Abstract
In this study, pulsed mode ultrasound assisted extraction (PUAE) was investigated as green technology for the extraction of bioactive compounds from oregano (Origanum vulgare) leaves. A Box–Behnken experimental design (BBD) combined with response surface methodology (RSM) was used to determine the optimum sonication parameters. Solvent concentration and feed to solvent (F:S) ratio were first optimized using single factor experiment, followed by the optimization of three pulsed mode sonication parameters, namely sonication time, amplitude percentage and duty cycle. RSM was used to simultaneously maximize all the four responses viz. total phenolic content (TPC), total flavonoid content (TFC) and antioxidant capacity in terms of gallic acid equivalent antioxidant capacity (GAEAC) and ferric reducing antioxidant power (FRAP). The experimental results were fitted to quadratic models and statistical analysis in terms of multiple regression and analysis of variance (ANOVA) was carried out for each response variable. The optimum values of response variables were obtained at a sonication time of 10 min, using a duty cycle of 0.8 at 80% of sonication amplitude. Multivariate analysis in terms of principal component analysis (PCA) and hierarchical cluster analysis (HCA) was done for better understanding of interactions among the response variables and treatment conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current scenario is focusing on the increased consciousness among consumers for the use of natural compounds for nutritional, nutraceutical and other health applications [1]. Earlier researches established the fact that plants provide a wide range of bioactive compounds, starting from phenolic metabolites, terpenoids and alkaloids, to name a few [2]. Among these, phenolic compounds are among the most abundantly found phytochemicals which serve immense health benefits in terms of their anti-oxidative, anti-inflammatory, anti-carcinogenic as well as anti-microbial properties [3]. Oregano (Origanum vulgare) leaves are a promising source of anti-oxidants, being used in the form of its essential oils and also as soluble phenolic extracts. It is a Mediterranean herb mainly used for culinary and medicinal purposes. It has found applications in treatment of various diseases like respiratory tract disorders, gastro-intestinal (GI) tract disorders, urinary tract disorders, menstrual cramps, etc. [4].
The major bioactive compounds of oregano are the isomer phenols carvacrol and thymol, along with their precursor monoterpenes, p-cymene and γ-terpinene, limonene, pinene, caryophyllene, rosmarinic acid, protocatechuic acid, etc. [5]. Carvacrol and thymol help in preventing cell damage induced by free radicals and improve the body’s ability to resist against infections [6]. One major problem encountered in industries is that, after obtaining the essential oils from oregano leaves by hydrodistillation, steam distillation or any other suitable methods, the spent leaves remain un-utilized which still contains some bioactive compounds, mostly in bound phenolics form. In large industrial applications, huge amount of spent leaves are generated, which do not have any significant utility. This needs for the development of an optimized extraction method for product valorization.
Several extraction techniques have been developed in due course of time for the extraction of these phytochemicals in a simple and efficient way [7]. They are mainly categorized as conventional and non-conventional extraction methods. The conventional methods include maceration [8], soxhlet extraction [9], hydro-distillation [10], etc. while non-conventional methods include ultrasound assisted extraction [11], enzyme-assisted extraction [12], pulsed electric field extraction [13], microwave-assisted extraction [14], ohmic heating [15], pressurized liquid extraction and supercritical fluid extraction [16] among others. Conventional methods are time-consuming, require high purity of solvents, which add to the cost, use a large amount of solvent and have less solvent recovery [17]. Hence, eventually they were replaced by non-conventional methods of extraction. Excluding few techniques like high pressure processing (HPP), pulsed electric Field (PEF) extraction, ultrasound-assisted extraction (UAE) and supercritical fluid extraction (SCFE)), most of the non-conventional methods are generally expensive and non-scalable. Ultrasound assisted extraction (UAE) was found to be a promising extraction technique, amenable to its automation, which holds the key to achieve sustainable green chemistry [11]. Probe type UAE proves to be a better extraction technique as compared to bath type UAE, producing bioactive compounds with high antioxidant properties [18]. It fulfills not only the advantages of ultrasound application, but also gives a direct application of sonication over the samples, thus resulting in effective and uniform extraction. Pulsed mode UAE (PUAE) is a modification in the sonication treatment, where sonication effect is provided in a discontinuous manner by application of ultrasound for a pre-determined duration, followed by a rest period. This pulsed effect is explained in terms of duty cycle, which is the ratio of duration of ultrasound effect to the total duration for one cycle. Due to discontinuous effect, PUAE gives better extraction as compared to continuous mode with low power consumption and better control on extraction temperature, which improves purity and extractability [19]. It also eliminates the requirement of an additional temperature controller, and improves the probe life [20].
