Abstract
Consumption of carotenoids may reduce the risk of certain chronic diseases, but their use in foods is currently limited because of their low bioavailability and physicochemical instability. The purpose of this research was to evaluate the physical stability of β-carotene (β-CE) in oil-in-water sodium caseinate nanoemulsion under the conditions of pH, temperature, ion strength and storage time. β-CE nanoemulsion is liable to aggregation at pH values close to the isoelectric point of the casein (pH 4–5). The β-CE nanoemulsion shows preferable physical stability under the conditions of heat at 80 °C for 90 min and ion strength (100–500 mmol/L). The physical stability of nanoemulsion in digestion model was also evaluated.Confocal images as well as droplet characterization in terms of size and charge indicate that the negative charge and Z-average of droplet size in different digestion stages are variant. β-CE nanoemulsion became highly aggregated when exposed to gastric condition. The extent of the FFA release is increased with the increase of lipolysis time. These results have significant implications for using proteins to fabricate emulsion-based delivery system for β-CE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotenoids are the most common natural pigments widely found in fruits and vegetables. Among these carotenoids, the highest pro-vitamin A activity is β-carotene (β-CE). β-CE is served as a good candidate for application in functional food because it has many biological activities, which can lower the risk of certain chronic diseases, such as heart disease [1], cancers [2], cardiovascular disease [3] and cataracts [4]. However, bioavailability of β-CE is constrained due to its poor solubility and digestibility in aqueous solution. Otherwise, because of multiple conjugated double bond structure, β-CE is also prone to oxidize and chemical degradation during food processing and storage due to various environmental effects, such as pH [5], temperature [6] and ion strength [7]. Therefore, facing the challenge is the way to improve the stability and bioavailability of β-CE. To solve these problems, various potential technologies, including nanoemulsification [7], microencapsulation [8], self-assemble, are being introduced to improve the stability and digestion of β-CE in foods [9].
Nanoemulsion technologies are especially suitable to deliver lipophilic components, such as carotenoids, curcumin and fat soluble vitamin. These technologies are prepared by dissolving the bioactive lipophilic components within an oil phase and then homogenizing it at high pressure with an aqueous phase containing a water-soluble emulsifier [7]. Nanoemulsions have been reported to have better stability to particle aggregation and gravitational separation due to their small droplet size [10, 11]. Previous studies have reported that the bioavailability of β-CE and its digestion in carotenoids can be enhanced by co-administering them with digestible lipids [12–14]. When the lipid is digested by gastric and pancreatic lipases, it forms free fatty acids that are incorporated along with bile acids and phospholipids into mixed micelles that can solubilise and transport carotenoids to epithelium cells [15].
Milk proteins are ideal materials which were used to deliver bioactive compounds and drugs not only because inexpensive, readily available, easily digestible, non-toxic and highly stable, but also because they have the ability to stabilize emulsions and some extent retard oxidation [16]. Milk proteins are mainly composed of casein and whey protein. Casein (CN) accounts for 80% of milk protein and comprises four principal proteins: αs1-CN, αs2-CN, β-CN and к-CN, the mass ratio of these four proteins is about 4:1:4:1. Sodium caseinate (SC) as a food-grade dairy protein emulsifier widely used in the food and beverage industry. Compare to other food proteins, SC can form a thicker spherically stabilizing layer on the emulsion droplet interface that protects newly formed droplets against flocculation and coalescence [17]. SC emulsion generally shows good stability and reduces particle size due to steric hindrance of the extended protein chain and electrostatic charge repulsion due to the phosphorescent protein [17]. Previous studies have investigated the factors that affect the bioaccessibility or bioavailability of carotenoids incorporated with emulsion-based delivery systems, such as droplet size, lipid phase physical state, and interfacial properties [18–21]. The digestibility of lipids within the gastrointestinal tract is a complicated process and its effect on the release and uptake of lipophilic components remains understood. In vitro digestion models was monitored as a simulated gastrointestinal tract condition. These models have recently gained much attention as a tool for understanding the basic physicochemical processes that occur during lipid digestion stage and the release of lipophilic components [22]. The objective of present research is to investigate the stability of β-CE in nanoemulsion delivery system for various environmental effects, including pH, temperature and ion strength. At the same time, by using a gastrointestinal model that simulates the mouth, stomach and small intestine to investigate the stability of β-CE in nanoemulsion delivery system. The results of this study will be useful for designing effective delivery systems to emulsify and stabilize β-CE for application in food and beverage industry.
