Abstract
Combination of low energy and high energy methods were studied as alternative process to individual spontaneous emulsification and ultrasonication for production of stable nanoemulsions in order to reduce the synthetic surfactant requirement. A three-step procedure was used: The dispersed oil phase containing hydrophilic surfactant (Tween 80) was titrated into an aqueous phase for formation of nanoemulsion by spontaneous method. Then, it was homogenized by a high shear homogenizer and sonicated to form final stable nanoemulsions. Influence of orange oil to sunflower oil ratio, surfactant emulsion ratio (SER), ultrasonication (US) time and temperature and high shear homogenization (HSH) time on particle size and polydispersity index (PDI) of nanoemulsions were determined. Orange oil/sunflower oil ratio, SER, US time and temperature and HSH time all had an appreciable effect on nanoemulsion formation, particle size distribution and stability. Translucent nanoemulsions (70 nm) was obtained under following specific conditions: 10 wt% oil phase (7 wt% orange oil + 3 wt% sunflower oil), 2 wt% SER (Tween 80), 5 min HSH and 10 min US in an ice bath. The selected nanoemulsion was stable for 35-day storage at ambient temperature. These findings demonstrate that stable orange oil nanoemulsions can be produced from food-grade ingredients using combined processing operations (spontaneous homogenization, HSH and US homogenization) and low synthetic surfactant concentration. This study provides important information for design and application of essential oil nanoemulsion-based delivery systems in food, beverage and other applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Orange (Citrus sinensis L.) essential oil which is produced usually by cold pressing of fruit peel, possess good antimicrobial activity (against various kinds of bacteria, molds and yeasts, and viruses) and antioxidant properties owing to high content of limonene and linalool (which are classified as terpenes) and has been proved to inhibit food spoilage by many researchers [1, 2]. Therefore, orange essential oil can be utilized as a suitable replacer for synthetic preservatives to produce green food that today’s public users prefer to consume owing to the increasing knowledge of potential adverse effects of artificial additives in foodstuffs [3, 4].
However, the high content of hydrophobic and low molecular weight constituents such as limonene and linalool in the orange essential oil makes it so insoluble in water, volatile and sensitive to oxidation. Consequently, it needs to be encapsulated as a delivery system so that it can be protected and readily dispersed in the water-based food and beverage products [5].
Oil in water (O/W) nanoemulsions are among the best delivery systems for plant essential oils. Nanoemulsions are emulsions with particle diameter of 20–200 nm which are prone to be transparent or translucent owing to very smaller droplets compared to the light wavelength (d ≪ λ) and have much better stability against creaming, sedimentation and flocculation than conventional emulsions. However, nanoemulsions are thermodynamically unsteady systems and will become unstable gradually over time due to coalescence and/or Ostwald ripening [6, 7].
Moreover, antimicrobial activity of plant essential oils can be improved appreciably by their submicron droplet size which leads to their easily penetration through the microorganism membrane and leakage of the cell contents out of the cell and finally the death of the microorganism [8, 9].
Different low energy and high energy approaches (including spontaneous emulsification and ultrasonication, respectively) have been applied for nanoemulsion preparation up to now [4,5,6, 8, 10]. Each of these approaches have some benefits and drawbacks [5, 6, 11]. There are few studies on different approaches for producing orange oil nanoemulsions. Orange and lemongrass oil loaded nanoemulsions were prepared by microfluidization (MF) and ultrasonication (US) methods, respectively [12, 13]. Chang and McClements examined the potential of spontaneous emulsification for preparation of orange oil nanoemulsion [14]. High pressure homogenization and ultrasonication were used to prepare orange oil in water nanoemulsions [15].
Previous studies have indicated that the composition of two aqueous and oil phases, the environmental conditions (e.g., temperature, pH, and ionic strength) and/or, the mixing conditions (e.g., stirring speed, rate of addition, and order of addition) may be effective on the droplet size in spontaneous emulsification method [5, 6]. On the other hand, the droplet size of nanoemulsions produced by ultrasonication depends upon some factors, including sonication amplitude, sonication time, as well as process temperature [15]. To the best of our knowledge, orange oil preparation by combined spontaneous and ultrasonic emulsification has not yet been reported. Therefore, the aim of this study was to design and fabrication of stable orange oil nanoemulsions by combination of two low and high energy methods with the goal of reduction in synthetic surfactant consumption. Also, the effect of oil phase composition, surfactant concentration, ultrasound time and temperature and high shear homogenization time on the particle size and polydispersity index of nanoemulsions were examined. Storage stability of selected nanoemulsion was also investigated at ambient temperature.
