Abstract
In this study, a pulsed mode ultrasound assisted extraction was performed for the extraction of bioactive compounds from Pomegranate (Punica granatum L.) peel. Box-Behnken experimental design combined with response surface methodology, was applied for the optimization of process conditions. The independent variables investigated were ultrasound amplitude (50–80%), sonication time (5–15 min), duty cycle (0.2–0.8), and methanol concentration (30–70%) to maximize the yield of total phenolic content, flavonoid content, total condensed and hydrolysable tannin (TCT and THT respectively), and antioxidant activity (DPPH and FRAP). The experimental results were fitted to quadratic models, and statistical analysis in terms of multiple regression, and analysis of variation was carried out for each response variable. The optimum predicted values of the response variables were obtained at 70% methanol concentration, 12.8 min of sonication treatment at a duty cycle of 0.58, and 80% ultrasound amplitude. A comparative analysis at optimized condition showed better recovery of bioactive compounds in pulsed mode, compared to continuous mode of sonication. Correlation analysis of the response variables was done to find the associations among the variables. Multivariate analysis using principal component analysis, and hierarchical cluster analysis was done for better understanding of interactions among experimental treatments and responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pomegranate (Punica granatum L.) fruit, known for its nutritional as well as nutraceutical values, belongs to Punicaceae family [1]. The fruit has around 52% of edible portion consisting of 78% juice and 22% seeds. The juice is known for its high bioactive properties, while the seeds are rich source of lipids, fibers, protein, and ash [2]. The remaining portion of fruit, consists of peel, which is generally considered as waste, and discarded. But, recent researches have proved high bioactive constituents in peel, making a wide scope for research. The pomegranate peel (PoP) is reported to have a lot of health promoting properties like anti-inflammatory, antioxidant, anti-microbial, anti-mutagenic properties, anti-viral, chemo-preventive, anti-proliferic, and anti-fungal properties etc [1,2,3,4].
The major bioactive compounds present in PoP consist of different classes of polyphenol compounds, including flavonoids, anthocyanin, phenolic acids, and hydrolysable tannins. The PoP has around 0.2–1% of flavonoids, with the major portion as anthocynidins (30%). Punicalagin and ellagic acid derivatives are the foremost important compounds in PoP, having very high antioxidant properties [5]. Ellagitannins, metabolites of gallotannins, are hydrolysable phenolics which, upon hydrolysis releases monomeric phenolics. Monomeric phenolics are known to have better anti-bacterial properties, compared to oligomeric, dimeric or trimeric tannins [6]. They have multiple hydroxyl groups, which helps in quenching free radicals effectively. Due to these properties, a selective and effective extraction of bioactive compounds from pomegranate peel is necessary [3].
Response surface methodology (RSM) is a very important statistical tool, which is used to find the relation between input and output variables, and to optimize the responses. Box-Behnken design (BBD) is a very popular experimental design used in RSM. It allows efficient estimation of first and second order coefficients of an empirical model, by suggesting a method to select points from 3 level factorial design. It is economic compared to full factorial design or central composite design, as it proposes lesser number of experiments, and has better efficiency. Generally, center-points are added in such designs to check for the curvature, as well as process stability and variability [7].
Pulsed-mode ultrasound assisted extraction (PUAE) is a modification in ultrasound extraction technique (UAE), where the ultrasound energy is provided in a discontinuous manner, with a cycle of on and off. It is considered better than continuous mode, as it increases horn life, preserves heat labile compounds, consumes less power, and causes better extraction due to non-steady state condition [8]. Duty cycle is generally used to define the pulsed mode in UAE, which is considered as the ratio of “on” time to total cycle time. Apart from the method of extraction, the bioactive recovery varies a lot with the variation in solvent concentrations, as well as solvent to feed ratio, which mainly depends on the solubility, and diffusion properties of respective solvents [3]. The combined effect of extraction solvent, time, duty cycle, and amplitude in PUAE of pomegranate peel has not been attempted so far. So, this study was intended to see the effect of these variables on total phenolic content (TPC), total flavonoid content (TFC), tannin content (condensed and hydrolysable), and antioxidant activity (2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay and ferric reducing antioxidant power (FRAP) assay). Furthermore, response surface methodology (RSM) was used for the optimization of independent variables.
