Abstract
To optimize storage quality of ‘d’Anjou’ pears (Pyrus communis L.) produced in the US Pacific Northwest regions, the recommended harvest maturity as indicated by flesh pressure is 67 to 58 N. Over-mature pears are more susceptible to superficial scald and inferior eating quality after storage. However, a significant portion of pears is harvested at an over-mature stage due to rapid on-tree maturation during years with labor shortages. 1-Methylcyclopropene (1-MCP) extends storage quality of pears but interferes their ultimate ripening capacity. The purpose of this work was to evaluate the effect of 1-MCP on over-mature ‘d’Anjou’ pears. Fruit from two orchards at fruit firmness (FF) 55.6 and 56.0 N were treated with 0.15 μL L−1 1-MCP, held at − 1.1 °C for up to 7 months. 1-MCP treatment significantly inhibited ethylene synthesis and respiration rate, reduced superficial scald, and maintained high storage quality of over-mature pears from two orchards during 7 months of storage. After 7 months of storage at − 1.1 °C, some of the pears from Orchard 1 recovered ripening capacity after 5 days at 20 °C. The development of superficial scald in over-mature ‘Anjou’ pears was associated with the accumulation of α-farnesene and conjugated trienols (CTols). 1-MCP significantly inhibited the development of scald, perhaps by alleviating membrane lipid peroxidation and enhancing total antioxidant capacity, antioxidant metabolites (i.e., total polyphenols (TP) and total flavonoids (TF)), and related enzyme activities (i.e., superoxide dismutase (SOD), catalase (CAT)) in pear skin. Overall, this study provides the preliminary basis for commercial application of 1-MCP in over-mature pears and demonstrates its potential to maintain fruit quality and reduce the incidence of superficial scald.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the US Pacific Northwest regions, ‘d’Anjou’ is the most widely produced European pear (Pyrus communis L.) with annual production of about 222 million kilograms. They are most enjoyed when ripened to a melting (buttery and juicy) texture at a warm temperature after removal from cold storage (Villalobos-Acuña and Mitcham 2008; Sugar and Basile 2013; Dong et al. 2018). Optimum storage conditions for ‘d’Anjou’ pears is ~ 5 months in regular air at − 1.1 °C or ~ 8 months in controlled atmosphere (CA) at − 1.1 °C with O2 at 1.5 kPa and CO2 at < 1 kPa. In optimal storage conditions, pears maintain quality attributes with minimal occurrence of storage disorders (Mellenthin et al. 1980; Chen et al. 1983). Superficial scald has been well documented as the physiological disorder of the greatest economic impact in ‘d’Anjou’ pears (Whitaker 2007; Yu and Wang 2017). It is the result of necrosis of the hypodermal cortical tissue and manifests as brown or black patches on the skin, appearing after storage (Lurie and Watkins 2012). Damaged to cells in the peel tissue is thought to be induced and exacerbated by conjugated trienols (CTols), the oxidation products of (E, E)-α-farnesene (Chen et al. 1990; Whitaker 2007). However, neither α-farnesene nor CTols accumulation is directly correlated with scald development (Whitaker et al. 2000; Calvo et al. 2015). A substantial number of references have shown that an efficient antioxidant system is involved in the development of scald (Barden and Bramlage 1994a, 1994b; Ahn et al. 2007; Tsantili et al. 2007; Rudell and Mattheis 2009; Zhao et al. 2016).
Current commercial control for scald in ‘d’Anjou’ pear relies on pre-storage application of antioxidant 1,2-dihydro-6-ethoxy-2,2,4-trimethyl-quinoline (ethoxyquin) (Hansen and Mellenthin 1979; Chen 1990). However, this control is inadequate and more significant storage losses due to scald occur in certain years (Wang 2016). Additionally, the European Union has withdrawn authorization for plant protection products containing ethoxyquin (Xie et al. 2014). Alternatives to ethoxyquin are required. Ethylene biosynthesis triggers the synthesis of α-farnesene and induces the accumulation of CTols, resulting in the development of scald (Fan et al. 1999; Gapper et al. 2006; Jung & Watkins 2008; Xie et al. 2014). 1-Methylcyclopropene (1-MCP) is an inhibitor of ethylene receptors that retards ethylene-dependent responses such as ripening, senescence, and physiological disorders (Sisler and Serek 1997; Sisler et al. 2003; Watkins et al. 2000). Based on the differences in European pear development, storability, and response to ethylene, cultivars are grouped into two categories: summer pears and winter pears. ‘d’Anjou’ pear belongs to the winter pears category and requires a minimum of 0.15 μL L−1 1-MCP for effective development scald control, while the summer pears require twofold 1-MCP concentration (Argenta et al. 2003; Chen and Spotts 2005; Bai et al. 2006; Xie et al. 2014; Xie et al. 2016).
Maturity at harvest plays a pivotal role in the optimization of fruit quality following storage. When harvested at the optimal maturity, ‘d’Anjou’ pears have a relatively long storage life, low storage loss, and develop a buttery and juicy texture with full flavor after storage at − 1.1 °C for 2 to 4 months (Chen et al. 1983; Sugar 2007). However, over-mature fruit are prone to reduce firmness and green color, more susceptible to scald, and develop ripening capacity earlier than the optimum harvested fruit (Chen and Mellenthin 1981; Chen et al. 1983; Calvo et al. 2015). In normal seasons, commercial harvest maturity for ‘d’Anjou’ pears begins as firmness decreases to ~ 67 N and ends when firmness reaches ~ 58 N over the course of ~ 14 days (Chen et al. 1983; Sugar 2007). However, during abnormally hot seasons, or during seasons with labor shortages, fruit remains on tree faster longer, and ripens more quickly, resulting in some fruit being harvested at an over-mature stage.