Response surface methodology (RSM) is widely used for process optimization, as it reduces the number of experimental trials and gives a better explanation among the interaction of responses with the input parameters [21]. Many experimental designs are formulated to solve the purpose, which includes full factorial design (FFD), central composite design (CCD), central composite rotatable design (CCRD), Box-Behnken design (BBD), Doehlert matrix (DM) etc. [22]. Among these, BBD is a superior model among others, because of fewer experimental runs and efficient experimental design [23]. It is a multivariate optimization technique on the basis of three level design, used for second order polynomial model [24]. It is also economical as it proposes lesser number of experimental trials with higher efficiency [20].
Apart from the extraction technique followed, the bioactive recovery depends on solvent properties, its concentration being used for extraction, feed to solvent (F:S) ratio, etc. which in turn depends on the extent of solubility and diffusivity of those solvents [25]. However, to the best of our knowledge, the effect of solvent concentration, F:S ratio, sonication amplitude, sonication time and duty cycle of PUAE for extraction of phenolic compounds from oregano leaves has not been attempted so far. Hence, the present study has focused on the effect of solvent as well as pulsed mode sonication parameters, i.e. sonication time, duty cycle, and amplitude on total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity (in terms of gallic acid equivalent anti-oxidant capacity (GAEAC) and ferric reducing antioxidant power (FRAP)). Optimization was carried out using BBD of RSM to maximize the response variables. Furthermore, multivariate analysis in terms of principal component analysis (PCA) and hierarchical cluster analysis (HCA) were done for better understanding of interactions among the response variables and treatment conditions.
Materials and methods
Raw materials
Freeze-dried oregano leaf shreds (moisture content of 13.72% (db)) were procured from Keya Foods International Pvt. Ltd., Mumbai, India. They were ground to fine powder using mixer grinder, and further sieved through BS 95 sieves to obtain a particle size less than 0.2 mm.
Chemicals
All chemicals and laboratory reagents used in the experimental work were of analytical grade, and were procured from Himedia and Merck, India. The chemicals and reagents were 2,2-diphenyl-1-picrylhydrazyl (DPPH), Folin–Ciocalteau (FC) reagent, sodium hydroxide, sodium carbonate, sodium nitrite, sodium acetate, acetic acid, ferric chloride, hydrochloric acid, 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), aluminum chloride, ethanol, methanol and acetone.
Ultrasound-assisted extraction
Oregano leaf extraction was performed using a probe sonicator (ATP 500, Athena Technologies, Pvt. Ltd., India), with power of 500 W and frequency of 40 kHz. The sonotrode of 6 mm diameter was used for extraction. In case of single factor experiments, sonication was carried out at affixed duty cycle of 0.8 for a sonication time of 10 min and 80% of amplitude (expressed as percentage of maximum power). The sonotrode was submerged to a depth of 1 cm within the sample. After extraction, the samples were centrifuged using a cooling centrifuge for 10 min at 10,000 rpm, maintaining a temperature of 4 °C throughout the centrifugation process. After centrifugation, the supernatant was filtered through Whatman No. 4 filter paper, and stored in air tight conditions at − 32 °C until further analysis.
Single factor experiments
Initially, single factor experiment was carried out to optimize the solvent concentration and F:S ratio. Solvent concentration was optimized using different solvents, namely, de-ionized water (DiW), methanol, ethanol and acetone with concentrations (except DiW, which was taken in pure form) of 25, 50, 75% (v/v) and pure solvent (99–100% purity), hence giving a total of thirteen solvent concentrations. These particular solvents were chosen because they have shown good extraction properties with a large number of raw materials, as observed in the previous literatures [26]. Moreover, acetone and ethanol belong to class-3 solvents while methanol belongs to class-2 solvents, which are safe for human health and pharmaceutical applications when used within permissible limits, as per FDA guidelines [27]. TPC, GAEAC and TFC were the measured responses. The concentration of solvent giving the maximum overall response was chosen as the optimum solvent concentration. In the next phase, keeping the solvent concentration fixed at the optimum value, F:S ratio was varied from 1:5 to 1:25 g/mL, and similarly the one with highest overall response was chosen as the optimum F:S ratio. The optimum solvent concentration and F:S ratio were fixed for further experimentation.
Experimental design
A three factor (k), three level (L) BBD of RSM, with five central points (C0) was applied for optimization of the extraction conditions for maximum bioactive recovery from oregano leaves. Total number of experiments (N) were determined using the formula given by Maran [28]:
Three independent variables, namely sonication time (\({x}_{1}\)), amplitude (\({x}_{2}\)) and duty cycle (\({x}_{3}\)) were optimized for the probe type sonicator. The natural and coded values of the independent variables are summarized in Table 1. The experimental combinations for the independent parameters are presented in Table 2. The levels of independent variables were fixed on the basis of preliminary experimentations. The responses measured were TPC, GAEAC, TFC and FRAP. The experimental data were fitted using the following second order polynomial function:
where \({y}_{j}\) represents different responses; \({\beta }_{0}\), \({\beta }_{i}\), \({\beta }_{ii}\) and \({\beta }_{ij}\) are the regression coefficients for the mean, linear, quadratic and interaction terms, respectively, and \({x}_{i}\) & \({x}_{j}\) are the independent variables in coded values, ranging from − 1 to + 1.
