Abstract
The release of aqueous residues generated by the extraction process of essential oils presents a real risk of environmental pollution. This work aims to reduce this risk and produce value-added materials. The aqueous residue of Dittrichia graveolens (D. graveolens) hydrodistillation has been reused in two valorization ways: 1/in the ecological dyeing. 2/in biological field. First, a phytochemical study of the aqueous residue was carried out by determining the content of polyphenols (237 mg EqAG g−1) and flavonoids (91 mg EqC g−1). Second, HPLC analysis allowed the identification and evaluation of catechin (5.92 mg g−1 of extract) and quercetin (4 mg g−1 of extract) as two of the coloring molecules present in this aqueous residue. Third, the eco-dyeing process with the aqueous residue was performed on the polyamide fabric, the process was optimized by the surface response methodology using Minitab software. Thus, the optimum dyeing conditions were evaluated at pH, temperature and duration of 3, 80 °C and 90 min, respectively, giving a maximum value of K/S color yield (equal to 7.5). Fourth, the dyeing process was evaluated by measuring fastness tests for the optimal conditions. Finally, the aqueous residue was assessed for its antioxidant, antibacterial, anti-inflammatory and cytotoxic potential from where it is proven that it could be a source of the bioactive compounds.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The extraction of essential oils generates significant amounts of colored wastewater which can constitute a risk of environmental pollution. In this context and for the first time, the aqueous residue from D. graveolens hydrodistillation process was reused as a dye bath for textile fabrics. The biological potential of this aqueous residue was also evaluated for the first time in a perspective of research on smart textiles in the health field. The promising coloring power and biological potential of the aqueous residue from D. graveolens hydrodistillation have shown that the latter could be an interesting source of environmentally friendly natural dyes and bioactive products.
Introduction
The textile dyeing and the essential oil extraction industries are among the most successful industries in the Mediterranean basin [1, 2]. Although their fields of activity are not related, these two types of industries have in common, in addition to their geographical proximity, their considerable volume of their colored water discharges. Indeed, the amount of water generated by dyeing one kilogram of fabric is about 60 L, depending on the dyeing process [3]. This water, strongly colored, represents a real ecological disaster that pushes more and more "eco-conscious" consumers to boycott textile products containing synthetic dyes. The same is true for buyers of essential oils who are generally health conscious and who realize that the product they have purchased is obtained through a non-ecological process. Indeed, the hydrodistillation extraction of one milliliter of oil requires the consumption of 0.5–1 L of water [4,5,6]. At the end of the extraction process, the colored residual water is totally discharged in the environment without appropriate color removal treatment.
In the face of this ecological awareness that characterizes the first half of the twenty-first century, scientific research has increasingly focused on the development of cleaner industrial processes while following the measures provided by cleaner production which aims to reduce waste and emissions while improving productivity and thus reducing overall production costs. The concept of ecological industry is considered as a strategy for cleaner production, it focuses on industrial exchange using waste or by-products of a process as an input for another process, it so contributes to increase the sustainability of resources and reduce human and environmental impacts [7]. Thus, in the field of textile dyeing, researchers such us Shu et al. [8] focused their research on replacing the pad-steam dyeing process by a cleaner process “pad-batch-steam dyeing”, which aims to reduce interactions between reactive dyes and molecules of water, which ultimately leads not only to a high speed and rate of the dyes fixation, but also to a reduction of the textile effluent amount. Other researchers have focused their attention on liquid and solid waste management strategies in leather processing using silica gel and boric acid as environmentally friendly methods of curing and cleaner preservation, as well as using enzymatic dehairing instead of sulfide and biodegradation combined with ozonation techniques to reduce the BOD, COD and the toxicity of effluents due to chrome, tannins, azo-dyes and phenols [9].
Several studies have demonstrated the threat that synthetic dyes can cause to the environment and human health. It is revealed that reactive dyes can be a factor of acute toxicity causing allergic respiratory or skin reactions, it is also suggested that azo molecules are responsible for genotoxicity [10,11,12]. By coming into contact with the skin during perspiration, the molecules absorbed release carcinogenic amines which lead to allergic and carcinogenic responses. On the other hand, studies have proven the infection of plants which is due to the synthetic dyes present in the dye effluents and therefore the consumption of infected vegetables affects human health as well as the toxicity of the reactive dyes lead to the death of soil microorganisms, which poses a threat to agricultural productivity. Organisms in the aquatic ecosystem are also exposed to the hazards related to synthetic dyes: Toxicological and histopathological impacts on fish and changes in biochemical parameters of algae have been proven. A decrease in photosynthesis and ecological imbalance in the food chain have been observed following the creation of a layer by the colored effluent which prevents the penetration of sunlight to aquatic organisms [12]. For these reasons, some researchers such us Ben Ticha et al. [13], Kan et al. [14] and Yusuf et al. [15, 16] have conducted extensive works to replace synthetic dyes, environmentally toxic, with natural dyes such as anthocyanins extracted from Brassica oleracea L., kamala dye from Mallotus philippensis and anthraquinone extracted from Rubia cordifolia while achieving excellent dyeing performance. Natural dyes not only have a high degradability, sustainability and respect for the environment [17], but also possess important biological and medical properties such us ultraviolet protective [18], anti-inflammatory, wound healing, anti-viral [19, 20], antimicrobial [21], antiallergic [14] … that can therefore serve as a green alternative to synthetic dyes. Other researchers have concentrated their efforts on minimizing water consumption and even replacing it with a so-called green solvent: supercritical CO2 [22]. Goto et al. [23] have extracted a fraction enriched in natural dyes such as chlorophyll and carotenoids like “lutein and β-carotene” from Algae using supercritical CO2 as an alternative liquid solvent.