The objectives of this study were to (1) evaluate the effect of 1-MCP on storage and eating quality and superficial scald development in over-mature ‘d’Anjou’ pears after long-term cold storage, and (2) to determine the effect of 1-MCP on the catabolism of α-farnesene and antioxidant system in fruit peel associated with scald development.
Materials and Methods
Fruit and Treatments
‘d’Anjou’ pears were harvested from mature trees in two commercial orchards with the different elevation (Orchard 1, Odell, fruit firmness ≈ 55.6 N, elevation 210 m; Orchard 2, Parkdale, fruit firmness ≈ 56.0 N, elevation 526 m) in Hood River, OR. Fruit from three blocks in each orchard were mixed within each block and defect-free fruit were packed in 24 wooden boxes (~ 80 fruit per box) with standard perforated polyethylene liners and immediately moved into cold storage at − 1.1 °C and > 90% relative humidity. After 24 h of storage, 12 boxes of each orchard were exposed to 1-MCP (SmartFresh®, AgroFresh, Spring House, PA, USA) in an airtight room (39.8 m3) with a circulation fan at − 1.1 °C for 24 h as recommended by the manufacturer. The initial concentration of 1-MCP was determined by gas chromatography and was 0.15 μL L−1 for 1-MCP-treated pears. After treatments, all fruit were stored at − 1.1 °C for 7 months. Three replicates of one box each per treatment were removed after 5, 6, or 7 months of storage and fruit were evaluated for physiological, biochemical, and fruit quality attributes. Fruit were held at 20 °C for 1, 3, or 5 days. Peel samples (2 mm in thickness) were removed and quick-frozen in liquid nitrogen, then stored at − 80 °C for subsequent analysis. Before analyses, the frozen peels were pulverized in mortars into a powder in liquid nitrogen.
Ethylene Production and Respiration Rates
Ethylene production and respiration rates of five fruit per replicate were measured from each treatment. Fruit were sealed in a 3.8-L air-tight jar for 1 h at 20 °C. Headspace gas samples were withdrawn with a 1-mL gas-tight syringe. The headspace ethylene concentration was measured by injecting the gas sample into a gas chromatograph (GC-8A; Shimadzu, Tokyo, Japan) equipped with a flame ionization detector and a Porapack Q column (80/100 mesh, 3 mm in diameter, 2 m long). The carrier gas was nitrogen at a flow rate of 0.8 mL s−1, the oven temperature was 90 °C, and the injector and detector temperatures were 140 °C. An external standard of ethylene was used for calibration. Ethylene production was expressed as ng kg−1 s−1. The headspace CO2 concentration was measured using a CO2 analyzer (900161; Bridge Analyzers Inc., Alameda, CA, USA) and expressed as μg CO2 kg−1 s−1.
Fruit Flesh Firmness (FF), Peel Chlorophyll Content, Soluble Solids Content (SSC), Titratable Acidity (TA), and Sensory Quality
Peel chlorophyll content was estimated at two opposite locations on the fruit surface near the equator using a DA meter (Sinteleia, Bolonga, Italy) and expressed as IAD value. FF was measured using a texture analyzer (GS-14, Güss Manufacturing Ltd., Strand, South Africa) equipped with a 8-mm probe, at 9 mm of penetration and at a speed of 9 mm s−1. The maximum force was recorded and expressed as newtons (N). Two FF were obtained per fruit on opposite sides of the equator after removing 2-mm thickness of peel disks. After firmness determination, 100 g of flesh tissue was macerated for 3 min using a juicer (6001; Acme Juicer Manufacturing Co, Sierra Madre, CA, USA) equipped with a uniform strip of milk filter (Schwartz Manufacturing Co., Two Rivers, WI, USA). SSC of the juice was determined using a digital refractometer (PAL-1; Atago, Tokyo, Japan). TA was determined by titrating 10 mL of the juice to pH 8.1 using 0.1 mol L−1 NaOH with a commercial titration system (T80/20; Schott-Gerate, Hofheim, Germany) and expressed as percentage (grams per 100 mL juice) of malic acid equivalent. After 5, 6, or 7 months of storage and 5 days at 20 °C, a panel of 13 taste testers was trained to recognize and score the sensory quality of the pears (Dong et al. 2018). Sensory quality (buttery-juicy texture score) was scored on a five-point hedonic scale. Fruit with highly, moderately, or slightly buttery-juicy texture were rated as 5, 4, or 3, respectively, and those rated as moderately or very firm or with moderately or very mealy flesh texture were rated as 2 or 1, respectively. Scale anchor points and definitions were determined in an orientation session before the first evaluation. An average score of 3 or higher was defined as commercially acceptable.
Evaluation of Superficial Scald
Superficial scald was assessed visually on 40 fruits of each replicate after 5 days at 20 °C upon removal from cold storage for 5, 6, or 7 months. Scald incidence was expressed as the percentage of fruit affected with commercially unacceptable scald (> 0.6 cm2) as described by Wang (2016).