Numerical optimization
Response variables were optimized using an optimization function given by Derringer and Suich [29], with a target to maximize TPC, TFC, GAEAC and FRAP. Each response was transformed into a dimensionless desirability function (di), which varied from lower to higher desirability values (0 to 1). The maximization of any response was denoted by the overall desirability function (G), which was obtained by combining the individual desirability values of each response (Eq. 3).
where, ‘di’ denotes the desirability of the response and ‘n’ is the number of responses in the experiment. The validity of the present developed model was performed under the optimal conditions as predicted by the model. The average values of the experiments were compared with the predicted values of the developed model and the accuracy was assessed for suitability of the established model.
Biochemical analysis of extracts
Total phenolic content (TPC)
TPC of the extract was determined by Folin Ciocalteau (FC) method proposed by Singleton et al. [30] with minor modifications. The method utilizes the reaction of phenolic compounds with FC reagent (FCR) in alkaline media to change the color of reaction mixture from yellow to blue. FCR is a redox reagent, which upon reaction with polyphenols forms a blue chromophore which is nothing but a phospho-tungstic-phospho-molybdenum complex. Briefly, 0.2 mL of the extract sample were taken in a test tube and 2.8 mL DiW was added to it, followed by the addition of 0.5 mL of FCR (10 times diluted) and 0.75 mL sodium carbonate (Na2CO3, 20% w/v). The samples were properly mixed and then incubated in dark for 90 min. Absorbance was measured at wavelength of 760 nm using UV–Vis spectrophotometer. Gallic acid was taken as the standard and TPC was expressed in terms of mg gallic acid equivalents per dry weight of sample (mg GAE/g sample). This method not only measures the phenolic compounds, but also measures the total reducing capacity of a sample.
Total flavonoids content (TFC)
TFC was determined using a colorimetric method based on the reaction of flavonoid with AlCl3, as proposed by Vuong et al. [31] with slight modifications. AlCl3 forms stable complexes with C-4 keto groups and either C-3 or C-5 hydroxyl group of flavones and flavonols. Briefly, 0.5 mL of diluted extract was taken in a test tube. 2 mL DiW was added to it, followed by addition of 0.15 mL NaNO2. After 4–5 min, 0.15 mL of 10% AlCl3 solution was added to it. 5 min later, 1 mL of 1 M NaOH was added, followed by addition of 1.2 mL DiW. The sample was incubated for about 10 min. The absorbance was measured at a wavelength of 510 nm using a UV–Vis spectrophotometer. Quercetin was taken as the standard with TFC recorded as mg quercetin equivalents per gram dry weight of sample (mg QE/g sample).
Antioxidant activity
Gallic acid equivalent antioxidant capacity (GAEAC)
DPPH method, as proposed by Vuong et al. [31], was followed with slight modifications. Briefly, 0.2 mL of the diluted extract was taken in a test tube and to it, 0.95 mL of DPPH (2,2–Diphenyl–1–Picrylhydrazyl) solution and 2.85 mL of pure methanol were added. The sample was incubated for half an hour in dark. Absorbance was measured at a wavelength of 517 nm using UV–Vis spectrophotometer. Zeroing was done by pure methanol. Gallic acid was taken as the standard and the results were expressed in terms of mg gallic acid equivalents antioxidant capacity per gram of sample (mg GAEAC/g sample). It was measured using the formula:
where C = concentration of gallic acid (g/mL), v = volume of sample taken (mL), w = weight of powder taken in the sample (g).
Ferric reducing antioxidant power (FRAP)
A FRAP assay was prepared according to Benzie and Strain [32] with some modifications. Briefly, 25 µL dilute extract was added to 2.975 mL DiW and 2 mL of FRAP reagent. The FRAP reagent constituted 100 mL of 300 mM acetate buffer of pH 3.6; 10 mL of 10 mM of TPTZ (2,4,6-Tri(2-pyridyl)-1,3,5-triazine) mixed in 40 mM hydrochloric acid (HCl) solution and 10 mL of 20 mM ferric chloride hexahydrate (FeCl3·6H2O) solution. The absorbance was measured at a wavelength of 593 nm after 30 min of incubation in dark. The results were expressed in terms of mg Trolox equivalent per gram dry weight of sample (mg TE/g sample).
Statistical analysis
All the experiments were done in triplicate and the results were given as mean value ± standard deviation. The difference between the pair of means were analysed using Tukey test within a confidence interval of 95%. All experiments were carried out in triplicate. The fitness of models were verified by computing the coefficient of determination (R2) and adjusted coefficient of determination (adj. R2). Design Expert 11 (Stat-Ease Inc., Minneapolis, USA) software was used for the optimization of extraction conditions and plotting the response surfaces of various factor–response interactions. It also aided in confirming the adequacy of fitted RSM models.