The idea behind this work is to reduce the environmental impact of the essential oil extraction process by two complementary ways of valorisation: textile fiber dyeing by the aqueous residue of the hydrodistillation rich in colored substances and highlight the biological potential of the aqueous extract.
Inula is a vegetal genus that belongs to the Asteraceae family. It includes a variety of about 100 species, and is widely distributed in the Mediterranean basin. Species of this genus have been reported in the literature as having ethnopharmacological applications, to treat a wide range of disorders, mainly respiratory, digestive, inflammatory, dermatological, cancer and microbial diseases [24] thanks to their secondary metabolites such as flavonoids, sesquiterpenes and essential oils… Inula graveolens (L.) Desf. (Synonym: Dittrichia graveolens L. Greuter) is an annual aromatic plant with a foul camphor odor, it blooms from June to August. It is a nitrophilic species, growing on cultivated land, abandoned fields, roadsides and rural areas. Its leaves are oval and pointed and its flowers have yellow petals. Several studies concerning D. graveolens from different geographic zones have been reported and revealed its important pharmacological effects. Indeed, the D. graveolens ethanolic and methanolic extracts from Iraq, Jordan, Tunisia or Turkey showed antioxidant [25], antiproliferative [26], allelopathic, antifungal [27], cytotoxic and antibacterial [28] activities respectively. Moreover, the D. graveolens essential oil thanks to its wide use in aromatherapy was developed on a commercial scale [29] under the name "odorous Inula"; this essential oil is recognized for its powerful actions on the respiratory system: mucolytic, expectorant, anticatarrhal and antitussive action and it is also known as a regulator and cardiac tonic. The distillation of the essential oil generates a considerable quantity of colored water discharge and according to the literature, no study has demonstrated the importance of these residues in textile dyeing. We therefore chose to focus for the first time on the reuse in an environmentally friendly way of the aqueous residue from the hydrodistillation of Tunisian "Inula graveolens" as a dye bath and evaluated its biological potential.
In this study and first of all, a chemical characterization of the aqueous residue was evaluated. Secondly, the aqueous residue was valorized as a dye bath for the polyamide fabric, the dyeing process was optimized thanks to Minitab 18 software using the response surface methodology (RSM). The color strength parameter (K/S) and the fastness values were determined for the optimum dyeing conditions. Finally, the antioxidant, antibacterial, cytotoxic and anti-inflammatory activities were evaluated in order to estimate the biological potential of the hydrodistillation aqueous residue.
Materials and Methods
The Plant
The plant Dittrichia graveolens was selected by Dr. Ridha El Mokni, a researcher in botany at the faculty of pharmacy of Monastir, Tunisia. The voucher specimen was deposited at the herbarium (Asteraceae, n°23) of horticulture and breeding school of Chott-Meriem (University of center, Sousse, Tunisia). The plant was harvested in November 2015 in an olive grove in Monastir (Tunisia) where it grows wild. The leaves and flowers were separated from the stems to be dried in the dark and at room temperature. The dried leaves and flowers were then milled and used for the remainder of this study.
Aqueous Residue Preparation
The previously dried and ground leaves and flowers (100 g) were added to distilled water (1 L) and placed in a Clevenger apparatus to hydrodistillate the solid material for 3 h. The essential oils obtained is the subject of an independent study. The residual mixture (plant material + water) was filtered under vacuum to recover a colored aqueous extract whose concentration is 100 mg of dry matter per mL of water.
Phytochemical Study of the Aqueous Residue of D. graveolens Hydrodistillation
The total polyphenols were estimated using the Folin–Ciocalteu reagent according to a protocol described previous [30]. On the other hand, the flavonoid contents were determined by the method developed by Bouzidi et al. [31].
The Ultraviolet–visible (UV–vis) spectrum of the aqueous extract was obtained using a Campspec M 108 spectrophotometer.
The HPLC spectra of the aqueous residue were performed using an Agilent 1200 Series HPLC System. The analysis was carried out according to the following protocol [32]: Initially, 10 µL of the extract to be separated are injected at the inlet of the column (reversed phase C18, 100 × 4.6 mm × 2.6 microns). The mobile phase, consisting of two eluents containing 0.1% acetic acid: phase A (water) and phase B (acetonitrile), flows at high pressure at 2 mL min−1. At the column outlet, the detection of two main phenolic compounds, quercetin and catechin, was carried out using an UV–vis detector set at 254 and 280 nm, respectively. For this purpose, the analytical method by standard addition was used [33].
The Infrared (IR) spectrum of coloring powder obtained after lyophilization of the aqueous D. graveolens extract was carried out by a Perkin Elmer FTIR infrared spectrometer.