α-Farnesene and Conjugated Trienols (CTols)
α-Farnesene and CTols were measured as describe by Anet (1972) with slight modification. Fruit peels of 2 mm thickness were taken from five fruit of each replicate after being held at 20 °C for 1, 3, or 5 days. Samples were weighed and immersed in 25 mL of hexane (HPLC-grade; Fisher Scientific, Fair Lawn, NJ, USA) in a covered beaker. The beakers were shaken with an orbital shaker (VCOS-4P; PRO Scientific, Oxford, CT, USA) at 20 °C for 30 min. After extraction, the solvent was filtered with Waterman filter paper. Absorbance at 232 nm (α-farnesene) and 281–290 nm (CTols) was recorded using a spectrophotometer (Ultrospec 3100 Pro, Biochrom Ltd., Cambridge, UK). Concentrations of α-farnesene and CTols were calculated using molar extinction coefficients ε232 nm = 27,740 for α-farnesene and ε281–290 nm = 25,000 for CTols and expressed as mg kg−1 fresh mass.
Malondialdehyde (MDA)
MDA was measured as described by Wang (2016). Briefly, 2 g of peel tissue powder were homogenized in 5 mL of 10% (w/v) trichloroacetic acid and centrifuged at 10,000g for 15 min at 4 °C. The supernatant (2 mL) was added to 2 mL of 0.67% (w/v) thiobarbituric acid. After boiling for 20 min, absorbance was measured at 450, 532, and 600 nm. The MDA concentration was calculated according to the formula 6.45 × (A532 − A600) − 0.56 × A450, and results were expressed as μmol kg−1 fresh mass.
Total Antioxidant Capacity, Total Phenolics (TP), and Total Flavonoids (TF)
Two grams of peel tissue were extracted in 3 mL of (7:3, v/v) ethanol/acetone (EtOH-ACE) at 37 °C for 1 h, then centrifuged at 10,000g for 20 min at 4 °C. The supernatants were collected and stored at − 20 °C for subsequent tests.
Total antioxidant capacity was determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH). The ability to scavenge DPPH free radicals was measured as described by Du et al. (2009) with slight modification. Briefly, a 25 μL sample of the extract was added to 2 mL of 6.25 × 10−5 mol L−1 DPPH in methanol. A control sample consisting of the same volume of solvent was used to measure the maximum DPPH absorbance. The mixture was shaken vigorously and incubated at 37 °C in the dark for 30 min, and absorbance at 517 nm was recorded to determine the concentration of remaining DPPH. Results were expressed in trolox equivalents (mg kg−1 fresh mass) using a trolox standard curve.
Total antioxidant capacity was determined by ferric reducing antioxidant power (FRAP). The FRAP assay was measured according to the method described by Du et al. (2009) with slight modification. The stock solutions included 0.3 mol L−1 acetate buffer (pH 3.6), 10 mmol L−1 2,4,6-tripyridyl-s-triazine (TPTZ) solution in 40 mmol L−1 HCl, and 20 mmol L−1 FeCl3. The fresh working solution was prepared by mixing 25 mL acetate buffer, 2.5 mL TPTZ, and 2.5 mL FeCl3. A sample of the extract (10 μL) in 1 mL distilled water was allowed to react with 1.8 mL of fresh working solution for 10 min at 37 °C. Absorbance was measured at 593 nm, and results were expressed in trolox equivalents (mg kg−1 fresh mass) using a trolox standard curve.
TP was measured as described by Du et al. (2009). In a 10-mL glass tube, a sample of the extract (0.1 mL) was added to 7.9 mL of distilled water and 0.5 mL of 0.25 mol L−1 Folin-Ciocalteu reagent. After mixing for 5 min at 20 °C, 1.5 mL of 1 mol L−1 sodium carbonate was added and incubated in the dark at 20 °C for 2 h. Absorbance was measured at 765 nm, and results were expressed in gallic acid equivalents (mg kg−1 fresh mass) using a gallic acid standard curve.
TF was measured as described by Du et al. (2009). In a 10-mL glass tube, a sample of the extract (0.15 mL) was added to 3.4 mL of ethanol, 0.15 mL of 0.5 mol L−1 NaNO2, and 0.15 mL of 0.3 mol L−1 AlCl3. After mixing for 5 min, 1 mL of 1 mol L−1 NaOH was added. Absorbance was measured at 506 nm, and results were expressed in rutin equivalents (mg kg−1 fresh mass) using a rutin standard curve.
Antioxidant Enzymes
Two grams of peel tissue were homogenized in 2 mL of 50 mmol L−1 potassium phosphate buffer (pH 7.8) containing 1 mmol L−1 ethylenediaminetetraacetic acid (EDTA), 0.3% (v/v) Triton X-100, and 1% (w/v) polyvinylpolypyrrolidone (PVPP), with the addition of 1 mmol L−1 ascorbate in the case of ascorbate peroxidase (APX) assay. The homogenate was centrifuged at 13,000g for 20 min at 4 °C, and the supernatant was used for the subsequent enzyme tests.
Superoxide dismutase (SOD) activity was measured as described by Giannopolitis and Ries (1977). Briefly, a 20 μL sample of the extract was added to 1 mL of assay reagent (50 mmol L−1 potassium phosphate buffer (pH 7.8), 6.5 mmol L−1 methionine, 50 mmol L−1 nitro blue tetrazolium (NBT), 20 mmol L−1 riboflavin, and 10 mmol L−1 EDTA). One unit of SOD activity was defined as the amount of enzyme that cause 50% inhibition of the reduction of NBT as monitored per min at 560 nm.
Catalase (CAT) activity was measured as described by Aebi (1984). Briefly, a 20 μL sample of the extract was added to 1 mL of 50 mmol L−1 potassium phosphate buffer (pH 7.8) containing 20 mmol L−1 H2O2. One unit of CAT activity was defined as 1 μmol L−1 of H2O2 decomposed per minute at 240 nm.