Materials and methods
Plant material and chemicals
Pomegranate fruits were purchased from local market of IIT Kharagpur, India. Peels were manually removed and washed in distilled water and surface moisture was wiped off. Peels were dried to a moisture content of 8% (db), and grinded to a particle size of less than 0.5 mm. Further, the dried peel powder was used for PUAE. Chemicals used were DPPH, Folin–Ciocalteu (FC) reagent, gallic acid, vanillin, trolox, tannic acid, and catechin from Merck specialties private limited (Mumbai, India), 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ), sodium carbonate, sodium hydroxide, sodium nitrite, aluminum chloride, ethanol, and methanol from Himedia Laboratories (Mumbai, India). Analytical grade reagents were used.
Extract preparation
Extract for analyzing the effect of solvent concentration and solvent to feed ratio was prepared by UAE using an ultrasonic bath (US-5LH; 40 kHz) at 40 °C for 30 min. The solvent used were methanol, ethanol, acetone, 50% aqueous solutions of each, and deionized water. The effect of solvent to feed ratio was evaluated by extracting dried sample in 50% aqueous methanol, and varying feed to solvent ratio (g/mL) as 5:1, 10:1, 15:1, 20:1, and 25:1. TPC, TFC and DPPH antioxidant activity of the extracts were analyzed in both cases.
Pulsed mode ultrasound assisted extraction from pomegranate peel
The dried peel powder was subjected to PUAE using a probe sonicator (ATP 500; 40 kHz), with a probe of 6 mm diameter, dipped upto 2 cm of sample depth. Duty cycle, which signifies total “on time” in second per 10 s of total cycle time, varied from 0.2 to 0.8 to check pulsation effect. Solvent to feed ratio was kept constant as 15:1 (g/mL). Methanol concentration was varied from 30 to 70%. The ultrasound amplitude and sonication time was varied from 50 to 80% and 5 to 15 min, respectively. The ranges of independent variables (Table 1) were taken from the preliminary experimentations.
Determination of bioactive content in extract
Total phenolic content
Total phenolic content (TPC) was evaluated using the procedure given by Singleton and Rossi [9], with few modifications. It is a spectrophotometric method, where blue color is developed with Folin–Ciocalteu Reagent (FCR) in an alkaline medium (20% sodium carbonate) due to phenolic compounds, which gives absorption maxima at 760 nm. Standards were prepared using gallic acid, and the results were expressed in terms of mg Gallic acid equivalent (GAE)/g of dried sample.
Total flavonoid content
TFC was determined using the procedure given by Vuong et al. [10] with slight modifications. Briefly, the method involved reaction of flavonoid in NaNO2 solution, in presence of AlCl3 in basic media. Quercetin was used as the reference standard, with TFC expressed as mg quercetin equivalents per gram of dried sample (mg QE/g).
DPPH assay
It was evaluated using the method suggested by Rani et al. [11] with some modifications. DPPH was used as the reagent which changes its colour from purple to yellow in presence of antioxidants. Gallic acid was used as standard, and the antioxidant activity was expressed in terms of gallic acid equivalent antioxidant capacity (GAEAC) per g of sample.
FRAP assay
It was evaluated using the protocol given by Benzie and Strain, [12]. The absorbance was measured at 593 nm. The results were expressed in mg of Trolox equivalent (TE)/g of sample.
Total condensed tannin
Total condensed tannin (TCT) or proanthocyanidin content of pomegranate peel extract was determined using vanillin-HCL method, as suggested by Broadhurst and Jones, [13]. Briefly, 0.25 mL of extract was added to 1.5 mL of 4% vanillin-methanol solution, followed by addition of 1.5 mL of hydrochloric acid. The absorbance of the mixture was measured at 500 nm. The final results were expressed as mg catechin equivalent (CE) per g dry weight of sample.