Materials and methods
Materials
Sodium caseinate from bovine milk and β-carotene (purity >99) were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Corn oil (Shangdong Xiwang Foodstuffs Co, Ltd, Shandong Province, China) was acquired from a local supermarket. Simulated saliva fluid was obtained from Guangdong Dongwan Keda Corporation (Guangdong, China). All other chemicals used were of analytical grade.
Preparation of β-CE nanoemulsion
SC was dispersed in water and stirred at 100 rpm for 3 h by a magnetic stirrer (Shanghai Mei Yingpu Instruments Manufacture Co, Ltd, Shanghai, China) to form a 2% solution. 0.1% β-CE was dissolved in 25 g corn oil and stirred at 100 rpm for 10 min at 50 °C by a magnetic stirrer, then left stand for 1 h at room temperature to ensure complete solubilization. A crude emulsion of 0.1 wt% of the β-CE, 25% corn oil solution in the 2% SC solution was homogenized at 6000 rpm for 2 min by a high-speed homogenizer (High-speed homogenizer, AD200-H, Angli Corporation, Shanghai, China). The crude emulsion was then further homogenized by a high pressure homogenizer (APV-1000 homogenizer, Wilmington, MA, USA) at 120 MPa for three times. After the preparation, sodium azide (0.02%, w/w) was added to inhibit the growth of microorganisms.
Emulsion characterization
The physical properties, stability of the nanoemulsion and digestion samples were monitored by measuring their mean particle diameter (Z-average) and polydispersity indices (PDI). Three parameters were measured by dynamic light scattering (Nano-ZS, Malven Instruments, Worcestershire, UK). Samples were diluted (1:100) with buffer solution prior to analysis to avoid multiple scattering effects. The buffers used for dilution had the same pH and ionic composition as the samples being tested.
The electrical charge (Zeta-potential) of the particle was determined by electrophoretic mobility measurements (Nano-ZS, Malven Instruments, Worcestershire, UK). Samples were diluted (1:100) with buffer solution and then placed in a capillary test tube that was loaded into the instrument. Samples were equilibrated for 1 min inside the instrument before data were collected over at least 10 sequential readings and processed using the Smoluchowski model.
Stability of β-CE nanoemulsion
Nanoemulsion susceptibility to pH
The pH of β-CE nanoemulsions were adjusted to various pH levels (2, 4, 6, 8 and 10) with 1 M HCl or NaOH. Following an overnight equilibration period, the Z-potential of samples were measured.
Nanoemulsion susceptibility to temperature
β-CE nanoemulsions were heated at 80 °C for 90 min, the samples were taken out at intervals of 15 min. After cooling to room temperature, the Z-Average droplet size and PDI of samples were measured.
Nanoemulsion susceptibility to ion environment
The nanoemulsion stability to NaCl was pursued through addition of controlled volumes of a 400 mL NaCl stock solution to reach NaCl concentration of 0–500 mM at pH 6.8. After equilibrating an overnight, the Z-Average droplet size and PDI of samples were measured.
Storage stability of nanoemulsion
β-CE nanoemulsions were stored at room temperature for 25 d, the samples were taken out at intervals of 5 d, the Z-average droplet size and PDI of samples were measured.
In vitro digestion
An in vitro gastrointestinal tract (GIT) model consisting of mouth, gastric and intestinal phases was used to simulate the biological fate of ingested samples. Emulsions were passed through a GIT, samples were taken out after each phase. This method has been reported in detail previously, and so we only give a brief description here [23–26].
Mouth phase
A 20 mL of simulated saliva fluid was preheated at 37 °C in a temperature-controlled (37 °C) chamber (KEMEG Technology Co., Ltd, Guangdong, Guangdong Province, China), and then mixed with 20 mL-aliquot of initial emulsion, so that the final mixture contained 1.25% (w/w) oil. The pH of mixture was adjusted to 6.8, and then incubated at 37 °C and stirred at 100 rpm for 10 min by a magnetic stirrer.
Gastric phase
The sample obtained after the mouth phase was mixed (50:50 volume ratio) with simulated gastric fluid so that the final mixture contained 0.625% (w/w) oil. The pH was adjusted to 2.5 and the sample incubated at 37 °C for 2 h with stirring (100 rpm).