Correlation analysis of the data was carried out using R studio (Version 1.2.5033, RStudio-PBC, Vienna, Austria) to find the correlation coefficient between the response variables. Multivariate analysis in terms of principal component analysis (PCA) and hierarchical cluster analysis (HCA) was also carried out using the same software to determine the association among different experimental variables.
Results and discussion
Effect of solvent concentration and F:S ratio on bioactive recovery from oregano leaves
Prior to response surface study, the effects of solvent concentration and F:S ratio on TPC, GAEAC and TFC were investigated. F:S ratio was fixed at 1:15 g/mL for optimization of solvent concentration. As shown in Table 3, aqueous solvents were found to have better extractability of bioactive compounds as compared to pure solvents. This is attributed to the mixed polarity of aqueous solvents, that has better extractability as compared to pure ones [33]. 75% aqueous acetone showed the highest extractability among other solvent concentrations. This is because of the presence of more polar bioactive compounds in oregano leaves having higher affinity towards aqueous acetone as compared to other solvents [34]. Piechocka et al. [35] have also found similar results for Mulberry (Morus alba) fruits.
In the next phase, solvent concentration was kept constant at the obtained optimum value of 75% aqueous acetone, and F:S ratio was optimized. For F:S ratio (Table 4), it was observed that the extractability (in terms of TPC, GAEAC and TFC) enhanced with increase in solvent volume. This result was in corroboration with the mass transfer principle, explaining that extractability increases with increase in concentration gradient between solid and solvent [36]. However, higher F:S ratio means higher solvent usage, and thus, higher energy utilization in further treatment [37]. Increasing the F:S ratio from 1:5 to 1:20 g/mL increased the values of TPC, GAEAC and TFC to about 22.86, 2.88 and 34.04%, respectively, while increasing it from 1:20 to 1:25 g/mL showed insignificant changes, increasing the respective responses to about 0.43, 0.04 and 0.6%. Hence, optimum solvent concentration of 75% aqueous acetone and F:S ratio of 1:20 g/mL were chosen for further experimentation.
Effect of process parameters on TPC
The variation in TPC at different processing conditions of sonication is represented in Table 2. TPC varied from 59.01–66.09 mg GAE/g sample. The regression coefficients and statistical parameters (ANOVA) of the obtained data are represented in Table 5. The regression analysis showed that the linear terms of amplitude and duty cycle had significant effect on TPC, while sonication time had a non-significant effect. Amplitude had a negative and highly significant (p < 0.01) effect, while duty cycle had a positive and significant (p < 0.05) effect. Similarly, the quadratic effects of time and duty cycle were insignificant, while that of amplitude had a positive and significant (p < 0.1) effect on TPC. The interaction term of amplitude and duty cycle was negative and significant (p < 0.05). The model quadratic equation for the effect of process variables on TPC, by taking the significant terms into consideration is represented as follows:
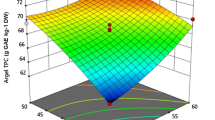
The response surface (RS) plots (Fig. 1) show the effect of various factors like sonication time, amplitude and duty cycle on TPC of the extract. The combination of amplitude and time showed positive correlation towards TPC yield. Martínez-Patiño et al. [23] observed similar trend in TPC for olive tree biomass. Amplitude and duty cycle were negatively correlated (Table 2), and it can be observed in RS plot that high amplitude at a low duty cycle resulted in higher TPC. Amplitude had a positive effect on TPC, but increase in duty cycle resulted in longer exposure of sample to high sonication effect, resulting in deteriorative effect of cavitation on the phenolic compounds [38]. Sonication time, however, showed a positive effect on TPC. Similar observations were made by Tabaraki et al. [39] for extraction of pomegranate peel antioxidants. The model F-value for TPC was 5.78, and was significant (p < 0.05) as suggested by ANOVA. R2 was found to be 0.88, showing a good accuracy of the model. Adjusted R2 was 0.73, suggesting a good correlation of experimental values with that of the predicted. Coefficient of variation (C.V.) of 1.52% confirmed good precision and accuracy of the model. Lack of fit (p–value) was found to be 0.20, which was insignificant (p > 0.05) and hence, desirable. The overall results showed that TPC increased with increase in input parameters, and the highest value of 66.09 mg GAE/g was obtained at 80% amplitude, 0.6 duty cycle and extraction time of 15 min.