Total hydrolysable tannin
Total hydrolysable tannins (THT) content of pomegranate peel was determined using a colorimetric method [14] with slight modifications. Briefly, 0.5 mL of diluted extract was added with 2.5 mL of 2.5% (w/w) KIO3 followed by incubation at room temperature for 7 min. After that the absorbance was taken at 550 nm. The standard curve was prepared using tannic acid, and the results were expressed in terms of mg tannic acid equivalent (TAE)/g of dried powder.
Experimental design
A four factor Box-Behnken design (BBD) with 5 center points was used for the optimization of PUAE conditions, with an aim to maximize the recovery of bioactive compounds, with high antioxidant activity. The independent variables were duty cycle (X1), amplitude (X2), sonication time (X3) and methanol concentration (X4). Each factor has three levels (Table 1), with coded and actual values. Second order polynomial equation was used for fitting all the responses, and a generalized second order polynomial model was proposed, which is given below:
where, Yi represents the response, βo is the constant term, β1, β2, β3 and β4 are regression coefficients for linear terms, β11, β22, β33 and β44 are regression coefficients of quadratic terms and β12, β13, β14, β23, β24 and β34 are regression coefficients of interaction terms. X1, X2, X3 and X4 are coded value of the independent variables.
Extraction in continuous mode ultrasound assisted extraction
The dried peel powder was further subjected to continuous mode of sonication, with a purpose to compare the results. The optimized PUAE conditions were used for comparison. A continuous mode sonication was given for a period of 7.5 min, which was taken on the basis of effective time obtained from the optimized condition of PUAE using the Eq. (2). The extraction was carried out in 70% aqueous methanol solution at 80% of the sonication amplitude. The experiments were conducted in triplicates.
Statistical analysis
Statistical analysis was carried out using the Design Exert 11.1.2.0 (Trial version, Stat-Ease Inc., Minneapolis, MN, USA) software, to analyze the RSM results. The model adequacy was determined by coefficient of determination (R2), adjusted and predicted R2, model p value, and p value for lack of fit. All the experiments were conducted in triplicate. The statistical significance was determined through one-way analysis of variance (ANOVA) by Tukey test (p < 0.05) using Origin Pro 8.5.0 (Origin Lab Corporation, Northampton, MA, USA). Correlation analysis and multivariate analysis using R studio (Version 1.2.5033), and Xlstat (Trial version, Addinsoft 1995–2020), respectively was performed on the results, to determine the relationship among different experimental treatments, and the responses.
Results and discussion
Effect of solvent concentration and solvent to feed ratio
Effects of solvent concentration, as well as solvent to feed ratio on TPC, TFC and DPPH assay were investigated before conducting RSM analysis (Fig. 1a, b). The extraction yield varied significantly with the variation in solvent concentration, as the bioactive compounds vary in their solubility. The higher solvation power of methanol can be attributed to the shorter methyl group present in methanol, compared to relatively longer ethyl group of ethanol [3]. The interaction of polar sites of phenolic compounds with the solvents by hydrogen bonding also improved solubility. Acetone gave least recovery which was mainly due to higher proton accepting property of acetone compared to proton donating capacity of other alcohols. Aqueous solvents were found better compared to pure organic solvents as the addition of water caused higher swelling of peel powder, which improved surface area between solid and solvent, thus enhanced mass transfer [15]. This suggested the use of aqueous methanol as solvent, for the extraction of PoP bioactive compounds. Similar results were found by Boeing et al. [15], Vega et al. [16] for phenolic compounds extraction from berries and Renealmia alpinia, respectively. Though, methanol comes under class 2 chemicals under FDA (food and drug administration), USA but the concentrated extract after vaporization in rotary evaporator has trace amount of methanol which was within permissible limit.
a Effect of solvent concentration on total phenolic content (TPC), total flavonoid content (TFC) and DPPH antioxidant activity of pomegranate peel, b Effect of solvent to feed ratio (mL/g) on TPC, TFC and DPPH antioxidant activity of pomegranate peel. All samples were analyzed in triplicate. A letter on the bar graph indicates the level of significance. Bars of each response denoted by the same letter are not statistically significant (p > 0.05)
The increase in extractability of bioactive compounds at higher solvent to feed ratio was due to increase in concentration gradient, which promoted mass transfer. The enhanced extraction can also be due to reduction in viscosity of solution, which reduces molecular interaction in medium and enhances cavitation effect [17]. However, higher solvent to solid ratio may mean more solvent usage in extraction and energy consumption for concentration in a later processing stage. Hence the optimum solvent to feed ratio was kept at 15:1 for further experimentations. The gradual decrease in extractability of polyphenols after solvent to feed ratio of 20:1, might be due to attenuation of ultrasound waves in the solution, due to increase in solvent to feed ratio [8].
