29, 63].
The antibacterial and antifungal activity of rosemary solid residue extracted by various ethanol/water concentrations were carried out against the two well-known bacteria Listeria innocua ATCC 33,090 (gram-positive) and Escherichia coli ACTCC 25,922 (gram-negative) as well as against the mold Geotrichum. sp and the yeast Rhodotorula glutinis. The results of the inhibition tests against the microbial strains are summarized in Table 4.
According to the statistical analysis, the antibacterial activity of rosemary solid residue extracts did not change significantly with increasing or decreasing ethanol concentration, for which the diameter measurements of the inhibition bacteria varied weakly from 7.08 to 8 mm and 7.08 to 7.35 mm for Listeria innocua and Escherichia coli, respectively. In contrast, a significant effect (p < 0.05) of ethanol/water concentration of rosemary solid residue extracts was revealed, resulting in a strong variation in antifungal activity, illustrated by an inhibition zone of 16.5–10.5 mm and 25.5–20.5 mm for Geotrichum. sp and Rhodotorula glutinis, respectively.
Despite the small differences detected in antibacterial activity, our results clearly show that the 20% ethanol extract exhibits greater inhibition against the two bacterial Listeria innocua (7.35 ± 0.05 mm) and Escherichia coli (8 ± 0.1 mm). Based on the literature data, conflicting results were found, among which some previous researchers revealed that the polar extracts had a weak effect on bacterial growth inhibition compared to apolar extracts [28], due to the high polarity of the extracted compounds [64, 65], while Shene et al. [63] reported that the antimicrobial activity of ethanolic extract was either absent compared to the aqueous extract, indicating that water-soluble compounds had the highest antimicrobial activity, which is in agreement with our results. The 20% ethanol extract of rosemary solid residue appeared to have a high recovery of the flavan-3-ol gallocatechin (17.35%) and caffeic acid (53.97%) as well as some phenolic diterpenes. From the characteristic compounds, we can assume that the antibacterial activity of rosemary solid residue extracted with 20% ethanol probably originates from these individual phenolic compounds. In addition, the mechanism of action of these phenolic compounds has not yet been thoroughly explained, however, only a few studies have assumed that phenolic compounds such as phenolic acids have the ability to prevent bacteria growth due to their pro-oxidant characteristics and alteration of hydrophobicity and cell surface charge, which ultimately leads to cytoplasmatic deposition and cell cracking and formation [66].
Compared to the antibacterial activity, the 50–20% ethanol range was found to be the best for the highest potential against the mold Geotrichum sp. and the yeast Rhodotorula glutinis. As can be seen in Table 4, the solid residue of rosemary extracted from a high-water content mixture inhibits fungi more than the high ethanol- mixture, and this could be related to the structure of the extracted phenolic compounds. Furthermore, the 20% ethanol extract was revealed to be the most effective against the mold Geotrichum sp., while the 50% ethanol extract was found to be the most repressive for the yeast Rhodotorula glutinis. These differences results can potentially be explained by the chemical profile of each extract. Taking into account the HPLC peak areas of the two extracts, the 20% ethanol extract represents over 54% of caffeic acid, tracked by some flavonoids and phenolic diterpenes including rosmanol, carnosol, and carnosic acid. Jordán et al. [67] studied the effect of phenolic diterpenes on the antimicrobial activity, founding a significant effect on improving antimicrobial activity of rosemary extracts. From these reports, we can conclude that the strong inhibition of the 20% ethanol extract against the mold Geotrichum sp. can be attributed not only to the high caffeic acid content, but also to the other phenolic compounds. On the contrary, the 50% ethanol extract was observed to be the only extract that recovered the most polar compounds, for which an equal amount of phenolic acid; caffeic acid and rosmarinic acid were found representing 50% of the total peak area, followed by some flavonoids. Based on the literature data, the combination of phenolic acids and flavonoids are already known for their high antifungal activity, as these polyphenolic compounds inhibit the growth of fungi by causing cell surface damage and breakage of the anterior septum in their fungi [68].
Therefore, the results of our study demonstrate that 20% ethanol is the best concentration for the inhibition of the pathogenic bacteria Listeria innocua ATCC 33,090 (gram-positive) and Escherichia coli ATCC25922 (gram-negative) as well as the mold Geotrichum sp, while 50% ethanol is strongly suggested for the yeast Rhodotorula glutinis.
It should be noted that this research focused primarily on solid residues of Rosmarinus tournefortii de Noé in the eastern region of Morocco. The lack of solid residue extracts indicates that our findings should be additionally explored in future work.