Effect of process parameters on TFC
The variations in TFC with processing conditions is also presented in Table 2. The values of TFC varied from 33.087–54.012 mg QE/g sample. The regression coefficients, as given in Table 5 suggests that all linear terms (i.e. time, amplitude and duty cycle) had highly significant effect (p < 0.01) on TFC. Time and amplitude had positive effect while duty cycle had a negative effect. The quadratic term of time had negative and highly significant effect (p < 0.01) while that of amplitude and duty cycle had insignificant effect. The interaction terms had insignificant effect on TFC. The model quadratic equation for TFC, considering the significant terms only, is as follows:
The RS curve of TFC showed a saddle point for the effect of time and amplitude (Fig. 2a) showing that increase in sonication time had detrimental effect on TFC. Longer exposure of compounds at high amplitude may result in increase in temperature, which causes thermal degradation of flavonoids, thus resulting in decrease of TFC [40]. Similar effect was also observed for duty cycle and time. Silva et al. [41] found similar response for time in the extraction of phenolics from Inga edulis leaves. However, amplitude and duty cycle showed a significant positive effect (Fig. 2c), showing that high amplitude and duty cycle resulted in higher TFC yield. The reason might be the synergism in the combined effect which enhanced the flavonoid recovery [42]. The model F–value was obtained as 14.54 and, was highly significant as suggested by ANOVA. R2 and adjusted R2 were 0.95 and 0.88, respectively, showing very high accuracy of the model as well as a high correlation between the predicted and experimental values. C.V. was 4.25%, suggesting good precision of the model with high accuracy. Lack of fit (p–value) was found to be insignificant (0.16). Highest TFC value of 54.012 mg QE/g was obtained at sonication time of 10 min, 80% amplitude and 0.8 duty cycle.
Effect of process parameters on GAEAC
GAEAC varied from 9.00–11.12 mg GAEAC/g sample (Table 2). The regression coefficients (Table 5) revealed that linear terms of amplitude, and duty cycle had significant effect while sonication time had an insignificant effect on GAEAC. Amplitude had a negative and highly significant effect (p < 0.01) while duty cycle had a positive and significant effect (p < 0.05). The quadratic terms of amplitude had positive and significant effect (p < 0.1) while that of sonication time and duty cycle had insignificant effect. Similarly, the interaction term of time and amplitude had a negative and highly significant effect (p < 0.01) on GAEAC. The model quadratic equation for GAEAC, considering the significant terms, is as follows:
As already confirmed by the regression analysis, the RS plot also clearly represented the negative correlation of time and amplitude (Fig. 3a). Longer extraction time has degrading effect on the anti-oxidative properties of the extract, as exposure of compounds for longer period may lead to formation of hydroxyl radical resulting in chemical decomposition, thus adversely affecting the radical scavenging activity [43]. Similar trend was followed in the extraction of bioactive compounds from olive tree biomass [23]. Model F–value for GAEAC was 6.91 and was highly significant as suggested by ANOVA. R2 was 0.90, suggesting good accuracy of the model and adjusted R2 was 0.77, suggesting good correlation between experimental and predicted values. C.V. was 2.55%. Since the value was less than 5%, it denotes good accuracy and precision of the model. The lack of fit (p–value) was also insignificant (0.36). The highest value of GAEAC was found to be 11.121 mg GAEAC/g at a lower sonication time of 5 min, 80% amplitude and intermediate duty cycle of 0.6.
Effect of process parameters on FRAP
Under different process conditions, FRAP values varied from 29.92–36.88 mg TE/g sample (Table 2). The regression coefficients (Table 5) showed all the linear terms to be highly significant for FRAP (p < 0.01). Sonication time and duty cycle had positive effect while amplitude had a negative effect. Among the quadratic terms, only time had a negative and highly significant effect (p < 0.01). Similarly, the interaction term of time and amplitude had a positive and highly significant effect (p < 0.01) on FRAP. All other terms had insignificant effect. The model quadratic equation for FRAP is as follows:
From the RS plots as observed in case of TFC, FRAP also showed a similar result. An intermediate time resulted in higher FRAP for both amplitude and duty cycle (Fig. 4a, b). Increase in sonication time had a detrimental effect on antioxidative compounds in the extract due to thermal degradation [40]. However, higher duty cycle and amplitude showed a higher FRAP value (Fig. 4c), possibly due to higher cavitation effect [44]. The model F–value was found to be 21.64 suggesting the model to be statistically significant (ANOVA). R2 was 0.96, suggesting very high accuracy of the model. Adjusted R2 was also high (0.92) suggesting a high correlation between the predicted and experimental values. C.V. was 1.8%, indicating good accuracy and precision of the model. The lack of fit (p–value) was 0.47, which was statistically insignificant and hence, desirable. The overall highest value of FRAP was obtained at a sonication time of 10 min, amplitude of 80% and duty cycle of 0.8, yielding a value of 36.879 mg TE/g sample.
Optimization of PUAE conditions
RSM was followed for maximizing the bioactive recovery from oregano leaves in terms of its TPC, TFC, GAEAC and FRAP. The values of different responses analyzed at varying sonication conditions are shown in Table 2. The regression coefficients (in terms of coded values) determined by analysis of variance (ANOVA) for each model, and other statistical parameters like F-values, coefficient of determination (R2), adjusted R2, coefficient of variation (C.V.) and lack of fit (p-value) are summarized in Table 5.