Effects of extraction parameters on TPC
The variation in TPC at different ultrasonic processing conditions is shown in Table 2. The TPC varied from 77.06 to 99.58 mg GAE/g dry weight of PoP. Regression analysis of the obtained data (Table 3) represented that, the linear term of methanol concentration and amplitude had significant effect (p < 0.05) on TPC. Methanol concentration was found to have positive, and highly significant effect (p < 0.01). The interaction of duty cycle and sonication time showed a significant positive effect on TPC. The increase in TPC at higher sonication time and duty cycle may be due to increase in micro bubble formation, which enhanced cell damage, thus caused more release of phenols [18]. Aguilar-Hernández et al. [19] and Tomsik et al. [20] also found higher extraction of phenolics, with increase in sonication time for Annona muricata and wild garlic, respectively. Pulsed mode also facilitated higher release of phenols from the core, as it facilitates better transportation, as well as control temperature. A second order polynomial equation (Eq. 3) fitted well to the experimental data with R2 of 0.76. The adjusted and predicted R2 were also in good agreement, as their difference was less than 0.2, showing a good predictability of model equation. An insignificant lack of fit suggested reliability of the model.
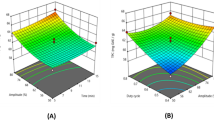
Visual analysis of response surface plots can be observed as per the regression analysis (Fig. 2a–d). Increase in amplitude at lower duty cycle gave better phenolic recovery, while with increase in duty cycle, TPC reduced which may be due to deteriorative effect of cavitation phenomena at longer exposure time [21]. The overall results showed that a higher amplitude condition with a medium duty cycle and sonication time gave better phenolic extract.
Effects of extraction parameters on TFC
The TFC varied from 13.28 to 33.62 mg QE/g dry weight of PoP (Table 2). As shown in Table 3, the quadratic model was found insignificant (p > 0.05) for TFC. The interaction of duty cycle and amplitude (X1X2) showed significant (p < 0.05) positive effect on TFC. The same effect can be visualized through the response surface curves (Fig. 3a, b). This may be due to weakening of phenolics bond matrix at higher amplitude and duty cycle, promoting more solvation of flavonoids. All other factors were found insignificant for flavonoid content. Based on the significant values of coefficients, the effect of process variables on TFC can be represented by Eq. (4).
A low adjusted R2, as well as predicted R2 obtained for flavonoids, suggested larger block effect. A high CV suggested data has more variability, thus lack precision and reproducibility. It can also be concluded that the independent variables might have better applicability on targeted bioactive compounds, than overall flavonoid content [22]. Furthermore, the major bioactive compounds in PoP consist of hydrolysable tannins with a very small proportion of flavonoids, out of total phenolics, which caused the insignificant effect of independent variables on extractability. A study by Pan et al. [23] reported better recovery of flavonoids from Hawthorn seeds, with increase in sonication time. In another research, Lim et al. [17] found an increase in flavonoid content from Phaleria macrocarpa fruit with increase in amplitude upto 60%, and then a decrease due to degradation of flavonoids at higher amplitude.
Effects of extraction parameters on antioxidant activity
The antioxidant activity of PoP was evaluated in terms of DPPH radical scavenging activity as well as FRAP. The DPPH antioxidant activity was found to vary from 9.40 to 9.89 mg GAEAC/g dry weight of pomegranate peel. The quadratic regression model was found to have satisfactory coefficient of determination (R2 = 0.76). A difference of less than 0.2 was obtained for predicted and adjusted R2 showing that there is no problem with data or the model. The regression coefficients of the fitted model (Table 3) showed a positive linear effect of methanol concentration as, higher methanol concentration reduced viscosity, and promoted cavitation [21, 22].The quadratic terms of duty cycle and ultrasonic time affected DPPH antioxidant activity significantly (p < 0.05). A higher duty cycle means more exposure of compounds to sonication which causes formation of hydroxyl radical leading to chemical decomposition that may adversely affect the radical scavenging activity [24]. The coefficient of variation (CV) was found very low (0.81) which represents higher reliability of the experiments. According to the significance values of coefficients, the effect of process variables on DPPH assay can be represented by Eq. (5).