Dyeing Quality Evaluation by the Aqueous Residue
Dyeing Protocol with the Aqueous Residue
The polyamide (jersey and weight of 302 g m−2) was chosen following the results obtained by the preliminary dyeing tests of a multifiber fabric (see Fig. S1). The dyeing process was carried out in a laboratory-dyeing machine (Ahiba Datacolor International, USA) at 60 °C for 60 min with a liquid ratio of 40:1. The dyed fabric was then rinsed with warm water and soaped with a nonionic detergent. It was finally washed again with cold water and dried at room temperature.
Infrared Spectroscopy Analysis
The IR spectra of the textile fibers before and after the dyeing were carried out using the Perkin Elmer FTIR infrared spectrometer.
Color Measurement and Fastness Testing
The dyeing quality was evaluated using the color strength parameter (K/S) measured by SpectroFlash SF300 spectrophotometer (Datacolor International, USA) using D65 and 10° standard observer. The (K/S) values were calculated at 410 nm using the Kubelka–Munk equation [34]:
where R is the decimal fraction of the reflectance of the dyed fabric, R0 is the decimal fraction of the reflectance of the undyed fabric, K is the absorption coefficient and S is the scattering coefficient.
Specific tests include color fastness to washing according to ISO 105-C06, colorfastness to rubbing ISO 105-X12 and colorfastness to light ISO 105-B02.
Experimental Design and Optimization
Optimisation studies were conducted using response surface methodology (RSM) and Minitab 18 software (Version18, State College, PA, USA). To evaluate the effect of each selected experimental parameter on the results obtained, regression and variance analysis (ANOVA) was used. The experiments were established based on a Central Composite Design Method (CCD) for three factors (temperature, duration and pH) and three levels.
Evaluation of the Biochemical Potential of the Aqueous Residue of D. graveolens Hydrodistillation
The sample performance for all the tests was assessed by performing triplicate assays in the same situation.
Anti-oxidant Activity
The antioxidant activity was evaluated by two methods: DPPH (2,2-diphenylpicrylhydrazyl) radical scavenging test which allows the evaluation of the anti-radical capacity of a sample and ORAC (Oxygen Radical Absorbance Capacity) test which measures the capacity of a sample to inhibit the oxidation of a target molecule (fluorescein) induced by a source of radicals.
Assay of DPPH Radical Scavenging Activity
The antioxidant activity of the aqueous extract of D. graveolens was evaluated by the DPPH radical scavenging assay as described by Yu et al., 2008 [35]. The inhibition percentage (% IP) of DPPH radicals was calculated by the following formula [36]:
where Abs0 is the absorbance of the negative control (0.1 mM DPPH solution) at 517 nm and Absi is the absorbance, at the same wavelength, of 0.1 mM DPPH solutions containing different concentrations (0–0.12 mg mL−1) of the sample to be tested.
The inhibitory concentration (IC50), which corresponds to the amount of the sample required to remove 50% of the DPPH groups, could thus be determined graphically.
ORAC Test
ORAC test was carried out according to the method developed by Cao et al. (1993) [37] and improved by Ou et al. (2001) [38], using 2,2′-azobis-(2-amidinopropane)-dihydrochloride (AAPH) as a peroxyl radical generator and fluorescein as a fluorescent probe.
AAPH (414 mg) was dissolved in 10 mL of 75 mM Phosphate Buffered Saline (PBS) (pH 7.4); a fluorescein stock solution 4 μM was prepared in 75 mM PBS buffer and kept at 6 °C; this stock solution was diluted 1000 times immediately prior to use. Measurements were performed using a 96-well microplate; 150 μL of the fluorescein solution was added in all experimental wells; 25 μL of sample solutions prepared at different concentrations were added while 25 μL of buffer was used for the blank. After incubation at 37 °C during 30 min, the reaction was initiated by the addition of 25 μL of the AAPH solution; the intensity of fluorescence was recorded every 1 min for 2 h at respective excitation and emission wavelengths of 483 and 530 nm, using a microplate reader of BMG LABTECH type, CLARIOstar.
The influence of a given sample on the degradation of fluorescein may be evaluated by measuring the area under the fluorescein quenching curve, with or without antioxidant.
The area under the curve (AUC) was calculated as follows:
where I is the intensity of the fluorescence, t0 is the time at 0 min and tn the time at n min. Interpreting the ORAC analysis data involves calculating the AUC net:
Antibacterial Activity
Antibacterial activity was evaluated for the aqueous extract against Vibrio parahaemolyticus ATCC17802, Vibrio alginolyticus ATCC17749, Staphylococcus epidermidis CIP3106510 and Escherichia coli ATCC35218 using disk-diffusion tests.
Bacterial suspensions (106 CFU mL−1) were inoculated on the surface of Mueller Hinton agar plates. A first test consists of placing 10 µL of the diluted extract (10 mg mL−1) on filter disks inoculated with Mueller Hinton agar (Biorad, France). The inhibition zone diameters around each disk were measured after 18 h of incubation at 37 °C. Chloramphenicol solution (Sigma-Aldrich, Switzerland) was used as a reference.
Anti-inflammatory Activity
Anti-inflammatory activity was conducted using Swiss mice (20–25 g) of 6–8 weeks old. The animals were treated in accordance with guidelines established by the European Union for the Use and Care of animals (CEC Council 86/609).