Materials and methods
Materials
Cold pressed Brazilian Orange oil 012210 (onefold) was a kind gift from Givaudan International SA (Switzerland) and was comprised primarily of d-limonene (97%), myrcene (1.6%), linalool (1%) and alpha-pinene (0.4%) as measured by gas chromatography. The non-ionic surfactant (Tween 80) was obtained from Sigma-Aldrich Co. (St. Louis). Sunflower oil was purchased from a local market. Citric acid (1 mM, pH 3.2) and sodium citrate were purchased from Sigma Chemical Co. (St. Louis, MO, USA) to prepare acidic emulsions similar to certain soft drinks and beverages (5). Deionized water was used for the production of nanoemulsions.
Preparation of nanoemulsions
Nanoemulsion formation was carried out using a combination of low energy (spontaneous emulsification) and high energy (ultrasonic emulsification) Methods. All emulsions were prepared through a three-stage process. At first, a mixture of 10% organic phase (different ratios of orange oil to sunflower oil, 50:50, 60:40, 70:30 and 80:20) and different surfactant emulsion ratio (SER % 0.5–3%) was titrated (1 mL/min) into an aqueous phase (5 mM citrate buffer at pH 3.5) while gently stirring (500 rpm) the system with a magnetic stirrer at temperature of 35 °C. Coarse emulsions were obtained by high speed stirring using an Ultra Turrax T25 (IKA Labortechnik, Jahnke und Kunkel, Germany) at 24,000 rpm for 5 min, maintaining the samples in an ice bath. Afterwards, the droplet size reduced further by symmetrically immersing a sonotrode (13 mm in diameter) 1 cm below the emulsion surface (batch ChromTech Ultrasonic Processor UP-100, 20 kHz, Taiwan). The sonication was carried out for different emulsification times (0–30 min, controlled by the software of the device) in ice bath (3 °C) or at room temperature (22 °C). All emulsions were produced in triplicates and were stored at 3 °C until experiments. The total amount of emulsion produced for each treatment was 50 grams.
Droplet size determination
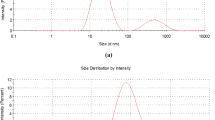
The mean droplet diameter (MDD) and particle size distribution of nanoemulsions were measured using static light scattering (SLS) instrument based on Mie and Fraunhofer scattering theories (CILAS particle size analyzer, model 1090, France) at ambient temperature. This instrument determines the particle size from intensity-time fluctuations of two laser beams (635 and 830 nm, 2 and 5 mV power respectively) scattered from a sample at angles of 173° and 90°. The result of each individual measurement was obtained from 10 runs in SLS instrument. Before measurements, all samples were diluted 10 times (1:10) using citrate buffer solution (5 mM, pH 3.5) to avoid multiple scattering effects and stirred continuously during the tests to ensure that homogeneous emulsions are obtained. Droplet size data is reported as the volume weighted mean diameter (d43) and was calculated from Eq. 1:
where ni is the number of droplets with diameter di.
Polydispersity index (PDI: the concentration of droplets in different size classes), which gives an indication of the width of the droplet size distribution, was calculated from Eq. 2:
where Dv0.1, Dv0.5, and Dv0.9 are diameters at which the cumulative volume of the droplets is under 10%, 50%, and 90%, respectively.
Each measurement was replicated twice and standard deviation was calculated.
Nanoemulsion stability measurements
Selected nanoemulsions prepared by combined low and high energy methods were centrifuged at 9,000 rpm for 30 min, and their creaming stability after centrifugation were evaluated. Intrinsic creaming stability was also determined by storing selected nanoemulsions at ambient temperature. The phase separation of nanoemulsion systems were subsequently detected visually. Moreover, the kinetic stability of the nanoemulsions was determined by measuring mean particle size and polydispersity index changes weekly during 35-days storage at refrigerator temperature (3 °C). Mean droplet diameter of the nanoemulsions was evaluated using a static light scattering device (CILAS particle size analyzer, model 1090, France) at fixed scattering angles of 173° and 90° at 25 °C. PDI was calculated from Eq. 2 (See “Droplet size determination” section). To avoid multiple scattering effects, all samples were diluted 10 times with citrate buffer solution (5 mM, pH 3.5) and stirred continuously to ensure they were well dispersed. A citrate buffer solution (5 mM, pH 3.5) was used as a blank.