Conclusion
The present study has demonstrated a significant effect (p < 0.05) of the ethanol/water concentration on the phenolic compound’s composition as well as the antioxidant and the antimicrobial activity of the hydrodistillated solid residues of Rosmarinus tournefortii de Noé. A different ratios of ethanol/water solvents were crucial for the efficient extraction of phenolic compounds using the traditional maceration technique. Moreover, 80% ethanol in water solvent showed the highest TFC and extraction yield, while the TPC value was found in the 40% ethanol extract. Taking into account the HPLC peak areas of the phenolic compounds of rosemary extracts, 80% and 100% ethanol were found containing the major phenolic compounds, which therefore suggest that there was no meaningful relation between the TPC value and the number of phenolic compounds. These differences can be explained by the fact that the highest recovery of phenolic compounds does not necessarily depend on the highest mass extraction yield, but also on the reaction degree of the phenolic compounds with the Folin-Ciocalteu reagent, adhering to the traditional theory of structure-activity relationship, according to which the order of activity is inversely related to the availability of hydroxyl groups on the aromatic ring. Considering the selectivity of the phenolic compounds’ extraction, the best ratio of individual phenolic compounds was found to be in the range of 40–0% ethanol, except rosmarinic acid, which was found to be maximized in the pure ethanol. Gallocatechin, caffeic acid, and carnosic acid were preferentially extracted with 20% ethanol, also the two flavonoids homoplantaginin, scutellarein, and apigenin were highly recovered with 40% ethanol. Indeed, cirsimaritin and carnosol were extremely extracted with 0% ethanol. These findings can be supported by the high selectivity of the presence of water in the system which can enhance the swelling of the plant materials, thus increasing the contact between the surface area of the plant matrix and the solvent.
Furthermore, the decrease in ethanol concentration from 60 to 40% increased the antioxidant activity of rosemary extracts, which is in the same line of TPC value, while for the antimicrobial activity the range of 50–20% ethanol concentration are revealed. Based on the obtained results, the 40% ethanol extract can be strongly recommended for its highly antioxidant and antimicrobial activities. In fact, 20% ethanol extract was the most promising antibacterial activity against Listeria innocua ATCC 33,090 (gram-positive) and Escherichia coli ACTCC 25,922 (gram-negative) as well as against the mold Geotrichum Sp., while 50% ethanol was found to be the most repressive for the yeast Rhodotorula glutinis. These results can be related to the reduction abilities of each phenolic compounds against the microbial strains and free radicals. Overall, this study reinforces the industry's interest in the importance of ethanol/water concentration on the desired target compounds to be extracted and that these solid residue extracts can also be used as a vital source of bioactive compounds with a strong antioxidant and antimicrobial activity.
References
A. Saha, B.B. Basak, Ind. Crops Prod. 145, 111979 (2020)
L. Hamelin, M. Borzęcka, M. Kozak, R. Pudełko, Renew. Sustain. Energy Rev. 100, 127 (2019)
P.S.N. Nigam, A. Pandey, Biotechnology for agro-industrial residues utilisation (Springer, 2009)
O. Santana-Méridas, A. González-Coloma, R. Sánchez-Vioque, Phytochem. Rev. 11, 447 (2012)
C. Bensouici, T. Boudiar, I. Kashi, K. Bouhedjar, A. Boumechhour, L. Khatabi, H. Larguet, J. Food Meas. Charact. 14, 632 (2020)
M. Rahmani, Romarin et thym Zrira, Saadia 2017 Examen national de l’export Vert du maroc: produits oléicoles. Cnuced 44 (2017).