A saddle point was observed in the response surface curve showing the effect of sonication time and duty cycle (Fig. 4b), as well as sonication time and amplitude (Fig. 4d) showing that some intermediate processing condition yielded higher antioxidant activity. Similar results were obtained by Shaterabadi et al. [25] for extract from black caraway, and Chakraborty et al. [26] for the extract from bitter gourd. This suggested, the selection of an appropriate duty cycle for the better recovery of compounds with high radical scavenging activity.
The results shown in Table 2 indicated the variation in FRAP from 74.81 to 96.25 mg TE/g with the variation in processing conditions. A quadratic model was fitted with a very good R2 value of 0.88, showing a better correlation between the observed and predicted values from the model (Table 3). A difference of less than 0.2 between the adjusted and predicted R2 suggested the accuracy of model for predicting the response. An insignificant lack of fit also, confirmed model reliability. The linear terms of amplitude, sonication time and methanol concentration were significant (p < 0.05). Tabaraki et al. [3] and Vu et al. [27] also found a significant effect of sonication time on FRAP of PoP extract and banana peel extract. The quadratic term of amplitude and the interaction of duty cycle and amplitude were found to have positive and significant effect on FRAP. The regression equation based on the significant values of coefficients is shown in Eq. (6).
The increase in FRAP with duty cycle, and amplitude (Fig. 5a) may be attributed to higher cavitation effect, which enhanced the reducing power of the compounds. Higher methanol concentration yielded more phenolics, thus yielded better antioxidant activity [21].
Effects of extraction parameters on total hydrolysable tannins
Hydrolysable tannins consist of major portion of tannins present in PoP. Ellagitannins, hydrolysable tannin, are metabolites of gallotannins, which can be hydrolyzed easily to form ellagic acids. They are found to have high antioxidant activity, hence a profitable extraction of THT is desirable [1]. The ultrasound assisted extraction significantly affected the THT content yield at varying conditions. The THT varied from 142.16 to 199.51 mg TAE/g dry weight of pomegranate peel (Table 2). A quadratic regression model (Eq. 7) was found to fit the data for THT with a good coefficient of determination (R2) of 0.86. Predicted R2 value of 0.58 gave satisfactory predictability of the quadratic model. Also, the model was found appropriate as the difference between predicted and adjusted R2 was found below 0.2.
The regression coefficients (Table 3) showed a highly significant (p < 0.01) linear effect of amplitude, sonication time and methanol concentration on THT. The interaction term of amplitude and methanol concentration was found significant. The increase in methanol concentration enhanced the recovery of hydrolysable tannins (Fig. 6b, d), which might be due to better solubility of hydrolysable tannins in organic solvents [1]. A better recovery of hydrolysable tannins with increase in amplitude, may be due to reduction in surface tension and viscosity, facilitating better effect of sonication [22]. The increase in methanol concentration at lower amplitude caused better extraction of tannins, while at higher amplitude the effect of methanol concentration is not significant. This may be due to more pronounced effect of ultrasonic power at a higher level compared to solvent concentration on extraction of hydrolysable tannins [22]. In a research by Kazemi et al. [18], Punicalagin which is a major ellagitannin of PoP, was found to extract better in PUAE, with duty cycle having least significant effect. On the contrary, Sousa et al. [28] found lesser recovery of ellagitannins by UAE compared to conventional extraction.
Effects of extraction parameters on total condensed tannins
Condensed tannins or proanthocyanidins does not have sugar residue, and yields anthocyanidins when depolymerized by oxidation [29]. In the present research, the TCT varied from 16.57 to 35.26 mg CE/g dry weight of pomegranate peel (Table 2). The fitted second order model was found to have a good coefficient of determination (R2 = 0.86). A difference of less than 0.2 in adjusted and predicted R2 suggested suitability of the model for prediction. A highly significant (p < 0.01) regression model p-value, suggested a good agreement of model data with the experimental results. A lower CV (5.38) showed a good reliability of experiments.