Experimental parameters and statistical analysis
The primary parameters affecting the droplet size and size distribution, i.e., orange oil in oil phase (wt%), SER (wt%), ultrasonication time and temperature, and high shear homogenization time were studied at different levels (Table 1). All measurements were carried out on two or three freshly prepared samples and the results were reported as means and standard deviations of these experiments. Analysis of variance (ANOVA) was conducted and the means were analyzed by Duncan’s multiple range test (for more than two samples) or Student’s t-test (for two samples comparison) (p < 0.05).
Results and discussion
Influence of the oil-phase composition on the particle size and polydispersity index
At first, the effect of oil-phase composition on the droplet size and polydispersity index of nanoemulsions prepared using combined spontaneous and ultrasonic emulsification, was examined. Previous studies showed that stable nanoemulsions could not be produced without using long chain triglycerides (such as corn, soy or sunflower oil) or medium chain triglycerides (such as Miglyol®) in the oil phase as Ostwald ripening (OR) inhibitor [5, 16]. These low water soluble molecules can retard OR by generating entropy of mixing effect that counter-balances the curvature effects [17]. Therefore, in this study the oil phase (10 wt%) was composed of different orange oil to sunflower oil ratios. Figure 1 shows the influence of the oil phase composition on the mean droplet diameter (MDD) and polydispersity index (span) of nanoemulsions stabilized by 1 wt% Tween 80.
Effect of the oil phase composition (wt% of orange oil in the oil phase) on the mean droplet diameter (MDD) and polydispersity index (span) of nanoemulsions produced by combined spontaneous and ultrasonic emulsification (n = 3). Nanoemulsions were prepared using 10 wt% oil phase (orange oil + sunflower oil), 1 wt% surfactant (Tween 80), and 89 wt% aqueous phase (citrate buffer solution, 5 mM, pH 3.5) at a stirring speed of 500 rpm at 35 °C
With 50 wt% orange oil in the oil phase, the mean droplet diameter was larger than 200 nm and no transparent or translucent nanoemulsion could be produced. When the orange oil level in the oil phase increased initially from 50 wt% to 70 wt%, there was a dramatic decrease in the mean droplet diameter (from 313.33 to 176.66 nm) and polydispersity index (from 0.65 to 0.52). This could be attributed to decrease in viscosity and interface tension of the dispersed phase to the continuous phase due to decrease in sunflower oil content in the oil phase [12]. With increasing the orange oil level in the oil phase from 70 to 90 wt%, MDD and span were appreciably increased (from 176.66 to 196.66 nm and from 0.52 to 0.6) This shows sunflower oil content of the oil phase is not enough to inhibit Ostwald ripening of the newly formed droplets in the homogenizer very rapidly after homogenization. Consequently, for obtaining the lowest particle size, there is a critical ratio of insoluble oil to the dispersed phase which depends on different factors such as mass volume of dispersed phase, interfacial tension and polarity and solubility [18]. The similar phenomenon in essential oil nanoemulsions was observed by other researchers [5, 12]. The results show that at low essential oil fractions (high long chain triglyceride oil fractions), Ostwald ripening can be inhibited by enough oil content in the formulation and smaller droplet size can be obtained,at high orange oil fractions (low sunflower oil fractions), there is not enough oil to prevent Ostwald ripening.