L.A. Conde-Hernández, J.R. Espinosa-Victoria, A. Trejo, J. Guerrero-Beltrán, J. Food Eng. 200, 81 (2017)
S. Karakaya, S.N. El, N. Karagozlu, S. Sahin, G. Sumnu, B. Bayramoglu, J. Food Sci. Technol. 51, 1056 (2014)
I. Psarrou, A. Oreopoulou, D. Tsimogiannis, V. Oreopoulou, Molecules 25, 4520 (2020)
O. Santana-Méridas, M. Polissiou, M.E. Izquierdo-Melero, K. Astraka, P.A. Tarantilis, D. Herraiz-Peñalver, R. Sánchez-Vioque, Ind. Crops Prod. 59, 125 (2014)
K. Rižnar, Š Čelan, Ž Knez, M. Škerget, D. Bauman, R. Glaser, J. Food Sci. 71, 425 (2006)
S. Cheung, J. Tai, Oncol. Rep. 17, 1525 (2007)
S.H. Choi, G.W. Jang, S. Il Choi, T.D. Jung, B.Y. Cho, W.S. Sim, X. Han, J.S. Lee, D.Y. Kim, D.B. Kim, O.H. Lee, Antioxidants 8, 76 (2019)
O. Yesil-Celiktas, C. Sevimli, E. Bedir, F. Vardar-Sukan, Plant Foods Hum. Nutr. 65, 158 (2010)
Y.L. Ngo, C.H. Lau, L.S. Chua, Food Chem. Toxicol. 121, 687 (2018)
F.V. Hassani, K. Shirani, H. Hosseinzadeh, Naunyn. Schmiedebergs Arch. Pharmacol. 389, 931 (2016)
P.C. Martins-Gomes, F.M. Nunes, P.A. Sampaio, E.B. Souto, P.A.M. Silva, Antioxidents 9, 34 (2019)
A. Sankara, J.C.W. Ouédraogo, L. Pignolet, M.F. Thévenon, Y.L. Bonzi-Coulibaly, J. Agric. Sci. 12, 245 (2020)
M. Jacotet-Navarro, M. Laguerre, A.S. Fabiano-Tixier, M. Tenon, N. Feuillère, A. Bily, F. Chemat, Electrophoresis 39, 1946 (2018)
N.E. Durling, O.J. Catchpole, J.B. Grey, R.F. Webby, K.A. Mitchell, L.Y. Foo, N.B. Perry, Food Chem. 101, 1417 (2007)
Y. Yilmaz, R.T. Toledo, J. Food Compos. Anal. 19, 41 (2006)
G.A. Akowuah, Z. Ismail, I. Norhayati, A. Sadikun, Food Chem. 93, 311 (2005)
Á. Rubio-Moraga, J. Argandoña, B. Mota, J. Pérez, A. Verde, J. Fajardo, J. Gómez-Navarro, R. Castillo-López, O. Ahrazem, L. Gómez-Gómez, J. Ethnopharmacol. 148, 287 (2013)
K. Waszkowiak, A. Gliszczyńska-Świgło, Eur. Food Res. Technol. 242, 777 (2016)
G.D.A.R. Oliveira, A.E. De Oliveira, E.C. Da Conceição, M.I.G. Leles, Food Chem. 211, 465 (2016)
E. Lesellier, T. Lefebvre, E. Destandau, TrAC Trends Anal. Chem. 135, 116158 (2021)
T. Lefebvre, E. Destandau, E. Lesellier, J. Chromatogr. A 1639, 461709 (2021)
F.D. Gonelimali, J. Lin, W. Miao, J. Xuan, F. Charles, M. Chen, S.R. Hatab, Front. Microbiol. 9, 1 (2018)
D.E. Blank, G.H. Alves, P.D.S. Nascente, R.A. Freitag, M.B. Cleff, J. Agric. Chem. Environ. 09, 85 (2020)
A. Navarrete, M. Herrero, A. Martín, M.J. Cocero, E. Ibáñez, J. Food Eng. 104, 196 (2011)
N. Ćujić, K. Šavikin, T. Janković, D. Pljevljakušić, G. Zdunić, S. Ibrić, Food Chem. 194, 135 (2016)
J. Zhishen, T. Mengcheng, W. Jianming, Food Chem. 64, 555 (1999)
T. Liu, X. Sui, R. Zhang, L. Yang, Y. Zu, L. Zhang, Y. Zhang, Z. Zhang, J. Chromatogr. A 1218, 8480 (2011)
Y. Shen, H. Zhang, L. Cheng, L. Wang, H. Qian, X. Qi, Food Chem. 194, 1003 (2016)
T.T.N. Tran, D.P. Tran, V.C. Nguyen, T.D.T. Tran, T.T.T. Bui, J.H. Bowie, J. Pept. Sci. 27, 1 (2021)
R. Van der Werf, C. Marcic, A. Khalil, S. Sigrist, E. Marchioni, LWT Food Sci. Technol. 58, 77 (2014)
S. Tohma, D. Günal-Köroğlu, S. Turan, M.F. Ramadan, Rend. Lincei 32, 585 (2021)
L. Barros, R.C. Calhelha, J.A. Vaz, I.C.F.R. Ferreira, P. Baptista, L.M. Estevinho, Eur. Food Res. Technol. 225, 151 (2007)
N. Gharred, N. Baaka, N. Bettache, A. Hamdi, A. Dbeibia, H. Dhaouadi, A. Morere, C. Menut, S. Dridi-Dhaouadi, Waste and Biomass Valorization 12, 5065 (2021)