The linear as well as quadratic effects of duty cycle, amplitude and sonication time were found significant (p < 0.05). A similar result was observed by Prakash Maran et al. [30] and Sun et al. [31] for Nephelium lappaceum L. fruit and Larix gmelinii Bark, respectively, where they found significant effect of sonication time and power on extraction of proanthocyanidin. Both the linear and quadratic effect of methanol concentration was found insignificant, which may be due to the presence of condensed tannins in different forms i.e. soluble, insoluble or pure, which interact differently with aqueous methanol [32]. Since, there were no significant interaction terms for the condensed tannin extraction, the response curves did not varied much (Fig. 7a–d).
Optimization and verification of the predictive models
An optimization study was conducted to evaluate the optimal conditions for the operating variables, aimed at the maximization of response variables. Table 4 shows the predicted and experimental values of the response variables at optimal condition. The optimal condition was obtained at a medium duty cycle of 0.58, at 80% amplitude for 12.8 min of extraction in 70% aqueous methanol. The desirability was found as 0.95, which is a better representation for the optimum solutions. Experimental verification of the optimum solution was also conducted, which showed a relative error of less than 10% for all of the responses, representing a good validation by RSM models.
Comparison with continuous mode of sonication
The optimized results were further compared with continuous mode of sonication The TPC in continuous mode of sonication was obtained as 73.06 ± 0.24 mg GAE/g, which was 28% lesser than the yield by PUAE. The TFC, TCT and THT in continuous mode was obtained as 34.10 ± 2.44 mg QE/g, 26.10 ± 0.58 mg CE/g, and 161.54 ± 2.14 mg TAE/g respectively, which were significantly lesser compared to PUAE. The bioactivity in terms of antioxidant activity was also found to decrease significantly in continuous mode of sonication, compared to PUAE. The DPPH antioxidant activity and FRAP was found to be 8.31 ± 0.04 mg GAEAC/g and 81.12 ± 1.17 mg TE/g respectively which were 15% (DPPH) and 18% (FRAP) lesser compared to PUAE. It can be concluded that, the PUAE is better in terms of yield of bioactive compounds than continuous mode of sonication. Apart from that, it is economic, saves energy, as well as prevent damage to probe.
Correlation matrix and principal component analysis
Correlation analysis of response variables was done to find the relation among all the response variables (Fig. 8). TFC and FRAP showed the highest correlation coefficient of 0.79. THT was least correlated to DPPH antioxidant activity with a correlation coefficient of 0.09. The better correlation of flavonoids, condensed tannins and hydrolysable tannins was found with FRAP compared to DPPH antioxidant activity, showing the major antioxidant mechanism in PoP was due to electron transfer rather than hydrogen atom transfer. Similar results were obtained by Youn et al. [33] and Vu et al. [27], showing high correlation of FRAP with TFC and TPC. The correlation can be further visualized in principal component analysis (PCA). PCA was conducted for getting additional information on the influence of response variables analyzed, in relation to treatment conditions [26, 27]. The PCA was executed using two principal components (F1 and F2) explaining about 73.59% of variability: F1 (53.86%) and F2 (19.73%). It can be observed that samples with high TPC are rich in DPPH antioxidant activity as they are grouped together while samples with high TCT, THT and TFC are rich in FRAP antioxidant activity (Fig. 9). The DPPH antioxidant activity and FRAP are poorly correlated. This may be due to the difference in the mechanism of action in both methods as DPPH method uses free radical mechanism while FRAP utilizes metal ions for oxidation [34]. The tannins (condensed and hydrolysable) and flavonoids showed better reducing power due to higher degree of hydroxylation, and extent of conjugation compared to monomeric phenolics, which enhanced the FRAP antioxidant activity [35]. Hierarchical cluster analysis (HCA) was used to cluster the data into groups, based on the hierarchy of their relationships. HCA results are represented in terms of dendrogram (Fig. 10). The results are corroborating with PCA results, with the overall treatments (experimental runs) are divided into two broad clusters. It can be visualized that treatments yielding lower TFC (S2, S9, S12, S13, S17 and S25) are placed in same cluster with nearly similar heights while those giving higher TFC (S5, S11, S24, S26 and S28) are in different cluster. Similarly the treatments with similar values of response variables are in having same height or lesser dissimilarity (S5, S11; S6, S28; S2, S9; S18, S19). This made a clear understanding of treatments or experimental runs on the response variables.