Effect of the surfactant concentration on the particle size and polydispersity index
To investigate the effect of the surfactant concentration on the particle size and polydispersity index, a series of nanoemulsions with a constant oil phase composition (7% orange oil + 3% sunflower oil) and surfactant type (Tween 80) and various SERs, were prepared and the mean droplet diameter and PDI were measured immediately after emulsion formation. Surfactant to emulsion ratio (SER) here expressed as: SER = 100 × mS/mE, where mS and mE are the mass of surfactant and the mass of emulsion, respectively [5]. As seen in Fig. 2, the mean droplet sizes were about 210, 180, 68.3 and 100 nm for emulsions containing 0.5, 1, 2 and 3 wt% Tween 80, respectively. MDD was decreased appreciably by increasing SER % from 0.5 to 2 wt%, and then increased at higher level. Polydispersity index was decreased from 0.97 to 0.6 with increasing SER %. The smallest particles with PDI lower than 1 were obtained at 2 wt% Tween 80. So this surfactant emulsion ratio was applied in the next stage of the study. These results show that SER % had an appreciable effect on mean droplet diameter and polydispersity index and translucent nanoemulsions could be produced by combination of low energy and high energy methods at very low SER %. In this study, nanoemulsions were produced by a three-stage production method. At the first stage, emulsions were prepared by spontaneous emulsification which usually needs a high concentration of synthetic surfactant (around 20 wt% or more) to produce nano-sized droplets. However, we used up to 3 wt% surfactant in this step and the droplet size reduced in the next two steps by high energy mechanical devices from around 600–800 nm at the first step (data not shown) to around 70–200 nm at the final step. At the first step, increasing surfactant level causes reducing interfacial tension and a highly dynamic interface which lead to a turbulent interface and spontaneous formation of relatively small droplets. On the other hand, by increasing the surfactant level, bigger oil–water interface can be stabilized and smaller droplets may be produced [5]. At the next two steps, high energy applied from high shear homogenization and ultrasonic homogenization produces much smaller droplets. This phenomenon may be attributed to the more and faster covering of recently developed droplets in the homogenizer and inhibition of droplet recoalescence in the homogenizer by more surfactant level [19]. But, increasing surfactant concentration to 3 wt%, may lead to increasing the surface viscosity and inhibition of surfactant diffusion to the aqueous phase. By increasing the concentration of surfactant, some of them may be placed in the mass instead of the surface and surfactant micelles could be formed which increase local osmotic pressure in the system. As a result, the continuous phase between droplets is transferred to this location and causes depletion flocculation between two droplets. Therefore, by combination of these two low and high energy methods, nano-sized droplets can be produced at very low synthetic surfactant concentration (2 wt%) successfully. Liu et al. reported that smaller droplet sizes and narrower size distributions were observed at higher surfactant concentrations for paraffin oil-in-water nanoemulsions prepared by the emulsion inversion point method [20].
Effect of the surfactant concentration (SER %) on the mean droplet diameter (MDD) and polydispersity index (span) of nanoemulsions produced by combined spontaneous and ultrasonic emulsification (n = 3). Nanoemulsions were prepared using 10 wt% oil phase (7 wt% orange oil + 3 wt% sunflower oil), varied SER % and aqueous phase % (citrate buffer solution, 5 mM, pH 3.5) at a stirring speed of 500 rpm at 35 °C
Effect of the ultrasound time and temperature on the particle size and polydispersity index