The investigation of anti-inflammatory properties was carried out according to the method described by Kou et al. (2005) [39] slightly modified. The mice are divided into several lots, each comprising 6 mices: one batch (negative control) receives nothing, two batches representing positive control (reference): the first receives dexamethasone and the second receives aspegic (15 mg Kg−1), the last batch receives the extract in different doses (5 and 10 mg Kg−1).
A thirty minutes period after intraperitoneal administration of the extract or dexamethasone, 30 µL of xylene (phlogogenic agent) were applied to the internal and external surfaces of the right ear of each mouse. The left ear was considered a witness. The thickness of the ear was measured using a digital caliper three hours after the induction of inflammation. The difference in thickness between the two ears was determined. The percentage inhibition of edema compared to the control group was calculated according the following formula:
where Et is the average edema in the treated groups and Ec is the average edema in the untreated group (control group).
Cytotoxic Test
A cell line of skin healthy human fibroblast CCD-45 SK ((ATCC® CRL 1506) was used in order to investigate the cytotoxicity effect of the D. graveolens aqueous extract. This test was carried out according to the method described by Noudogbessi et al. (2014) [40], using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) as reagent. This method is based on the capacity cells to reduce MTT to a colored product: formazan. The cells were seeded in a 96-well microplate at the rate of 2000 cells per well in 150 µL of culture medium, followed by a 24 h incubation. The cells were then incubated with the aqueous extract for 72 h at concentrations between 0.005 and 0.2 mg mL−1. Briefly, 15 µL was added per well of MTT (0.5 mg mL−1) followed by a 4 h incubation. The appearance of crystals is proportional to the number of living cells. The supernatant was removed, the crystals were dissolved in 150 μL of an ethanol/dimethyl sulfoxide (DMSO) solution (50:50) and its absorbance was measured by a Multiskan microplate reader (Thermo Scientific, Courtaboeuf, France) at 540 nm.
Results and Discussion
Phytochemical Study of the Hydrodistillation Aqueous Residue
The aqueous residue of the hydrodistillation of D. graveolens, characterized by a yellowish-brown color, has a concentration of total polyphenols and flavonoids of 237 mg EqGA g−1 extract and 91 mg EqC g−1 extract, respectively. Figure A.1 shows the UV–visible and infrared spectra of the aqueous residue which confirm the presence of flavonoids. Indeed, the UV–visible spectrum (Figure A.1(a)) has two characteristic absorbance peaks at 290 and 330 nm. The first peak can be attributed to the benzoyl function while the second one is related to the cinnamoyl acid form of the molecules [41]. On the other hand, the infrared spectrum of the aqueous residue (Figure A.1(b)) reveals the characteristic bands relating to flavonoids such as: a broad band attributable to the hydroxyl functions at 3400 cm−1.
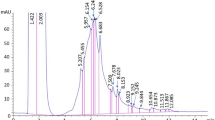
A more specific exploration of flavonoid content in the aqueous residue was carried out by HPLC using two standards: quercetin and catechin. Indeed, these two molecules are known to be responsible for the yellow color of certain flowers [42]. Thus, Fig. 1 shows the chromatograms of the hydrodistillation aqueous residue in which quercetin and catechin (Fig. 2) were, respectively, identified as coloring molecules [43]. The amount of quercetin and catechin in the aqueous extract was evaluated at 4 mg g−1 of extract and 5.92 mg g−1 of extract respectively.
Textile Dyeing Using the Hydrodistillation Aqueous Residue
Dyeing of Multifiber Fabric: Tests of Validation
The aqueous extract of the mixture of leaves and flowers of D. graveolens was tested to dye a piece of multifibre fabric. Figure A.2 gives the result of this test and shows that brown, light yellow and mustard yellow colorations can be obtained on wool, cellulose acetate and polyamide, respectively. Based on the preliminary tests, further studies are only focused on the polyamide fibers dyeing which gave the deepest shade when dyed with hydrodistillation aqueous residue.
Catechin and quercetin are the two main coloring molecules identified during the chemical characterization of the aqueous extract. In order to validate their contributions in the dyeing process, three synthetic dyebaths were prepared containing a known amount of each of the molecules taken alone and as a mixture. Table 1 shows the results of the polyamide dyeing by the three synthetic dyebaths as well as that performed by the hydrodistillation aqueous residue. When comparing the different shades of color obtained, it seems that catechin and quercetin, present in the aqueous residue of the hydrodistillation of the leaves and flowers of the D. graveolens plant, are the main molecules responsible for the dyeing of polyamide fabrics.
Influence of the pH on the Coloring Power
Once the coloring molecules identified, it becomes possible to study the influence of the pH on the coloring power and to propose a dyeing mechanism. Indeed, Table 2 shows the effect of varying the pH of the aqueous extract of D. graveolens hydrodistillation on polyamide fiber dyeing. The results reveal that the aqueous extract loses its coloring power at neutral and basic pH.
The UV spectra of the aqueous extract at different pHs presented in this same Table 2, highlight the effect of pH on the dyeing process. Indeed, the pH increase causes a decrease in the absorbance of the aqueous residue. So according to this observation, supported by the literature [16], hydroxyl groups (OH) of the dye molecules and the amide functions of the fiber are directly involved in the dyeing mechanism, probably through hydrogen bonds.
Modelling and Optimization of the Dyeing Process
The experimental design included fifteen polyamide dyeing experiments, which were conducted according to the scheme mentioned in Table 3.