Correlation matrix showing relationship between response variables in the factor analysis. Each variables distribution is shown in the diagonal, bottom of which is the distribution of each variable shown by scatter plot and top of it shows Pearson correlation coefficient. TPC total phenolic content; TFC total flavonoid content; FRAP ferric reducing antioxidant power; THT total hydrolysable tannin; TCT total condensed tannin)
Principal component analysis of response variables at different experimental runs (For codes of experimental runs, see Table 2; F1: principal component 1; F2: principal component 2; TPC total phenolic content; TFC total flavonoid content; FRAP ferric reducing antioxidant power; THT total hydrolysable tannin; TCT total condensed tannin)
Dendrogram of hierarchical cluster analysis of experimental runs based on the extraction of bioactive compounds (for codes of experimental runs, see Table 2)
Conclusions
Pulsed mode ultrasound assisted extraction was found very effective in the extraction of bioactive compounds from pomegranate peel. The experiments based on Box- Behnken design was found satisfactory and reliable for the analysis of UAE parameters on TPC, TFC, TTC, THT and antioxidant activity (DPPH and FRAP). A multi-response optimization based on RSM, enabled to find the optimal condition for the maximum recovery of bioactive compounds with high antioxidant activity. The optimum predicted values of the response variables were obtained at 70% methanol concentration, 12.8 min of sonication treatment at a duty cycle of 0.58, and 80% ultrasound amplitude. A comparative analysis of PUAE with continuous mode sonication showed that a phenolic recovery increased by 28% with PUAE also higher antioxidant activity of 18% (DPPH) was achieved in PUAE. The optimization results showed a good concurrence with the experimental results with a very low relative error (< 10%). A multivariate approach, based on PCA, suggested better correlation of tannins and flavonoid extracted from PoP with FRAP compared to DPPH antioxidant activity. Future study is recommended for the extraction and purification of individual compounds from the peel, their bioavailability, as well as other potential health benefits.
References
S. Akhtar, T. Ismail, D. Fraternale, P. Sestili, Food Chem. 174, 417 (2015)
S.E. El-Nemr, I.A. Ismail, M. Ragab, Food/Nahrung 34, 601 (1990)
R. Tabaraki, E. Heidarizadi, A. Benvidi, Sep. Purif. Technol. 98, 16 (2012)
S.R. Kanatt, R. Chander, A. Sharma, Int. J. Food Sci. Technol. 45, 216 (2010)
M.N. Clifford, A. Scalbert, J. Sci. Food Agric. 80, 1118 (2000)
J. Živković, K. Šavikin, T. Janković, N. Ćujić, N. Menković, Sep. Purif. Technol. 194, 40 (2018)
S.L.C. Ferreira, R.E. Bruns, H.S. Ferreira, G.D. Matos, J.M. David, G.C. Brandão, E.G.P. da Silva, L.A. Portugal, P.S. dos Reis, A.S. Souza, W.N.L. dos Santos, Anal. Chim. Acta 597, 179 (2007)
A.J. Purohit, P.R. Gogate, Sep. Sci. Technol. 50, 1507 (2015)