The influence of ultrasound time at fixed oil phase composition (7 wt% orange oil + 3 wt% sunflower oil) and constant SER (2 wt% Tween 80) on droplet size and size distribution was also examined. As shown in Fig. 3, with increasing the duration of ultrasound treatment from 0 to 10 min, the mean droplet diameter is decreased from 126.67 to 63.33 nm, respectively and then it is increased to 93.33 nm and 96.67 nm in 20 min and 30 min sonication, respectively. Polydispersity index is decreased from 1.25 to 0.55 in 10 min and then with increasing ultrasound time to 30 min, PDI is significantly increased (p < 0.05). So, translucent nanoemulsion with minimum droplet size and minimum PDI is reached within 10 min ultrasonication. There are two fundamental mechanisms in ultrasonication for nanoemulsion preparation, namely droplet distruption and droplet recoalescence which kinetis of each of them separately influences the final particle size [21]. High frequency (more than 20 kHz) ultrasonic waves produces severe mechanical vibrations that causes droplet formation, droplet growth and collapse of tiny bubbles (cavitation effects). So, the temperature increases within the emulsion and the dispersion of the oil phase in the aqueous phase is facilitated owing to decrease in viscosity, interfacial tension and Laplace pressure (droplet resistance to deformation) [21] and intense turbulence occurs at high speed. This provisional turbulence causes high shear rate and leads to droplet decomposition [6, 15, 22]. Previous studies have shown that the more the residence time in the disruption zone, the smaller the droplets [6, 23]. In this research, increasing the emulsion residence time in disruption zone up to 10 min, appreciably decreases the MDD and PDI (p < 0.05), mainly since more surfactant can adsorb to the droplet surfaces during longer sonication and thermodynamic equilibrium is established. Furthermore, increasing the sonication time causes increasing in shear forces exerted on the droplets, therefore, decomposition of the droplets occurs more. Usually it is postulated that the size of droplets decreases by increasing the sonication time [13, 15, 24]. However, in this study sonication more than 10 min causes larger droplets and bigger PDI probably due to reduction in emulsifier adsorption on the droplet surfaces, and droplet recoalescence phenomenon [15, 22]. So, 10 min’ sonication was found to produce optimum results. Similar trends have been observed when orange peel oil or d-limonene nanoemulsions prepared by ultrasonic emulsification [6, 15, 22]. Also, Salvia et al. showed that the mean droplet size of lemongrass oil–alginate nanoemulsions decreased with increasing sonication amplitude and time [13]. Gaikwad and Pandit stated that smaller droplet sizes and narrower size distributions were observed at longer sonication times and higher power applied, which is proportional to the amplitude [25]. Spinelli et al. reported the same behavior in synthetic oil (decane/ toluene/cyclohexane, 50:30:20) in water emulsions stabilized with nonionic surfactants and they obtained a minimum average droplet size of 30 nm by increasing the sonication time up to 8 min [26].
Effect of the ultrasound time on the mean droplet diameter (MDD) and polydispersity index (span) of nanoemulsions produced by combined spontaneous and ultrasonic emulsification (n = 3). Nanoemulsions were prepared using 10 wt% oil phase (7 wt% orange oil + 3 wt% sunflower oil), 2 wt% Tween 80 and 88 wt% aqueous phase % (citrate buffer solution, 5 mM, pH 3.5) at a stirring speed of 500 rpm at 35 °C
Figure 4 shows the impact of ultrasonication process temperature on the particle size and polydispersity index of nanoemulsions with fixed oil phase (7 wt% orange oil + 3 wt% sunflower oil) and constant SER (2 wt% Tween 80) produced by combined method of spontaneous emulsification and 10 min ultrasonication. As can be seen, particle size and PDI are increased significantly (p < 0.05) with increasing the process temperature from 3 °C (while cooling the emulsion by maintaining it in an ice bath during sonication) to 22 °C (while removing the ice bath). Process temperature seems to have considerable effect on the droplet size owing to its influence on the phase viscosity and interfacial tension at the oil–water boundary [15]. At higher temperatures, the droplet thermal energy and collision frequency increases. So, interface viscosity decreases and the rate of droplet coalescence increases [27]. In the other words, lowering the treatment temperature while sonication can decrease the rate of droplet coalescence and increase the emulsion kinetic equilibrium [15]. It should be noted that ultrasonication usually increases the emulsion temperature owing to some heat generation during the process. So it is mandatory to control and decrease the temperature of the container to protect any heat sensitive ingredients (e.g. essential oils) in the emulsion [28].