The performance of the dyeing process of polyamide fabric using the aqueous extract of D. graveolens was evaluated by measuring the strength parameter (K/S), which is dependent on the following input factors: the pH of the dye bath (3, 5 and 7), the dyeing temperature (40, 60 and 80 °C) and the dyeing duration (30, 60 and 90 min).
Response Surface Methodology Regression
The matrix design and the corresponding results of RSM experiments were shown in Table 3. The regression analysis of the experimental data displayed that the correlation between the response variables and the test variables was established by the following second-order polynomial equations:
where K/S is the strength parameter, T(°C) is the dyeing temperature and t(min) is the dyeing duration.
The model has a regression coefficient R2 = 98.98%, which indicates good predictability in the chosen range of variables.
Variance Analysis (ANOVA)
A variance analysis was performed to evaluate the main effects of the factors influencing the performance of the dyeing process. Indeed, p values (given in Table A.1) lower than 0.05 indicate that the model and the estimated parameters are statistically significant [44]. According to Table A.1, the regression model obtained from Eq. (3) is highly significant. Moreover, it seems that the factors pH and temperature are statistically significant followed by the factor duration (p = 0.047). However, the interaction between temperature and duration dyeing is statistically not significant (p = 0.11).
Analysis of the Contour Plots
The analysis of the effects of the different parameters on the dyeing performance is detailed in the supplementary data (Annex A.1). The results are given in Fig. 3 Analysis of the contour plots was used to estimate the optimal values for the studied response (K/S). Figure 3 shows the variation of the color yield as a function of two variables while keeping the third constant. The results suggest that high K/S values can be reached for high temperatures (80 °C) and low pH (3) while the variation in the dyeing time did not significantly affect the response.
Response Optimization and Validation of the Model
Optimization of the response was also performed using the Minitab software. The objective is to determine the optimal experimental conditions that give the maximum strength color (K/S) obtained by dyeing the polyamide with the D. graveolens aqueous extract. The results are summarized in Fig. 4 and indicates that the optimum level is reached for a dye bath at pH 3, for a temperature of 80° C and a dyeing duration time of 90 min. These optimal conditions lead theoretically to a colour strength (K/S) equal to 7.50. To validate this result, a polyamide dyeing test with the aqueous extract of D. graveolens under the optimum conditions given above was carried out in triplicate. An average value of K/S equal to 7.49 was found, which indicates that the experimental result corresponds to the theoretical optimum.
Hence, the model is validated. Fastness properties (washing, light and rubbing) of the polyamide fabric dyed with the aqueous extract of the D. graveolens are given in Table 4. The results show that the resistance to washing and rubbing are excellent. However, the light resistance is less good, which, according to the literature, is one of the main limits of natural dyeing [45]. However, post-treatments can be considered to improve the light resistance [46].
Evaluation of biochemical activities
Anti-oxidant Activity
Assay of DPPH Radical Scavenging Activity
The chemical characterization of the aqueous extract generated by the hydrodistillation of D. graveolens revealed the presence of polyphenols and flavonoids which are known for their antioxidant power; the latter has been evaluated for the aqueous extract and the result is given in Fig. 5.
Compared to the quercetin solution, which is the positive reference, the aqueous extract has a significant antioxidant power. Indeed, the inhibitory concentration IC50, was evaluated at 0.022 mg mL−1 for the aqueous extract against 0.013 mg mL−1 for the standard solution.
ORAC Test
The principle of the ORAC test is to assess the "protective" effect of an antioxidant sample on the degradation process of fluorescein, initiated by the addition of AAPH as radical peroxyl generators (ROO·). The reaction is simple in concept but complex in practice. A competition between the reaction of targets and antioxidants with ROO· forms the basis of the test: Fluorescein is an intensely fluorescent target in its native form. When attacked by peroxyl radicals, the fluorescence is lost. The antioxidants slow the loss of fluorescence by quenching peroxyl radicals via the transfer of hydrogen atoms or the addition of radicals [47]. The reaction is followed by recording the fluorescence over time as shown in the Fig. 6a. The curve in the Fig. 6b represents the measurement of the AUCnet at different concentrations of the aqueous extract. A strong reactivity of the aqueous extract is observed at concentrations between 0.01 and 0.05 mg mL−1, leading to AUCnet of 90 at 0.05 mg mL−1. This can be explained by the high content of polyphenols present in the aqueous extract and which are known for their antioxidant potential.
The evaluation of the anti-free radical and antioxidant power revealed important results at convergent concentrations and this could be correlated to the data obtained according to the test of the content of phenols/reducing agents.
Antibacterial Activity
The main results of the antibacterial tests of the aqueous extract are gathered in Table 5.
These tests highlight the important antibacterial activity of the aqueous extract mostly against Vibrio alginolyticus, Vibrio parahaemolyticus and Staphylococcus epidermidis with inhibition zone diameters of 21, 18 and 17 mm respectively at concentration of 10 mg mL−1.
Anti-inflammatory Activity
The in-vivo anti-inflammatory activity of the aqueous residue was evaluated by xylene-induced ear edema in mice model. As the Fig. 7 shows, a weak inhibition of the control due to a developed increase in the average thickness of the ear following the topical application of xylene on the right ear of the control group. Whereas, the aqueous extract, 30 min before the application of xylene, significantly suppressed the edema of the ear compared to the control group with the highest percentage of inhibition at the 10 mg kg−1 (75.59%). The aqueous extract also showed a high anti-inflammatory potential compared to those of dexamethasone, the reference drug, the effect of which did not exceed 53.49%, contrary to aspegic reference which presents the highest anti-inflammatory potential (91.48%).