APX activity was measured as described by Nakano and Asada (1981). Briefly, a sample of the extract (20 μL) was added to 1 mL of assay reagent (50 mmol L−1 HEPES-KOH (pH 7.6), 0.1 mmol L−1 EDTA-Na2, 0.5 mmol L−1 ascorbate, and 0.2 mmol L−1 H2O2). The reaction was initiated by adding H2O2. One unit of APX activity was defined as 1 μmol L−1 of ascorbate oxidized per minute at 290 nm.
Statistical Analysis
Experiments were performed using a completely randomized design. One-way analysis of variance (ANOVA) was carried out to determine the significance of differences between means using Fisher’s protected least significant difference (LSD) test (P < 0.05). The data were subjected to analysis using IBM SPSS Statistics (IBM Co., Armonk, NY, USA).
Results and Discussion
Stage of maturity at harvest is the key factor affecting the fruit’s ripening behavior and storage potential (Chen et al. 1983; Sugar 2007). If pears are harvested at an early maturity stage, their smaller size with low levels of SSC and TA limits maximum storage life. In addition, these immature pears are more susceptible to shrivel, scald, scuffing, and friction discoloration during the handing process. If harvested too late, over-mature fruit will be subjected to rapid quality deterioration during storage and show a higher rate of decay during marketing (Chen et al. 1983; Villalobos-Acuna and Mitcham 2008). ‘d’Anjou’ pears harvested between 58 and 67 N require ~ 60 days of low temperature chill to attain ripening capacity after exposure to warmer temperature (Hansen and Mellenthin 1979; Sugar and Einhorn 2011); when harvested between 53 and 58 N, pears need only ~ 30 days of low temperature chill to achieve ripening capacity and develop a coarse-dry texture after regular storage periods (Chen et al. 1983). In this study, Orchard 1, fruit were picked at FF of ~ 56.0 N, peel chlorophyll content of 1.88 IAD, SSC of 12.2%, TA of 2.21%, and respiration rate of 1.88 μg CO2 kg−1 s−1; Orchard 2, fruit were picked at FF of ~ 55.6 N, peel chlorophyll content of 1.85 IAD, SSC of 12.6%, TA of 2.14%, and respiration rate of 1.83 μg CO2 kg−1 s−1. No ethylene production was detected in fruit from either orchard at harvest.
Effect of 1-MCP on Ethylene Production and Respiration Rates of Over-Mature Pears After Long-Term Storage
Ethylene production rate in the control fruit from the two orchards dramatically increased during ripening at 20 °C for 5 days after 5, 6, or 7 months of storage, while 1-MCP inhibited the increase of ethylene production in the treated fruit (Fig. 1). However, after 7 months of storage and 5 days at 20 °C, the efficacy of 1-MCP weakened against ethylene production; similar results were observed in 1-MCP-treated ‘Comice’ pear after long-term storage (Dong et al. 2018). Compared with the untreated control, respiration rates of the 1-MCP-treated fruit from the two orchards were significantly lower after removal from cold storage and during ripening at 20 °C.
Ethylene production rate (a, b) and respiration rate (c, d) influenced by 0.15 μL L−1 1-MCP in over-mature ‘d’Anjou’ pears in two orchards following 5, 6, or 7 months of storage at − 1.1 °C and 1, 3, or 5 days at 20 °C. Values are presented as the means ± SD, n = 3. Vertical bars represent LSD values at the 5% level for effects of orchard location, treatment, storage time, and poststorage ripening periods
Effect of 1-MCP on Storage and Eating Quality of Over-Mature Pears After Long-Term Storage
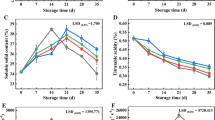
After receiving a minimum chilling, ‘d’Anjou’ fruit attain the ability to soften to between 14 and 23 N with extractable juice below 650 mL kg−1, and to develop a buttery-juicy texture with a characteristic full flavor after removal from cold storage and 7 days at 20 °C (Chen and Borgic 1985). Compared with the initial, the control fruit declined in FF and peel chlorophyll content during ripening at 20 °C for 5 days following 5, 6, or 7 months of storage (Fig. 2). Pears turned to buttery-juicy texture at ripening after 5 months of storage. However, after 7 months of storage at − 1.1 °C and 5 days at 20 °C, sensory scores of textural quality in the control fruit decreased to 2.7 and 2.9 for Orchard 1 and 2, respectively, and a coarse-dry texture was observed in these over-mature fruit. By contrast, 1-MCP-treated fruit maintained higher SSC and TA than the control fruit during 5, 6, or 7 months of storage. 1-MCP slowed the decline of FF and peel chlorophyll content during cold storage or ripening. After 7 months of storage and 5 days at 20 °C, some of 1-MCP-treated fruit in Orchard 1 developed ripening capacity and buttery-juicy texture.