Effect of the ultrasound process temperature on the mean droplet diameter (MDD) and polydispersity index (span) of nanoemulsions produced by combined spontaneous and ultrasonic emulsification (n = 3). Nanoemulsions were prepared using 10 wt% oil phase (7 wt% orange oil + 3 wt% sunflower oil), 2 wt% Tween 80 and 88 wt% aqueous phase% (citrate buffer solution, 5 mM, pH 3.5) at a stirring speed of 500 rpm at 35 °C at the first step and 10 min’ sonication at the final step
Effect of the high shear homogenization time on the particle size and polydispersity index
The impact of high shear homogenization on the mean droplet diameter and PDI of the emulsions prepared by combined spontaneous and ultrasonic emulsification has also been studied and the results have been shown in Fig. 5. All emulsions were generated with 7 wt% orange oil and 3 wt% sunflower oil in the oil phase, 2 wt% Tween 80 as nonionic surfactant and 10 min ultrasonication in an ice bath. Mean droplet diameter of nanoemulsions without and with 5 min high shear homogenization was around 103.33 nm and 70 nm, and their PDI was around 0.87 and 0.63, respectively. Therefore, MDD and PDI obtained with high shear homogenization was drastically smaller than without any high shear homogenization treatment (p < 0.05). High shear homogenization is one the most common methods for mixing the oil and water phases in the food industry to produce course emulsions. The mixing head of the rotor–stator device rotates at high speed and creates a mixture of radial, longitudinal, and rotational velocity gradients in the liquids, which causes the destruction of the oil–water boundary and makes the fluids to mix together and fractures the larger droplets to the smaller ones. Usually, the more the homogenization time or the mixer head speed, the smaller the droplets. The nature and concentration of the components and the intermingling capability of the mixer determine the lowest droplet size which can be obtained [28]. High shear homogenization delivers more energy to the system that lowers the emulsion viscosity and the emulsion resistance to deformation which facilitates further disruption of emulsion droplets and leads to smaller droplets. In the other words, disruptive forces in ultrasonication final step may not be capable of viscos liquid decomposition at applied amplitude and processing time. Moreover, possibly there is not enough time for the emulsifier to adsorb to the droplet surfaces during ultrasonication. Energy input increase in emulsions with high shear homogenization step, decreases the PDI. Oppositely, increasing PDI for emulsions with no high shear homogenization can be attributed to non-uniform emulsification conditions created by ultrasonication alone. Because by lower energy input to the system from ultrasonication, only the oil droplets in the vicinity of ultrasound probe disrupt and farther oil droplets remain intact. These findings indicated that ultrasonication always needs a high shear homogenization device to break up the interface strongly before using an acoustic field [24]. Tang et al. studied the Impact of process parameters in the generation of novel aspirin nanoemulsions produced by two different methods of ultrasound cavitation and microfluidization. In the case of using the microfluidizer, it has been indicated that pre-homogenization had a negligible effect in increasing the droplet size. Whereas, in the case of ultrasound emulsification, the prepared emulsion was found to be dependent on the pre-homogenization. These researchers found that the mean droplet diameter could be decreased drastically with the help of pre-homogenization [24].
Effect of the high shear homogenization time on the mean droplet diameter (MDD) and polydispersity index (span) of nanoemulsions produced by combined spontaneous and ultrasonic emulsification (n = 3). Nanoemulsions prepared using 10 wt% oil phase (7 wt% orange oil + 3 wt% sunflower oil), 2 wt% Tween 80 and 88 wt% aqueous phase % (citrate buffer solution, 5 mM, pH 3.5) at a stirring speed of 500 rpm at 35 °C at the first step and 10 min’ sonication at final step
Storage stability of the combined spontaneously and ultrasonic emulsified orange oil nanoemulsions
Selected nanoemulsions with the smallest droplet size and the lowest PDI, were stable after centrifugation at 9,000 rpm for 30 min, and no phase separation was observed after a storage time of 35-days. Figure 6 represents the visual appearance of selected formulation which is optically translucent without any phase separation. Long-term stability of nanoemulsion-based delivery systems has great importance in the food industries and commercial applications, since it indicates the further applicability of the system in food and beverage industries [5]. These systems are thermodynamically unstable, which means that the free energy of colloidal dispersion is higher than the free energy of individual oil and water phases and therefore, the droplet size will increase and the system will breakdown over time. The rate at which the system will decompose, depends on the height of the energy barrier between final nanoemulsion and separated phases and this energy barrier determines the kinetic stability of the system [16]. Consequently, we determined particle size and PDI changes during 35-day refrigerated storage of selected nanoemulsion (orange oil to sunflower oil ratio of 7:3, SER: 2%, 10 min ultrasonication in an ice bath) at 1-week intervals. This system was chosen because it had the smallest mean droplet diameter and polydispersity index and was translucent at room temperature after combined spontaneous and ultrasound emulsification. Figure 7 shows that MDD was increased considerably (p < 0.05) from 63.33 nm at production day (day 0) to 103.33 nm at day 7 and then it was not changed significantly until the last day of storage (day 35) (p > 0.05). Nevertheless, during 35-day storage, the size of droplets was still within the scale of nanoemulsions (20–200 nm) [28]. Polydispersity index was around 0.62, 0.58, 0.71, 0.81, 0.73, 0.72 and 0.68 at day 0, 1, 7, 14, 21, 28 and 35, respectively.