Previous research on the genus Inula has indicated that the characteristic compounds of this genus are sesquiterpenes lactones [24]. The major part of experimental studies is focused on these products, in particular their cytotoxic and anti-inflammatory activities [24]. In addition, experimental studies of D. graveolens from Turkey have focused on isolated lactone sequiterpenes such as ivalin, 8-epi-inuviscolide, 8-epi-xanthatin-1β, 5β-epoxide, which are involved in the cytotoxic and antibacterial effects [28], in addition to flavonoids and terpenoids which revealed an antiproliferative effect of Jordan D. graveolens [26]. Consequently, the significant biological effects (antibacterial, cytotoxic and anti-inflammatory effects) of the aqueous residue obtained from the hydrodistillation of D. graveolens could be attributed to the presence of these products.
Cytotoxic Test
The cytotoxicity of the aqueous extract was carried out against healthy skin fibroblasts CCD-45 SK (ATCC® CRL 1506). After having studied the dye power and the biological potential of the aqueous residue of hydrodistillation, it is interesting to know its toxicity on healthy fibroblast cells of the skin. The results shown in Fig. 8 proved the non-cytotoxicity of the aqueous extract towards healthy skin fibroblasts in concentration range (≤ 0.2 mg mL−1) showing anti-free radical and antioxidant activities. This could be a factor which reinforces its added value.
Conclusion
The aqueous extract generated by the hydrodistillation of D. graveolens, the discharge of which presents a risk of environmental pollution, was used for the dyeing of the polyamide fiber and was evaluated for its biochemical potential. Thanks to its high content in polyphenolic compounds such as flavonoids, the aqueous residue revealed a good tinctorial power and the dyeing process was consequently optimized. Moreover, significant antioxidant, antibacterial and anti-inflammatory activities and non-cytotoxicity against healthy skin fibroblasts have been found for the aqueous residue.
Finally, the results obtained in this study are promising to revive the age-old art of dyeing with natural stuffs and reduce the risks of pollution that these liquid residues can cause. By the way, this encourage us to go ahead in the development of research on smart textiles taking advantage of the biological safety that this Tunisian D. graveolens aqueous residue revealed.
Availability of Data and Materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HPLC:
-
High performance liquid chromatography
- D. graveolens :
-
Dittrichia graveolens
- UV–vis:
-
Ultraviolet–visible
- IR:
-
Infrared
- RSM:
-
Response surface methodology
- CCD:
-
Central composite design
- ANOVA:
-
Analysis of variance
- DPPH:
-
2,2-Diphenylpicrylhydrazyl
- ORAC:
-
Oxygen Radical Absorbance Capacity
- IC:
-
Inhibitory Concentration
- AAPH:
-
2,2-Azobis-(2-aminopropane)-dihydrochloride
- PBS:
-
Phosphate buffered saline
- AUC:
-
Area under the curve
- CFU:
-
Colony forming unit
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- DMSO:
-
Dimethyl sulfoxide
- mg Eq GA g− 1 extract:
-
Milligram of equivalents of gallic acid per gram of extract
- mg Eq C g− 1 extract:
-
Milligram of equivalents of catechin per gram of extract
- T:
-
Temperature
- t:
-
Duration
References
Mediterranean Action Plan. Pollution prevention in the textile industry within the Mediterranean region (2002)
Baser, K.H.C., Buchbauer, G. Sources of essential oils. In: Handbook of Essential Oils: Science, Technology, and Applications, Second Edition (2015). https://doi.org/10.1201/b19393
Bhatt, P., Rani, A.: Textile dyeing and printing industry: An environmental hazard. Asian Dye 10, 51–54 (2013)
Wu, C., Wang, F., Liu, J., Zou, Y., Chen, X.: A comparison of volatile fractions obtained from Lonicera macranthoides via different extraction processes: ultrasound, microwave, Soxhlet extraction, hydrodistillation, and cold maceration. Integr. Med. Res. 4, 171–177 (2015)
Filly, A., Fabiano-Tixier, A.S., Louis, C., Fernandez, X., Chemat, F.: Water as a green solvent combined with different techniques for extraction of essential oil from lavender flowers. C. R. Chim. 19, 707–717 (2016)
Gharred, N., Dbeibia, A., Falconieri, D., Hammami, S., Piras, A., Dridi-Dhaouadi, S.: Chemical composition, antibacterial and antioxidant activities of essential oils from flowers, leaves and aerial parts of Tunisian Dittrichia Viscosa. J. Essent. Oil Res. 6, 582–589 (2019)
Jayasooriya, V.M.: Reducing anthropogenic environmental stresses: a review on cleaner production and industrial ecology. Manag. Environ. Qual. 29(3), 7–16 (2020)