B. Sik, E.L. Hanczné, V. Kapcsándi, Z. Ajtony, J. Pharm. Biomed. Anal. 184, 113173 (2020)
P. Panja, Curr. Opin. Food Sci. 23, 173 (2017)
C. Sun, Z. Wu, Z. Wang, H. Zhang, Evidence-based complement. Altern. Med. 2015, 1–10 (2015)
M. Herrero, M. Plaza, A. Cifuentes, E. Ibáñez, J. Chromatogr. A 1217, 2512 (2010)
S. Rodríguez-Rojo, A. Visentin, D. Maestri, M.J. Cocero, J. Food Eng. 109, 98 (2012)
S. Vijayalaxmi, S.K. Jayalakshmi, K. Sreeramulu, J. Food Sci. Technol. 52, 2761 (2014)
M.E. Cuvelier, H. Richard, C. Berset, JAOCS J. Am. Oil Chem. Soc. 73, 645 (1996)
L. Almela, B. Sánchez-Muñoz, J.A. Fernández-López, M.J. Roca, V. Rabe, J. Chromatogr. A 1120, 221 (2006)
J.A. Barreto Peixoto, G. Álvarez-Rivera, R.C. Alves, A.S.G. Costa, S. Machado, A. Cifuentes, E. Ibáñez, M.B.P.P. Oliveira, Antioxidants 10, 1 (2021)
E. Ibañez, A. Kubátová, F.J. Señoráns, S. Cavero, U. Reglero, S.B. Hawthorne, J. Agric. Food Chem. 51, 375 (2003)
F.J. Señoráns, E. Ibañez, S. Cavero, J. Tabera, G. Reglero, J. Chromatogr. A 870, 491 (2000)
S. Cavero, L. Jaime, P.J. Martín-Álvarez, F.J. Señoráns, G. Reglero, E. Ibañez, Eur. Food Res. Technol. 221, 478 (2005)
Y. Zhang, J.P. Smuts, E. Dodbiba, R. Rangarajan, J.C. Lang, D.W. Armstrong, J. Agric. Food Chem. 60, 9305 (2012)
R.G. Bitencourt, F.A. Cabral, A.J.A. Meirelles, J. Chem. Thermodyn. 103, 285 (2016)
M. Plaza, T. Pozzo, J. Liu, K.Z. Gulshan Ara, C. Turner, E. Nordberg Karlsson, J. Agric. Food Chem. 62, 3321 (2014)
C.G. Pereira, L. Barreira, S. Bijttebier, L. Pieters, C. Marques, T.F. Santos, M.J. Rodrigues, J. Varela, L. Custódio, Sci. Rep. 8, 1 (2018)
İ Gulcin, Antioxidants and antioxidant methods: an updated overview. Arch. Toxicol. 94, 615 (2020)
F.Y. Ning Meng, K. Chen, Y. Wang, J. Hou, W. Chu, S. Xie, C. Sun, Molecules 2022, 1 (2022)
A. Tajner-Czopek, M. Gertchen, E. Rytel, A. Kita, A.Z. Kucharska, A. Sokół-Łętowska, Antioxidants 9, 1 (2020)
G.P. Amaral, C.R. Mizdal, S.T. Stefanello, A.S.L. Mendez, R.L. Puntel, M.M.A. de Campos, F.A.A. Soares, R. Fachinetto, J. Tradit. Complement. Med. 9, 383 (2019)
N. Erkan, Food Chem. 133, 775 (2012)
H.C. Yurttas, H.W. Schafer, J.J. Warthesen, J. Food Sci. 65, 276 (2000)
R. Gyawali, S.A. Ibrahim, Food Control 46, 412 (2014)
E. Bouloumpasi, M. Hatzikamari, A. Lazaridou, P. Chatzopoulou, C.G. Biliaderis, M. Irakli, Biol. Life Sci. 6, 47 (2022)
J. Del Campo, M.J. Amiot, C. Nguyen, J. Food Prot. 63, 1359 (2000)
Q. Liu, X. Meng, Y. Li, C.N. Zhao, G.Y. Tang, H. Bin Li, Int. J. Mol. Sci. 18, 1 (2017)
M. Matejczyk, R. Świsłocka, A. Golonko, W. Lewandowski, E. Hawrylik, Adv. Med. Sci. 63, 14 (2018)
V. Lax, M.C. Rota, S. Lora, J.A. Sotomayor, Antioxidants 3, 439 (2012)
M. Nadeem, M. Imran, T.A. Gondal, A. Imran, M. Shahbaz, R.M. Amir, M.W. Sajid, Appl. Sci 9, 3139 (2019)
Acknowledgements
The authors sincerely thank the FSO analytical platform for its help in the analyses and we would like also to thank Cluster Valbiom Maroc for their funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ziani, I., Bouakline, H., Yahyaoui, M.I. et al. The effect of ethanol/water concentration on phenolic composition, antioxidant, and antimicrobial activities of Rosmarinus tournefortii de Noé hydrodistillation solid residues. Food Measure 17, 1602–1615 (2023). https://doi.org/10.1007/s11694-022-01722-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01722-6