Effect of storage time on the mean droplet diameter (MDD) and polydispersity index (Span) of nanoemulsions produced by combined spontaneous and ultrasonic emulsification (n = 3). Nanoemulsions were prepared using 10 wt% oil phase (7 wt% orange oil + 3 wt% sunflower oil), 2 wt% Tween 80 and 88 wt% aqueous phase % (citrate buffer solution, 5 mM, pH 3.5) at a stirring speed of 500 rpm at 35 °C at the first step and 10 min’ sonication at final step. *Values with different letters are significantly different (p < 0.05) between columns
Physicochemical mechanisms of nanoemulsion instability are gravitational separation (creaming or sedimentation), flocculation, coalescence and partial coalescence, and Ostwald ripening [29]. Creaming or sedimentation occurs when emulsion droplets (dispersed phase) move upward or downward, because of lower or higher density than the continuous phase, respectively. Flocculation is the mechanism by which two or more droplets join together to create an aggregate composed of droplets with individual integrity. Coalescence is the procedure whereby two or more droplets integrate together and produce an individual larger droplet. The process whereby two or more partially crystalline droplets combine together and produce a distinct asymmetrical aggregate, is called partial coalescence. Ostwald ripening occurs when larger droplets develop at the cost of smaller ones because of dispersed phase molecular propagation via the interfering continuous phase. It is believed that nanoemulsions owing to their nano-range droplets, usually are resistant to gravitational separation and flocculation, but they are prone to coalescence and Ostwald ripening (due to higher surface to volume ratio). Essential oils have higher water solubility than lipids constituted of medium- or long- chain triglycerides [29]. Consequently, very non polar compounds (e.g. sunflower oil, corn oil, sesame oil, canola oil or MCT) are incorporated with essential oil as ripening inhibitor [9, 14]. It is necessary to mention that addition of these medium or long chain triglycerides may lead to the decrease of essential oil level in the oil phase and so the reduction of antimicrobial activity [3]. As it is obvious from Fig. 7, at the first week of storage, the droplet size is increased drastically owing to the movement of the dispersed droplets via the continuous phase, increase in droplets collision frequency and consequently droplets coalescence or Ostwald ripening. The droplet growth remains relatively stable after a certain time (after 7, 14, 21, 28 and 35 days). This phenomenon is perhaps due to the high energy applied for nanoemulsion production at the two last steps thereby the newly formed droplets get so mobile and unstable and need time to attain kinetic balance [15, 22]. It should be noted that no phase separation or creaming was observed during 35-day storage. Similar results have been obtained by other researchers [14].
Conclusion
In this research, we have explained the effect of oil composition, surfactant concentration, high shear homogenization and ultrasonication conditions on the preparation and stability of orange oil nanoemulsions formed by combined spontaneous and ultrasound emulsification. We observed that oil-phase composition (Orange oil: sunflower oil ratio), surfactant concentration, Ultra Turrax time and ultrasonication time and temperature, all had a considerable effect on the particle size and polydispersity index. Translucent stable nanoemulsions could be formed under certain composition and process conditions: 7 wt% orange essential oil, 3 wt% sunflower oil, 2 wt% Tween 80 and 88 wt% aqueous phase (citrate buffer solution, 5 mM, pH 3.5) at a stirring speed of 500 rpm at 35 °C at the first step, 5 min high shear homogenization at the second step and 10 min’ sonication in an ice bath at the final step. The droplet size of these nanoemulsions was not changed significantly during 35-days storage at 3 °C. High shear homogenization or ultrasonication alone cannot produced nano-sized droplets, waste lots of energy as heat and increase nanoemulsion droplets PDI. On the other hand, spontaneous emulsification needs a high synthetic surfactant concentration to produce nanoemulsion-based delivery systems. By combined low energy and high energy methods, spontaneous emulsification reduces the droplet size to some extent at the first step. Therefore, this combined low energy and high energy method can overcome the above-mentioned disadvantages of individual methods and produce nano-sized translucent stable emulsions especially suitable for application in certain beverages. Likewise, there is no need to inaccessible high pressure or microfluidizer homogenizers in this method.