Shu, D., Fang, K., Liu, X., Cai, Y., Zhang, X., Zhang, J.: Cleaner coloration of cotton fabric with reactive dyes using a pad-batch-steam dyeing process. J. Clean. Prod. 196, 935–942 (2018)
Kanagaraj, J., Senthilvelan, T., Panda, R.C., Kavitha, S.: Eco-friendly waste management strategies for greener environment towards sustainable development in leather industry: a comprehensive review. J. Clean. Prod. 89, 1–17 (2015)
Kasiri, M.B., Safapour, S.: Natural dyes and antimicrobials for green treatment of textiles. Environ. Chem. Lett. 12, 1–13 (2014)
Zahedi, M., Shakerian, A., Rahimi, E., Sharafati Chaleshtori, R.: Determination of synthetic dyes in various food samples of Iran’s market and their risk assessment of daily intake. Egypt J. Vet. Sci. 51(1), 23–33 (2020)
Yusuf, M.: Synthetic dyes: a threat to the environment and water ecosystem. In: Shabbir, M. (ed.) Textiles and Clothing, pp. 11–26 (2019)
Ben Ticha, M., Haddar, W., Meksi, N., Guesmi, A., Mhenni, M.F.: Improving dyeability of modified cotton fabrics by the natural aqueous extract from red cabbage using ultrasonic energy. Carbohydr. Polym. 154, 287–295 (2016)
Khan, S.A., Khan, M.I., Yusuf, M., Shahid, M., Mohammad, F., Khan, M.A.: Natural dye shades on woollen yarn dyed with Kamala (Mallotus philippinensis) using eco-friendly metal mordants and their combination. Colourage 58(11), 38–44 (2011)
Yusuf, M., Mohammad, F., Shabbir, M.: Eco-friendly and effective dyeing of wool with anthraquinone colorants extracted from Rubia cordifolia roots: optimization, colorimetric and fastness assay. J. King Saud Univ. Sci. 29(2), 137–144 (2017)
Yusuf, M., Mohammad, F., Shabbir, M., Khan, M.A.: Eco-dyeing of wool with Rubia cordifolia root extract: assessment of the effect of Acacia catechu as biomordant on color and fastness properties. Text. Cloth. Sustain. 2(1), 1–9 (2017)
Yusuf, M. (ed.): Handbook of Renewable Materials for Coloration and Finishing. Wiley, Hoboken (2018)
Yusuf, M., Shabbir, M., Mohammad, F.: Natural colorants: historical, processing and sustainable prospects. Nat. Prod. Bioprospect. 7(1), 123–145 (2017)
Khan, S.A., Yusuf, M., Agarwal, P., Prasad, L.: Chlorophylls as pigment: a contemporary approach. In: Handbook of Renewable Materials for Coloration and Finishing, pp. 115–123. Wiley, Hoboken (2018)
Yusuf, M., Khan, M.A., Mohammad, F.: Investigations of the colourimetric and fastness properties of wool dyed with colorants extracted from Indian madder using reflectance spectroscopy. Optik. 127(15), 6087–6093 (2016)
Comlekcioglu, N., Aygan, A., Kutlu, M., Kocabas, Y.Z.: Antimicrobial activities of some natural dyes and dyed wool yarn. Iran. J. Chem. Chem. Eng. 36(4), 137–144 (2017)
Baaka, N., Dhouibi, N., Dridi-Dhaouadi, S., Dhaouadi, H.: Comparative study between supercritical carbondioxide fluid technology and conventional water-based processing to dye minor elastin containing cotton fabrics. J. Nat. Fibers (2020). https://doi.org/10.1080/15440478.2020.1787922
Goto, M., Kanda, H., Machmudah, S.: Extraction of carotenoids and lipids from algae by supercritical CO2 and subcritical dimethyl ether. J Supercrit. Fluids 96, 245–251 (2015)
Seca, A.M.L., Grigore, A., Pinto, D.C.G.A., Silva, A.M.S.: The genus Inula and their metabolites: from ethnopharmacological to medicinal uses. J. Ethnopharmacol. 154, 286–310 (2014)
Al-Fartosy, A.J.: Antioxidant properties of methanolic extract from Inula graveolens L. Turk. J. Agric. For. 35, 591–596 (2011)
Abu-Dahab, R., Afifi, F.: Antiproliferative activity of selected medicinal plants of Jordan against a breast adenocarcinoma cell line (MCF7. J. Pharm. Sci. 75, 121–136 (2007)
Omezzine, F., Ladhari, A., Rinez, A., Haouala, R.: Allelopathic potential of Inula graveolens on crops and weeds. Allelopathy J. 28, 63–76 (2011)
Topçu, G., Öksüz, S., Shieh, H., Cordell, G.A., Pezzuto, J.M., BozokJohansson, C.: Cytotoxic and antibacterial sesquiterpenes from Inula graveolens. Phytochemistry 33, 407–410 (1993)
Blanc, M.C., Muselli, A., Bradesi, P., Casanova, J.: Chemical composition and variability of the essential oil of Inula graveolens from Corsica. Flavour Frag J. 19, 314–314 (2004)
Boveiri Dehsheikh, A., Mahmoodi Sourestani, M., Boveiri Dehsheikh, P., Vitalini, S., Iriti, M., Mottaghipisheh, J.: A comparative study of essential oil constituents and phenolic compounds of Arabian Lilac (Vitex Trifolia var. Purpurea): an evidence of season effects. Foods 8, 52 (2019)
Bouzidi, A., Benzarti, A., Arem, A.E., Mahfoudhi, A., Hammami, S.: Chemical composition, antioxidant and antimicrobial effects of Tunisian Limoniastrum guyonianum Durieu ex Boiss extracts. Pak. J. Pharm. Sci. 29, 1299–1305 (2016)