Fruit flesh firmness (FF, a, b) and peel chlorophyll content (c, d) influenced by 1-MCP in over-mature ‘d’Anjou’ pears in two orchards following 5, 6, or 7 months of storage at − 1.1 °C and 1, 3, or 5 days at 20 °C. Soluble solid content (SSC, e, f) and titratable acidity (TA, g, h) of ‘d’Anjou’ pears treated with 1-MCP in two orchards following 5, 6, or 7 months of cold storage and 1 days at 20 °C. Vertical bars represent LSD values at the 5% level for effects of orchard location, treatment, storage time, and poststorage ripening periods. Sensory scores of textural quality (i, j) of ‘d’Anjou’ pears treated with 1-MCP in two orchards following 5, 6, or 7 months of cold storage and 5 days at 20 °C. Different letters indicates significant differences between treatments according Fisher’s protected LSD test at P = 0.05. Values are presented as the means ± SD, n = 3
Postharvest application of 1-MCP at 0.1–0.3 μL L−1 maintained and improved storability of ‘d’Anjou’ pears by blocking ethylene receptors, alleviating reduction in green color, and reducing ethylene-dependent physiological disorders during storage. However, the efficacy of 1-MCP entirely interfered the fruit’s ripening capacity in that these fruit were not able to develop a buttery-juicy texture after subjected to room temperature (Chen and Spotts 2005; Xie et al. 2014; Wang 2016; Dong et al. 2018). In this study, although treating fruit with 1-MCP inhibited ethylene synthesis during 5 and 6 months of storage, some of these pears, particularly in Orchard 1, produced amounts of ethylene and recovered ripening capacity during ripening after 7 months. Results indicated that 1-MCP applied to over-mature pears offset the existing ethylene receptors when pears stored for 6 months at − 1.1 °C, but new ethylene receptors might be formed after longer-term storage. As a result, these new ethylene receptors acted in a coordinated manner to activate ethylene responses and ripening processes.
Xie et al. (2014) found that ‘d’Anjou’ pears harvested between 62 and 55 N and treated with 0.15 μL L−1 1-MCP did not develop ripening capacity within 7 days at 20 °C following 8 months of storage at − 1.1 °C. However, in this study, some 1-MCP-treated pears recovered ripening capacity and began to synthesize more ethylene after 7 months of storage and 5 days at 20 °C. Therefore, another possibility for recovering of ripening capacity in 1-MCP-treated fruit might be attributed to the high accumulated cold units (ACUs) which is calculated as the total numbers of hours, in the previous 42 days prior to the first day of commercial maturity, based on FF at 65 N. Generally, ACUs are positively linearly correlated with the increase of elevation (Ma et al. 2001). Pears produced at higher elevation accumulated greater internal ethylene concentration (Wang and Sugar 2015). This indicated that 1-MCP efficacy was affected by harvest maturity and production elevation, with a reduced efficacy to bind tightly with ethylene receptors in more mature fruit produced at higher elevations.
Effect of 1-MCP on Superficial Scald, α-Farnesene, and CTols of over-Mature Pears After Long-Term Storage
Previous research had demonstrated that 1-MCP was effective against the development of superficial scald in ‘d’Anjou’ pears for up to 6 months in air at − 1.1 °C (Xie et al. 2014), and at least 9 months in controlled atmosphere under O2 at 1.5 kPa (Bai et al. 2006). In this study, control fruit from Orchard 1 and 2 developed 51 and 55% superficial scald incidence, respectively, after 5 months of storage, while after 7 months, the superficial scald incidence increased to 63 and 66% (Fig. 3). Treating fruit with 1-MCP significantly reduced the appearance of scald and developed only 5% for Orchard 1 and 2, alike, after 7 months of storage. The amount of α-farnesene and CTols in the control fruit from the two orchards increased dramatically following storage and was higher than in the 1-MCP-treated fruit even in ripening. This confirmed that 1-MCP effectively decreased the accumulation of α-farnesene and CTols during cold storage or in ripening (Xie et al. 2014; Wang 2016; Yu and Wang 2017). However, after 5 months of storage, 1-MCP-treated pears showed a slight incidence of scald, which increased thereafter, indicating that long-term storage time and late harvest maturity impaired the efficacy of 1-MCP.
Superficial scald incidence (a, b) of over-mature ‘d’Anjou’ pears treated with 1-MCP in two orchards following 5, 6, or 7 months of storage at − 1.1 °C and 5 days at 20 °C. Different letters indicates significant differences between treatments according Fisher’s protected LSD test at P = 0.05. α-Farnesene (c, d) and conjugated trienols (CTols, e, f) influenced by 1-MCP in over-mature ‘d’Anjou’ pears in two orchards following 5, 6, or 7 months of storage at − 1.1 °C and 1, 3, or 5 days at 20 °C. Values are presented as the means ± SD, n = 3. Vertical bars represent LSD values at the 5% level for effects of orchard location, treatment, storage time, and poststorage ripening periods
Effect of 1-MCP on MDA, Total Antioxidant Capacity, and Antioxidants of Over-Mature Pears After Long-Term Storage
Scald development is associated with an imbalance of oxidative metabolism caused by reactive oxygen species (ROS) and resulting in lipid peroxidation, protein denaturation, and degradation of cellular organs. Fortunately, plants developed the capacity to eliminate oxidative stress via antioxidant systems which included antioxidant metabolites and antioxidant enzymes (Lurie and Watkins 2012). In apple, primary oxidative damage in membrane systems is attributed to lipid peroxidation (Rao et al. 1998). In this study, MDA content in the control fruit increased from 3.3 to 4.4 μmol kg−1 and from 2.3 to 3.7 μmol kg−1 for Orchard 1 and 2, respectively, after 7 months of storage; this upward trend continued during ripening at 20 °C (Fig. 4). However, 1-MCP treatment reduced the accumulation of MDA in the two orchards, probably due to scavenging ROS and protecting membrane integrity (Li and Wang 2009). Barden and Bramlage (1994a and 1994b) reported that late harvest apples had high antioxidant contents at harvest. However, after long-term storage, these fruit had lower antioxidant capacity and higher scald incidence than fruit harvested at commercial maturity (Calvo et al. 2015). In this study, 1-MCP treatment maintained higher antioxidant capacity (DPPH and FRAP) and antioxidants (TP and TF) than the controls in either orchard over the entire cold storage and ripening periods (Figs. 4 and 5). These greater antioxidant capacity and antioxidant contents in 1-MCP-treated fruit curtailed the oxidation of α-farnesene to CTols and provided benefits in reducing the development of scald.