References
M. Viuda-Martos, Y. Ruiz-Navajas, J. Fernández-López, J. Pérez-Álvarez, Food Control 19, 1130–1138 (2008)
S. Rojas-Moreno, F. Cárdenas-Bailón, G. Osorio-Revilla, T. Gallardo-Velázquez, J. Proal-Nájera, Food Meas. 12, 650–660 (2018). https://doi.org/10.1007/s11694-017-9678-z
O.K. Topuz, E.B. Özvural, Q. Zhao, Q. Huang, M. Chikindas, M. Gölükçü, Food Chem. 203, 117–123 (2016)
Y. Chang, L. McLandsborough, D.J. McClements, Food Chem. 172, 293–304 (2015)
Y. Chang, D.J. McClements, J. Agric. Food Chem. 62, 2306–2312 (2014)
D.J. McClements, Soft Matter 7(6), 2297–2316 (2011)
Y. Zhang, S. He, Y. Li, H. Tang, Food Meas. 11, 864–871 (2017)
V. Ghosh, A. Mukherjee, N. Chandrasekaran, Colloids Surf. B Biointerfaces 114, 392–397 (2014)
F. Donsi, A. Cuomo, E. Marchese, G. Ferrari, Innov. Food Sci. Emerg. Technol. 22, 212–220 (2014)
Y. Li, Z. Zhang, Q. Yuan, H. Liang, F. Vriesekoop, J. Food Eng. 119, 419–424 (2013)
S. Calligaris, S. Plazzotta, F. Bot, S. Grasselli, A. Malchiodi, M. Anese, Food Res. Int. 83, 25–30 (2016)
J. Zhang, L. Bing, G. A. LWT-. Food Sci. Technol. 64, 1063–1070 (2015)
L. Salvia-Trujillo, A. Rojas-Graü, R. Soliva-Fortuny, O. Martín-Belloso, Food Bioprocess. Technol. 6, 2439–2446 (2013)
Y. Chang, L. McLandsborough, D.J. McClements, J. Agric. Food Chem. 60, 12056–12063 (2012)
A. Mirmajidi Hashtjin, S. Abbasi, Food Hydrocoll. 44, 40–48 (2015)
D.J. McClements, Soft Matter 8, 1719–1729 (2012)
J. Rao, D.J. McClements, Food Hydrocoll. 26, 268–276 (2012)
A.S. Kabalnov, A.V. Pertzov, E.D. Shchukin, J. Colloid Interface Sci. 118(2), 590–597 (1987)
S.M. Jafari, Y. He, B. Bhandari, Eur. Food Res. Technol. 225, 733–741 (2007)
W. Liu, D. Sun, C. Li, Q. Liu, J. Xu, J. Colloid Interface Sci. 303, 557–563 (2006)
O. Kaltsa, C. Michon, I. Mandala, Ultrason. Sonochem. 20, 881–891 (2013)
P.-H. Li, B.H. Chiang, Ultrason. Sonochem. 19, 192–197 (2012)
B. Abismail, J.P. Canselier, A.M. Wilhelm, H. Delmas, C. Gourdon, Ultrason. Sonochem. 7, 187–192 (2000)
S.Y. Tang, P. Shridharan, M. Sivakumar, Ultrason. Sonochem. 20, 485–497 (2013)
S.G. Gaikwad, A.B. Pandit, Ultrason. Sonochem. 15, 554–563 (2008)
L.S. Spinellia, C.R.E. Mansura, G. Gonzálezb, E.F. Lucasa, Colloid J. 72(1), 56–65 (2010)
P.-H. Li, W.C. Lu, Food Hydrocoll. 53, 218–224 (2015)
D.J. McClements, Food emulsions: Principles, Practices and Techniques, 3rd edn. (CRC Press, Boka Rayton, 2016), pp. 260–261
D.J. McClements, Crit. Rev. Food Sci. Nutr. 47, 611–649 (2007)
Acknowledgements
Gratitude is expressed to Givaudan International SA (Iran Branch) for kindly providing the orange essential oil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asadinezhad, S., Khodaiyan, F., Salami, M. et al. Effect of different parameters on orange oil nanoemulsion particle size: combination of low energy and high energy methods. Food Measure 13, 2501–2509 (2019). https://doi.org/10.1007/s11694-019-00170-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00170-z