Farías-Campomanes, A.M., Rostagno, M.A., Coaquira-Quispe, J.J., Meireles, M.A.A.: Supercritical fluid extraction of polyphenols from lees: overall extraction curve, kinetic data and composition of the extracts. BIOB 2, 45 (2015)
Pistos, C., Vlachou, M., Theiakodimitri, V., Philippou, E., Petrou, M., Middleton, N., Athanaselis, S., et al.: Standard addition HPLC method for the determination of a-tocopherol in plasma samples of adolescent swimmers. Pharmakeftiki 26, 115–122 (2014)
Haddar, W., Elksibi, I., Meksi, N., Mhenni, M.F.: Valorization of the leaves of fennel (Foeniculum vulgare) as natural dyes fixed on modified cotton: a dyeing process optimization based on a response surface methodology. Ind. Crop. Prod. 52, 588–596 (2014)
Yu, L., Zhao, M., shui Wang, J., Cui, C., Yang, B., Jiang, Y., Zhao, Q.: Antioxidant, immunomodulatory and anti-breast cancer activities of phenolic extract from pine (Pinus massoniana Lamb) bark. Innov. Food Sci. Emerg. Technol. 9, 122–128 (2008)
Tian, W., Lin, Q., Liu, G.-Q.: In vitro antioxidant capacities of rice residue hydrolysates from fermented broth of five mold strains. J. Med. Plants Res. 6, 2396–2401 (2012)
Cao, G., Alessio, H.M., Cutler, R.G.: Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 14, 303–311 (1993)
Ou, B., Hampsch-woodill, M., Prior, R.L.: Development and validation of an improved oxygen radical absorbance capacity assay using Fluorescein as the fluorescent probe. J. Agric. Food Chem. 49, 4619–4626 (2001)
Kou, J., Ni, Y., Li, N., Wang, J., Liu, L., Jiang, Z.H.: Analgesic and antiinflammatory activities of total extract and individual fractions of Chinese medicinal ants Polyrhachis lamellidens. Biol. Pharm. Bull. 28, 176–180 (2005)
Noudogbessi, J.-P., Gary-Bobo, M., Adomou, A., Adjalian, E., Alitonou, G.A., Avlessi, F., Garcia, M., Sohounhloue, D.C.K., Menut, C.: Comparative chemical study and cytotoxic activity of Uvariodendron angustifolium essential oils from Benin. Nat. Prod. Commun. 9, 261–264 (2014)
Kheyar-kraouche, A.N., Bento, A.: Characterization by liquid chromatography-mass spectrometry and antioxidant activity of an ethanolic extract of Inula viscosa leaves. SC. J. Pharm. Biomed. Anal. 156, 297–306 (2018)
Bechtold, T., Mussak, R. (ed.): Handbook of Natural Colorants (2009)
Baliarsingh, S., Panda, A.K., Jena, J., Das, T., Das, N.B.: Exploring sustainable technique on natural dye extraction from native plants for textile: identification of colourants, colourimetric analysis of dyed yarns and their antimicrobial evaluation. J. Clean. Prod. 37, 257–264 (2012)
Enid, M., Carmona, R., Ferreira, S.G.: Biosorption of chromium using factorial experimental design. Process Biochem. 40, 779–788 (2005)
Faidi, K., Baaka, N., Hammami, S., ElMokni, R.: Extraction of carotenoids from Lycium ferocissimum fruits for cotton dyeing optimization survey based on a central composite design method extraction of carotenoids from Lycium ferocissimum fruits for cotton dyeing: optimization survey based on a central. Fiber Polym. 17, 36–43 (2016)
Moussa, I., Baaka, N., Khiari, R., Moussa, A., Mortha, G., Mhenni, M.F.: Application of Prunus amygdalus by-products in eco-friendly dyeing of textile fabrics. J. Renew. Mater. 6, 55–67 (2018)
Prior, R.L., Wu, X., Schaich, K.M.: Standard methods for the determination of antioxidant capacity and phenolics in foods and dietery supplements. J. Agric. Food Chem. 53, 4290–4302 (2005)
Acknowledgements
The authors thank the Tunisian Ministry of Higher Education and Scientific Research for its financial support. The authors are grateful for: Mr. Ridha Mokni (botanist from the faculty of pharmacy of Monastir, Tunisia) for providing the plant, Dr. Nizar Meksi (textile engineering teacher from national school of engineers of Monastir) for having revised the "textile" part of the manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization, Methodology, Writing—Original Draft: NG; Investigation NB, NB, AH and AD; Resources: HD; Supervision, Resources: AM and CM; Supervision, Project administration: SD-D.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical Approval
The animals were treated in accordance with guidelines established by the European Union for the Use and Care of animals (CEC Council 86/609).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gharred, N., Baaka, N., Bettache, N. et al. Wastewater to Ecological Dyeing Process and Bioactive Compounds Resources: Case Study of Dittrichia graveolens Hydrodistillation Aqueous Residue. Waste Biomass Valor 12, 5065–5077 (2021). https://doi.org/10.1007/s12649-021-01375-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01375-4