Malondialdehyde (MDA, a, b) and total antioxidant capacity determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH, c, d) or ferric reducing antioxidant power (FRAP, e, f) of over-mature ‘d’Anjou’ pears treated with 1-MCP in two orchards following 5, 6, or 7 months of storage at − 1.1 °C and 1, 3, or 5 days at 20 °C. Values are presented as the means ± SD, n = 3. Vertical bars represent LSD values at the 5% level for effects of orchard location, treatment, storage time, and poststorage ripening periods
Total phenolics (TP, a, b) and total flavonoids (TF, c, d) influenced by 1-MCP in over-mature ‘d’Anjou’ pears in two orchards following 5, 6, or 7 months of storage at − 1.1 °C and 1, 3, or 5 days at 20 °C. Values are presented as the means ± SD, n = 3. Vertical bars represent LSD values at the 5% level for effects of orchard location, treatment, storage time, and poststorage ripening periods
Effect of 1-MPC on Antioxidant Enzymes of Over-Mature Pears After Long-Term Storage
In pear fruit, 1-MCP enhances ROS scavenging systems and superficial scald resistance by maintaining high activities of SOD, CAT, and peroxidase (POX) and low activities of glutathione reductase (GR) and monodehydro-ascorbate reductase (MDHAR) (Gao et al. 2015; Zhao et al. 2016; Zhou et al. 2017). In this study, SOD activity was lower in all fruit from the two orchards after 7 months of cold storage than that after 5 or 6 months, and dramatically decreased during ripening at 20 °C (Fig. 6). Compared with the control, 1-MCP-treated fruit maintained higher levels of SOD activities after 7 months of storage and ripening at 20 °C. For Orchard 1, CAT activity increased in the control and 1-MCP-treated fruit during cold storage. However, during ripening at 20 °C after 6 and 7 months, CAT activity in control fruit progressively decreased, while 1-MCP treated fruit maintained a stable high CAT activity. For Orchard 2, although no significant changes were observed in CAT activities of control fruit following 7 months of storage and 5 days at 20 °C, 1-MCP treatment sustained higher CAT activities, and increased after 6 and 7 months of storage and 5 days at 20 °C. Results indicated that the higher activities of SOD and CAT might more efficiently convert the superoxide anion (O2·−) and hydrogen peroxide (H2O2) into water and oxygen resulting in alleviation of oxidative damage (Apel and Hirt 2004). In addition, H2O2 is converted into water by the action of APX and glutathione peroxidase (GPX) via the ascorbate-glutathione cycle. However, lower activities of APX were observed in 1-MCP-treated pears during cold storage or in ripening, indicating that 1-MCP differentially affected the activities of antioxidant enzymes (Shaham et al. 2003). Therefore, activities of SOD and CAT might contribute to the control of scald development.
Superoxide dismutase (SOD, a, b), catalase (CAT, c, d), and ascorbate peroxidase (APX, e, f) influenced by 1-MCP in over-mature ‘d’Anjou’ pears in two orchards following 5, 6, or 7 months of storage at − 1.1 °C and 1, 3, or 5 days at 20 °C. Values are presented as the means ± SD, n = 3. Vertical bars represent LSD values at the 5% level for effects of orchard location, treatment, storage time, and poststorage ripening periods
Conclusion
The results of the present study demonstrate that 1-MCP at concentration of 0.15 μL L−1 could be applied in over-mature ‘d’Anjou’ pears (FF at 55~56 N) harvested from higher elevations to reduce the development of superficial scald. 1-MCP suppressed ethylene production and respiration rates, delayed losses in storage and eating quality, and inhibited the accumulation of α-farnesene and CTols. And some of 1-MCP-treated pears recovered ripening capacity with a buttery-juicy texture after 7 months of storage at − 1.1 °C and 5 days at 20 °C. In addition, higher antioxidant capacity (DPPH and FRAP), antioxidants (TP and TF), and antioxidant enzyme activity (SOD and CAT) in 1-MCP-treated pears had the positive effect of reducing α-farnesene catabolism and scavenging oxidative damage after long-term storage. When properly used, 1-MCP has the potential to extend storability, expand the marketing season, control scald, and support recovery of ripening capacity of over-mature ‘d’Anjou’ pears picked in hot seasons or during labor shortages.
References
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Ahn, T., Paliyath, G., & Murr, D. P. (2007). Antioxidant enzyme activities in apple varieties and resistance to superficial scald development. Food Research International, 40(8), 1012–1019.
Anet, E. F. L. J. (1972). Superficial scald, a functional disorder of stored apples. IX. Effect of maturity and ventilation. Journal of Science of Food and Agriculture, 23(6), 763–769.
Apel, K., & Hirt, H. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55(1), 373–399.
Argenta, L. C., Fan, X., & Mattheis, J. P. (2003). Influence of 1-methylcyclopropene on ripening, storage life, and volatile production by d’Anjou cv. pear fruit. Journal of Agricultural and Food Chemistry, 51(13), 3858–3864.