V.L. Singleton, J.A.J. Rossi, Am. J. Enol. Vitic. 16, 144 (1965)
Q.V. Vuong, S. Hirun, P.D. Roach, M.C. Bowyer, P.A. Phillips, C.J. Scarlett, J. Herb. Med. 3, 104 (2013)
P. Rani, A. Kumar, S.R. Purohit, P.S. Rao, LWT Food Sci. Technol. 89, 155 (2018)
I.F.F. Benzie, J.J. Strain, Anal. Biochem. 239, 70 (1996)
R.B. Broadhurst, W.T. Jones, J. Sci. Food Agric. 29, 788 (1978)
H. Saad, F. Charrier-El Bouhtoury, A. Pizzi, K. Rode, B. Charrier, N. Ayed, Ind. Crops Prod. 40, 239 (2012)
J.S. Boeing, É.O. Barizão, B.C. e Silva, P.F. Montanher, V. de Cinque Almeida, J.V. Visentainer, Chem. Cent. J. 8(1), 48 (2014)
A.J.D. Vega, R.-E. Hector, L.-G. Juan Jose, L.-G. Maria, H.-C. Paola, Á.-S. Raúl, O.-V. Carlos Enrique, Czech J. Food Sci. 35, 456 (2017)
Y.P. Lim, S.F. Pang, M.M. Yusoff, S.K. Abdul Mudalip, J. Gimbun, J. Appl. Res. Med. Aromat. Plants 14, 100224 (2019)
M. Kazemi, R. Karim, H. Mirhosseini, A. Abdul Hamid, Food Chem. 206, 156 (2016)
G. Aguilar-Hernández, M. De Lourdes García-Magaña, M. De los Ángeles Vivar-Vera, S.G. Sáyago-Ayerdi, J.A. Sánchez-Burgos, J. Morales-Castro, L.M. Anaya-Esparza, E.M. González, Molecules 24, 904 (2019)
A. Tomšik, B. Pavlić, J. Vladić, M. Ramić, J. Brindza, S. Vidović, Ultrason. Sonochem. 29, 502 (2016)
W. Chen, W.-P. Wang, H.-S. Zhang, Q. Huang, Carbohydr. Polym. 87, 614 (2012)
S.U. Kadam, B.K. Tiwari, T.J. Smyth, C.P. O’Donnell, Ultrason. Sonochem. 23, 308 (2015)
G. Pan, G. Yu, C. Zhu, J. Qiao, Ultrason. Sonochem. 19, 486 (2012)
C. Zhu, X. Zhai, L. Li, X. Wu, B. Li, Food Chem. 177, 139 (2015)
D. Shaterabadi, M. Aboonajmi, Food Sic Nutr. (2020). https://doi.org/10.1002/fsn3.1733
S. Chakraborty, R. Uppaluri, C. Das, Food Bioprod. Process. 120, 114 (2020)
H.T. Vu, C.J. Scarlett, Q.V. Vuong, J. Food Process. Preserv. 41, 1 (2017)
A.D. Sousa, A.I.V. Maia, T.H.S. Rodrigues, K.M. Canuto, P.R.V. Ribeiro, R. de Cassia Alves Pereira, R.F. Vieira, E.S. de Brito, Ind. Crops Prod. 79, 91 (2016)
H. Liu, T. Zou, J. Gao, L. Gu, Food Chem. 141, 488 (2013)
J. Prakash Maran, S. Manikandan, C. Vigna Nivetha, R. Dinesh, Arab. J. Chem. 10, S1145 (2017)
X. Sun, Z. Jin, L. Yang, J. Hao, Y. Zu, W. Wang, W. Liu, J. Chem. (2013). https://doi.org/10.1155/2013/541037
P.E. Shay, J.A. Trofymow, C.P. Constabel, Plant Methods 13, 1 (2017)
J.S. Youn, Y.J. Kim, H.J. Na, H.R. Jung, C.K. Song, S.Y. Kang, J.Y. Kim, Food Sci. Biotechnol. 28, 201 (2019)
A. Floegel, D.-O. Kim, S.-J. Chung, S.I. Koo, O.K. Chun, J. Food Compos. Anal. 24, 1043 (2011)
A.E. Hagerman, K.M. Riedl, G.A. Jones, K.N. Sovik, N.T. Ritchard, P.W. Hartzfeld, T.L. Riechel, J. Agric. Food Chem. 46, 1887 (1998)
Acknowledgements
This work was financially supported by the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, A., Srinivasa Rao, P. Optimization of pulsed-mode ultrasound assisted extraction of bioactive compounds from pomegranate peel using response surface methodology. Food Measure 14, 3493–3507 (2020). https://doi.org/10.1007/s11694-020-00597-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00597-9