The F-value for all responses (as shown in Table 5) indicated that the model obtained was statistically significant (p < 0.05). High R2 values also confirmed good accuracy of the chosen model. The adjusted R2 values were close to that of R2 values, confirming a high degree of correlation between the predicted and the experimental values. Also, in every case, the C.V. were less than 5%, confirming good precision and high reproducibility of the model, hence validating RSM. Moreover, the p-value (for lack of fit) was insignificant (p > 0.05) in all cases, suggesting that dispersion of experimental data was model–independent measure of pure error [23].
Table 6 represents the predicted values of the factors, responses and their corresponding experimental values. The relative error was less than 10% for all cases, suggesting that the model was suitable and the values thus obtained were optimized values. Higher amplitude percentage and duty cycle ensured desirable sonication effect, while intermediate exposure time for sonication kept the thermal degradation under control. This combination, along with optimum solvent parameters resulted in maximum bioactive recovery from oregano leaves. Hence, the optimum values of time, amplitude and duty cycle were 10 min, 80% and 0.8, respectively, with TPC, TFC, GAEAC and FRAP values of 63.07 ± 0.96 mg GAE/g, 53.61 ± 1.59 mg QE/g, 9.50 ± 0.06 mg GAEAC/g, and 37.25 ± 1.02 mg TE/g, respectively.
Correlation analysis of the response variables
Correlation analysis of the response data showed maximum correlation of GAEAC with TFC (0.66) followed by TPC (0.65) (Fig. 5). This clearly showed that phenolic compounds as well as flavonoids in oregano leaves have radical scavenging ability. The lower correlation of FRAP with TFC (0.55) and TPC (0.59), as compared to GAEAC, suggested that a better hydrogen atom transfer property was found in the bioactive phenolics compared to electron transfer property [45]. Furthermore, a lower correlation coefficient of 0.26 was observed for FRAP and GAEAC, showing a difference in the mechanism of antioxidant activities, which caused different interactions with the bioactive compounds. Similar correlation results were observed by Elufioye et al. [34] for Bryophyllum pinnatum extract, Deng et al. [46] for young barley grass extract and Ma et al. [47] for soymilk extract.
Correlation matrix showing relationship among response variables in the factor analysis. Each variables’ distribution is shown in the diagonal, bottom of which is the distribution of each variable shown by scatter plot and top of it shows Pearson correlation coefficient. TPC total phenolic content, TFC total flavonoid content, GAEAC Gallic acid equivalent antioxidant capacity, FRAP ferric reducing antioxidant power)
Multivariate analysis
Multivariate analysis of the obtained data was carried out using two popular statistical tools, i.e. PCA and HCA. PCA was conducted for getting additional information on the influence of response variables analyzed, in relation to treatment conditions. PCA was executed using two principal components (PC 1 and PC 2), explaining about 83.37% of variability: PC 1 explaining about 64.79% and PC 2 about 18.58% of the total variability. From Fig. 6, it can be observed that all the response variables were positively correlated with the PC 1. TPC and FRAP were positively correlated while TFC and GAEAC were negatively correlated with PC 2. It can be observed that GAEAC and FRAP were poorly correlated. This is due to the difference in the mechanism of action in both methods, as GAEAC uses free radical mechanism while FRAP utilizes metal ions for oxidation [48]. Similar result was obtained with correlation matrix. Treatments at the centre point (1, 2, 4 and 13) have lower values of all the response variables except treatment 9. Treatments at higher amplitude and higher duty cycle (6 and 7) were found to have higher FRAP, while the treatments with low duty cycle and amplitude (3, 8 and 10) were found to have low values of FRAP and TFC. HCA was used to cluster the data into groups, based on the hierarchy of their relationships. HCA results were represented in terms of dendrogram (Fig. 7). The results were corroborating with PCA results, with the overall treatments (experimental runs) being divided into three broad clusters. It can be visualized that treatments yielding lower TFC and FRAP (3, 10 and 11) were placed in same cluster with nearly similar heights, while those giving higher values of TFC and FRAP (2, 7, 12, 16, 17) were in a different cluster. The third cluster had two sub-clusters with the treatments at centre points (1, 4, 9 and 13) being placed in one sub-cluster. The results shown by PCA, as well as HCA can be seen in accordance with RSM results discussed earlier. This made a clear understanding of treatments on the response variables.