Bai, J., Mattheis, J. P., & Reed, N. (2006). Re-initiating softening ability of 1-methylcyclopropene-treated ‘Bartlett’ and ‘d’Anjou’ pears after regular air or controlled atmosphere storage. The Journal of Horticultural Science and Biotechnology, 81(6), 959–964.
Barden, C. L., & Bramlage, W. J. (1994a). Accumulation of antioxidants in apple peel as related to preharvest factors and superficial scald susceptibility of the fruit. Journal of the American Society for Horticultural Science, 119, 264–269.
Barden, C. L., & Bramlage, W. J. (1994b). Relationships of antioxidants in apple peel to changes in α-farnesene and conjugated trienes during storage, and to superficial scald development after storage. Postharvest Biology and Technology, 4(1-2), 23–33.
Calvo, G., Candan, A. P., Civello, M., Giné-Bordonaba, J., & Larrigaudière, C. (2015). An insight into the role of fruit maturity at harvest on superficial scald development in ‘Beurré D’Anjou’ pear. Scientia Horticulturae, 192, 173–179.
Chen, P. M., & Mellenthin, W. M. (1981). Effects of harvest date on ripening capacity and postharvest life of d’Anjou’ pears. Journal of the American Society for Horticultural Science, 106, 38–42.
Chen, P. M., & Borgic, D. M. (1985). Changes in water soluble polyuronides in the pulp tissue of ripening ‘bosc’ pears following cold storage in air or in 1% oxygen. Journal of the American Society for Horticultural Science, 110, 667–671.
Chen, P. M., Mellenthin, W. M., & Borgic, D. M. (1983). Changes in ripening behavior of d’Anjou’ pears (Pyrus communis L.) after cold storage. Scientia Horticulturae, 21(2), 137–146.
Chen, P. M., & Spotts, R. A. (2005). Changes in ripening behaviors of 1-MCP-treated ‘d’Anjou’ pears after storage. International Journal of Fruit Science, 5(3), 3–18.
Chen, P. M., Varga, D. M., Mielke, E. A., Facteau, T. J., & Drake, S. R. (1990). Control of superficial scald on ‘Anjou’ pears by ethoxyquin: Oxidation of α-farnesene and its inhibition. Journal of Food Science, 55(1), 171–175.
Dong, Y., Zhang, S., & Wang, Y. (2018). Compositional changes in cell wall polyuronides and enzyme activities associated with melting/mealy textural property during ripening following long-term storage of ‘Comice’ and d’Anjou’ pears. Postharvest Biology and Technology, 135, 131–140.
Du, G., Li, M., Ma, F., & Liang, D. (2009). Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chemistry, 113(2), 557–562.
Fan, X., Mattheis, J. P., & Blankenship, S. (1999). Development of apple superficial scald, soft scald, core flush, and greasiness is reduced by MCP. Journal of Agricultural and Food Chemistry, 47(8), 3063–3068.
Gao, M., Zhou, S., Guan, J., & Zhang, Y. (2015). Effects of 1-methylcyclopropene on superficial scald and related metabolism in ‘Wujiuxiang’ pears during cold storage. Journal of Applied Botany and Food Quality, 88, 102–108.
Gapper, N. E., Bai, J., & Whitaker, B. D. (2006). Inhibition of ethylene-induced α-farnesene synthase gene PcAFS1 expression in d’Anjou’ pears with 1-MCP reduces synthesis and oxidation of α-farnesene and delays development of superficial scald. Postharvest Biology and Technology, 41(3), 225–233.
Giannopolitis, C. N., & Ries, S. K. (1977). Superoxide dismutases I. Occurrence in higher plants. Plant Physiology, 59(2), 309–314.
Hansen, E., & Mellenthin, W. M. (1979). Commercial handling and storage practices for winter pears. Oregon State University, Agricultural Experiment Station Special Report 550.
Jung, S. K., & Watkins, C. B. (2008). Superficial scald control after delayed treatment of apple fruit with diphenylamine (DPA) and 1-methylcyclopropene (1-MCP). Postharvest Biology and Technology, 50(1), 45–52.
Li, Z. Q., & Wang, L. J. (2009). Effect of 1-methylcyclopropene on ripening and superficial scald of Japanese pear (Pyrus pyrifolia Nakai, cv. Akemizu) fruit at two temperatures. Food Science and Technology Research, 15(5), 483–490.
Lurie, S., & Watkins, C. B. (2012). Superficial scald, its etiology and control. Postharvest Biology and Technology, 65, 44–60.
Ma, S., Varga, D. M., & Chen, P. M. (2001). Using accumulated cold units to predict the development of superficial scald disorder on d’Anjou’pears during cold storage. The Journal of Horticultural Science and Biotechnology, 76(3), 305–310.
Mellenthin, W. M., Chen, P. M., & Kelly, S. B. (1980). Low oxygen effects on dessert quality, scald prevention, and nitrogen metabolism of ‘d’Anjou’ pear fruit during long-term storage. Journal of the American Society for Horticultural Science, 105, 522–527.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 867–880.
Rao, M. V., Watkins, C. B., Brown, S. K., & Weeden, N. F. (1998). Active oxygen species metabolism in ‘White Angel’ × ‘Rome Beauty’ apple selections resistant and susceptible to superficial scald. Journal of the American Society for Horticultural Science, 123, 299–304.
Rudell, D. R., & Mattheis, J. P. (2009). Superficial scald development and related metabolism is modified by postharvest light irradiation. Postharvest Biology and Technology, 51(2), 174–182.
Shaham, Z., Lers, A., & Lurie, S. (2003). Effect of heat or 1-methylcyclopropene on antioxidative enzyme activities and antioxidants in apples in relation to superficial scald development. Journal of the American Society for Horticultural Science, 128, 761–766.
Sisler, E. C., & Serek, M. (1997). Inhibitors of ethylene responses in plants at the receptor level: Recent developments. Physiologia Plantarum, 100(3), 577–582.
Sisler, E. C., Alwan, T., Goren, R., Serek, M., & Apelbaum, A. (2003). 1-Substituted cyclopropenes: Effective blocking agents for ethylene action in plants. Plant Growth Regulation, 40(3), 223–228.
Sugar, D. (2007). Postharvest handlings of winter pears. In: Mitcham, E.J., Elkins, R.B. (Eds.), Pear production and handling manual. University of California.
Sugar, D., & Basile, S. R. (2013). Integrated ethylene and temperature conditioning for induction of ripening capacity in ‘Anjou’ and ‘Comice’ pears. Postharvest Biology and Technology, 83, 9–16.
Sugar, D., & Einhorn, T. C. (2011). Conditioning temperature and harvest maturity influence induction of ripening capacity in ‘d’Anjou’ pear fruit. Postharvest Biology and Technology, 60(2), 121–124.
Tsantili, E., Gapper, N. E., Arquiza, J. A., Whitaker, B. D., & Watkins, C. B. (2007). Ethylene and α-farnesene metabolism in green and red skin of three apple cultivars in response to 1-methylcyclopropene (1-MCP) treatment. Journal of Agricultural and Food Chemistry, 55(13), 5267–5276.
Xie, X., Song, J., Wang, Y., & Sugar, D. (2014). Ethylene synthesis, ripening capacity, and superficial scald inhibition in 1-MCP treated d’Anjou’ pears are affected by storage temperature. Postharvest Biology and Technology, 97, 1–10.
Xie, X., Zhao, J., & Wang, Y. (2016). Initiation of ripening capacity in 1-MCP treated green and red ‘Anjou’ pears and associated expression of genes related to ethylene biosynthesis and perception following cold storage and post-storage ethylene conditioning. Postharvest Biology and Technology, 111, 140–149.
Villalobos-Acuna, M., & Mitcham, E. J. (2008). Ripening of European pears: The chilling dilemma. Postharvest Biology and Technology, 49(2), 187–200.
Wang, Y. (2016). Storage temperature, controlled atmosphere, and 1-methylcyclopropene effects on α-farnesene, conjugated trienols, and peroxidation in relation with superficial scald, pithy brown core, and fruit quality of ‘d’Anjou’ pears during long-term storage. Journal of the American Society for Horticultural Science, 141, 177–185.
Wang, Y., & Sugar, D. (2015). 1-MCP efficacy in extending storage life of ‘Bartlett’ pears is affected by harvest maturity, production elevation, and holding temperature during treatment delay. Postharvest Biology and Technology, 103, 1–8.
Watkins, C. B., Nock, J. F., & Whitaker, B. D. (2000). Responses of early, mid and late season apple cultivars to postharvest application of 1-methylcyclopropene (1-MCP) under air and controlled atmosphere storage conditions. Postharvest Biology and Technology, 19(1), 17–32. https://doi.org/10.1016/S0925-5214(00)00070-3.
Whitaker, B. D., Nock, J. F., & Watkins, C. B. (2000). Peel tissue α-farnesene and conjugated trienol concentrations during storage of ‘White Angel’ × ‘Rome Beauty’ hybrid apple selections susceptible and resistant to superficial scald. Postharvest Biology and Technology, 20(3), 231–241. https://doi.org/10.1016/S0925-5214(00)00139-3.
Whitaker, B. D. (2007). Oxidation products of α-farnesene associated with superficial scald development in d’Anjou pear fruits are conjugated trienols. Journal of Agricultural and Food Chemistry, 55(9), 3708–3712. https://doi.org/10.1021/jf063710i.
Yu, J., & Wang, Y. (2017). The combination of ethoxyquin, 1-methylcyclopropene and ethylene treatments controls superficial scald of ‘d’Anjou’ pears with recovery of ripening capacity after long-term controlled atmosphere storage. Postharvest Biology and Technology, 127, 53–59. https://doi.org/10.1016/j.postharvbio.2017.01.012.
Zhao, J., Xie, X., Shen, X., & Wang, Y. (2016). Effect of sunlight-exposure on antioxidants and antioxidant enzyme activities in ‘d’Anjou’ pear in relation to superficial scald development. Food Chemistry, 210, 18–25. https://doi.org/10.1016/j.foodchem.2016.04.045.
Zhou, S., Cheng, Y., & Guan, J. (2017). The molecular basis of superficial scald development related to ethylene perception and α-farnesene metabolism in ‘Wujiuxiang’ pear. Scientia Horticulturae, 216, 76–82. https://doi.org/10.1016/j.scienta.2016.12.025.
Acknowledgments
We are grateful to the Columbia Gorge Fruit Growers Association and the NW Fresh Pear Research Committees for financial support. The authors are also grateful for the expert assistance provided by Drs. Yan Wang and Paul M. Chen for writing support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhi, H., Dong, Y. Effect of 1-Methylcyclopropene on Superficial Scald Associated with Ethylene Production, α-Farnesene Catabolism, and Antioxidant System of Over-Mature ‘d’Anjou’ Pears After Long-Term Storage. Food Bioprocess Technol 11, 1775–1786 (2018). https://doi.org/10.1007/s11947-018-2141-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2141-2