Principal component analysis of response variables at different experimental runs (For codes of experimental runs, see Table 2; Comp 1: principal component 1 (PC 1); Comp 2: principal component 2 (PC 2); TPC: total phenolic content; TFC: total flavonoid content; GAEAC: Gallic acid equivalent antioxidant capacity; FRAP: ferric reducing antioxidant power)
Dendrogram of hierarchical cluster analysis (HCA) of experimental runs based on the extraction of bioactive compounds (for codes of experimental runs, see Table 2)
Conclusions
In this work, a pulsed mode sonication was carried out for extraction of phenolic rich bioactive compounds from oregano leaves. The combination of optimum extraction conditions were obtained for TPC, TFC, GAEAC and FRAP. Single factor experimentation on optimum solvent concentration and F:S ratio gave the most effective solvent (75% aqueous acetone at 1:20 g/mL) to be used for extraction. BBD of RSM proved to be an efficient statistical tool for optimizing process parameters. According to the design, the optimum extraction conditions were a sonication time of 10 min with 80% amplitude and 0.8 duty cycle. There was a very good correlation of the experimental data with that of the predicted, with relative error < 10% for each case, suggesting the suitability and reliability of the model. PUAE, along with the satisfactory results in extraction, also ensured temperature control, low power consumption, reduced extraction time and enhancement of probe life. This method is particularly helpful for extraction of thermo-sensitive bioactive compounds. Correlation analysis of the responses confirmed radical scavenging ability (RSA) of oregano leaf polyphenols and flavonoids. Furthermore, multivariate analysis in terms of PCA explained about 83.37% of the overall variability in two major principal components. PC 1 explained majority of the variability (64.79%), and showed a positive correlation of TPC with FRAP. HCA divided the responses into three broad clusters, mainly based on the lower, intermediate and higher values of TFC and FRAP. Higher extractability of bioactive compounds with reduced time and minimal thermal degradation shows the supremacy of ultrasound assisted extraction. Moreover, its working on the principle of green chemistry favors this method over other non-conventional methods. Most importantly, considering the industrial constraints with generation of huge amount of un-utilized spent leaves (after essential oil extraction), this protocol will be highly helpful for achieving product valorization. It will also pave way for efficient extraction of bioactive compounds from other plant sources, especially with high phenolic and flavonoid compounds to be used in various food formulations and other processing applications.
References
U. Hölker, M. Höfer, J. Lenz, Appl. Microbiol. Biotechnol. 64, 175 (2004)
J.B. Harborne, Phytochemistry 51, 417 (1999)
M.J. Carvalho, C.S. Gouveia, A.C. Vieira, A.C. Pereira, M. Carvalho, J.C. Marques, J. Food Sci. 52, 98 (2017)
E.P. Gutiérrez-Grijalva, M.A. Picos-Salas, N. Leyva-López, M.S. Criollo-Mendoza, G. Vazquez-Olivo, J.B. Heredia, Plants 7, 1 (2018)
S. Kokkini, R. Karousou, A. Dardioti, N. Krigas, T. Lanaras, Phytochemistry 44, 883 (1997)
A. Guarda, J.F. Rubilar, J. Miltz, M.J. Galotto, Int. J. Food Microbiol. 146, 144 (2011)
J. Azmir, I.S.M. Zaidul, M.M. Rahman, K.M. Sharif, A. Mohamed, F. Sahena, M.H.A. Jahurul, K. Ghafoor, N.A.N. Norulaini, A.K.M. Omar, J. Food Eng. 117, 426 (2013)
N. Ćujić, K. Šavikin, T. Janković, D. Pljevljakušić, G. Zdunić, S. Ibrić, Food Chem. 194, 135 (2016)
M. A. López-Bascón-Bascon and M. D. Luque de Castro, (2019) Liq. Extr., 327 (2019).
A. Wollinger, É. Perrin, J. Chahboun, V. Jeannot, D. Touraud, W. Kunz, Comptes Rendus Chim. 19, 754 (2016)
F. Chemat, N. Rombaut, A.G. Sicaire, A. Meullemiestre, A.S. Fabiano-Tixier, M. Abert-Vian, Ultrason. Sonochem. 34, 540 (2017)
S.S. Nadar, P. Rao, V.K. Rathod, Food Res. Int. 108, 309 (2018)
S. El Kantar, N. Boussetta, N. Lebovka, F. Foucart, H.N. Rajha, R.G. Maroun, N. Louka, E. Vorobiev, Innov. Food Sci. Emerg. Technol. 46, 153 (2018)
M. Llompart, C. Garcia-Jares, M. Celeiro, T. Dagnac, in Encycl. Anal. Sci. 116, 136 (2019)
N. Kaur, A.K. Singh, Crit. Rev. Food Sci. Nutr. 56, 2338 (2016)
R.P.F.F. da Silva, T.A.P. Rocha-Santos, A.C. Duarte, TrAC -. Trends Anal. Chem. 76, 40 (2016)
M.D. Luque de Castro, L.E. García-Ayuso, Anal. Chim. Acta 369, 1 (1998)
M. Güney, A. Elik, Commun. Soil Sci. Plant Anal. 48, 1741 (2017)
Z. Pan, W. Qu, H. Ma, G.G. Atungulu, T.H. McHugh, Ultrason. Sonochem. 19, 365 (2012)
A Kumar, P Srinivasa Rao (2020) J. Food Meas. Charact. 14: 3493
P. Pongsumpun, S. Iwamoto, U. Siripatrawan, Ultrason. - Sonochemistry 60, 104604 (2020)
S.L.C. Ferreira, R.E. Bruns, H.S. Ferreira, G.D. Matos, J.M. David, G.C. Brandão, E.G.P. da Silva, L.A. Portugal, P.S. dos Reis, A.S. Souza, W.N.L. dos Santos, Anal. Chim. Acta 597, 179 (2007)
J.C. Martínez-Patiño, B. Gullón, I. Romero, E. Ruiz, M. Brnčić, J.Š Žlabur, E. Castro, Ultrason. Sonochem. 51, 487 (2019)
T.F. Zhang, J.F. Yang, D.K.J. Lin, Stat. Probab. Lett. 81, 1027 (2011)
N. Medina-Torres, T. Ayora-Talavera, H. Espinosa-Andrews, A. Sánchez-Contreras, N. Pacheco, Agronomy 7, 47 (2017)
Q.D. Do, A.E. Angkawijaya, P.L. Tran-Nguyen, L.H. Huynh, F.E. Soetaredjo, S. Ismadji, Y.H. Ju, J. Food Drug Anal. 22, 296 (2014)
FDA, Microsoft Word 9765, 1 (2017).
J.P. Maran, Int. J. Biol. Macromol. 73, 92 (2015)
G. Derringer, R. Suich, J. Qual. Technol. 12, 214 (1980)
V. Singleton, R. Orthofer, R. Lamuela-Raventos, Methods Enzymol. 299, 152 (1999)
Q.V. Vuong, S. Hirun, P.D. Roach, M.C. Bowyer, P.A. Phillips, C.J. Scarlett, J. Herb. Med. 3, 104 (2013)
I.F.F. Benzie, J.J. Strain, Anal. Biochem. 239, 70 (1996)
I.E. Juárez-Rojop, C.A. Tovilla-Zárate, D.E. Aguilar-Domínguez, L.F.R. de la Fuente, C.E. Lobato-García, J.L. Blé-Castillo, L. López-Meraz, J.C. Díaz-Zagoya, D.Y. Bermúdez-Ocaña, Brazilian. J. Pharmacogn. 24, 341 (2014)
T. O. Elufioye, D. M. Olusola, and A. O. Oyedeji, Pharmacogn. J. 11 (2019).
J. Piechocka, P. Szulc, M. Dziedziński, J. Kobus-Cisowska, O. Szczepaniak, and D. Szymanowska-Powałowska, Cienc. Rural 50 (2020).
M. Pinelo, M. Rubilar, M. Jerez, J. Sineiro, M.J. Núñez, J. Agric. Food Chem. 53, 2111 (2005)
R. Raviadaran, M. Han, S. Manickam, D. Chandran, Ultrason. Sonochemistry 64, 104995 (2020)
W. Chen, W.-P. Wang, H.-S. Zhang, Q. Huang, Carbohydr. Polym. 87, 614 (2012)
R. Tabaraki, E. Heidarizadi, A. Benvidi, Sep. Purif. Technol. 98, 16 (2012)
B. Wang, J. Qu, S. Luo, S. Feng, T. Li, M. Yuan, Y. Huang, J. Liao, R. Yang, C. Ding, Molecules 23, 2513 (2018)
E.M. Silva, H. Rogez, Y. Larondelle, Sep. Purif. Technol. 55, 381 (2007)
G. Aguilar-Hernández, M. De Lourdes García-Magaña, M. De los Ángeles Vivar-Vera, S. G. Sáyago-Ayerdi, JA Sánchez-Burgos, J. Morales-Castro, LM Anaya-Esparza, EM González (2019) Molecules 24: 904.
C. Zhu, X. Zhai, L. Li, X. Wu, B. Li, Food Chem. 177, 139 (2015)
G. Pan, G. Yu, C. Zhu, J. Qiao, Ultrason. Sonochem. 19, 486 (2012)
B. Ou, D. Huang, M. Hampsch-Woodill, J.A. Flanagan, E.K. Deemer, J. Agric. Food Chem. 50, 3122 (2002)
L.N. Deng, G.N. Feng, Y. Gao, Y.X. Shen, H.S. Li, Y. Gu, H.Y. Luan, Agronomy 10, 37 (2020)
Y. Ma, M. Zhou, H. Huang, Eur. Food Res. Technol. 239, 637 (2014)
A. Floegel, D.-O. Kim, S.-J. Chung, S.I. Koo, O.K. Chun, J. Food Compos. Anal. 24, 1043 (2011)
Acknowledgements
This work was financially supported by the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India. The authors thankfully acknowledge SERB and DST for their valuable support for the successful completion of this research. They also thank the Department of Agricultural and Food Engineering (AgFE), Indian Institute of Technology Kharagpur, India, for providing necessary laboratory facilities for carrying out the experimental works.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest regarding finance or personal relationships, which would have influenced the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rout, R.K., Kumar, A. & Rao, P.S. A multivariate optimization of bioactive compounds extracted from oregano (Origanum vulgare) leaves using pulsed mode sonication. Food Measure 15, 3111–3122 (2021). https://doi.org/10.1007/s11694-021-00902-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00902-0