Small intestinal phase
An aliquot of the sample obtained from the gastric phase (300 mL) was adjusted to pH 7.0 and placed in a temperature-controlled (37 °C) chamber. Then, 40 mL bile extract (46.87 mg/mL) and 10 mL calcium chloride (110 mg/mL) buffer solutions were inserted. Then, 25 mL of freshly prepared lipase suspension (24 mg/mL) was added. The pH of the mixture was monitored and the volume of 0.05 M NaOH to neutralize the free fatty acids (FFA) released from the lipid digestion was recorded using a pH-stat (Metrohm USA Inc., Riverview, FL) over 2 h. The amount of FFA released was calculated from the titration curves as described previously [27].
Confocal laser scanning microscopy (CLSM)
Confocal imaging of emulsion structures was carried out at room temperature with a confocal laser scanning microscope (Leica TCS SP5, Leica Microsystems, Wetzlar, Germany) using a HCX PLAPO ×60 objective. Nile Red dissolved in ethanol and FITC dissolved in dimethyl sulphoxide, were mixed into the samples for fat and protein staining with final concentrations in the samples of 1 and 10 μg/mL, respectively. A small aliquot of the sample was placed on a slide for visualization. The fluorescent dyes were excited by an Argon 488 nm laser and the emitted light was collected at 515 nm for protein and 621 nm for the fat phase.
Statistical analysis
All the experiments and determinations were carried out at least in triplicates. Data were analyzed by the ANOVA method using SPSS software, version 10.0 (SPSS, Inc., Chicago, IL, USA). The differences among the means of the analysis data were compared at a significance level of p < 0.05.
Results and discussion
Zeta-potential of β-CE nanoemulsion
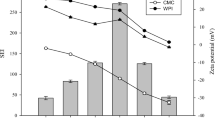
Figure 1 shows the zeta-potential of β-CE nanoemulsion, SC and pure β-CE at different pH (pH 2–10). Figure 1 clearly presents that the zeta-potential of β-CE nanoemulsion undergoes significant changes (p < 0.05) compared to SC. In general, SC is highly liable to aggregation around the isoelectric point (pI = 4.6), so casein-based emulsion have turned out to be unstable to droplet flocculation at pH value (4.0–5.0) close to the isoelectric point of SC. β-CE nanoemulsion significantly decreases (p < 0.05) the zeta-potential to a negative value of pH from 5 to 10 compared to SC, the increase of negative charges of β-CE nanoemulsion may be contribute to the interaction of protein charge and β-CE negative charge. The differences might be attributed to the carboxyl groups of the proteins under alkaline conditions were negatively charged (COO−) and the amino groups were neutral (NH2). When the pH value was less than pI value of proteins, COO− became neutral (COOH) and NH2 became positively charged (NH3+). Thus, the negative charge density on the surface decreased [28].These results indicate that the pH can influence the stability of β-CE nanoemulsion. Previous studies also reported that the changes of pH could lead to the instability of the emulsion system [29–31].
Stability of β-CE nanoemulsion
Effect of heat treatment on β-CE nanoemulsion stability
The Z-average droplet size and PDI of β-CE nanoemulsion at different heating time is shown in Fig. 2. The Z-average droplet size and PDI of β-CE nanoemulsion at heating time (0–90 min) has no significant difference (p > 0.05). The PDI measures the width of particle distribution, β-CE nanoemulsion has PDI value of <0.5 indicates that the sample has a narrow size distribution, and will probably be stable. The result suggested that β-CE nanoemulsion tends to be stable to heating. This result may be due to casein is the relatively flexible casein molecule doing not undergo appreciable heat-induced conformational changes [32]. Similar researchers have reported that the heating process had no significant effect on the physical and stability of emulsion [33, 34].
Effect of NaCl concentration on β-CE nanoemulsion stability
In order to study the effect of ion strength on β-CE nanoemulsion stability, the nanoemulsions were diluted by different concentration of NaCl solutions (0–500 mM) instead of deionized water. The Z-average droplet size and PDI of β-CE nanoemulsion at different NaCl concentrations are shown in Fig. 3. The Z-average droplet size and PDI of β-CE nanoemulsion undergo no significant change (p > 0.05) as the concentration of NaCl changed. The result may be due to electrolyte solution had an electrostatic screen effect on the negatively charged groups of the absorbed proteins. The result also demonstrated that the electrostatic interaction was not the main reason to influence the stability of β-CE nanoemulsion. This result maybe due to the electrostatic repulsion at these salt levels (100–500 mmol/L) is still strong enough to overcome the van der Waals and hydrophobic attraction. Thereby, steric stabilization might play a significant role in stabilizing the emulsions. Interestingly, the study of casein-based emulsions had a good stability under high ion strength condition which was desirable for functional food preparation [28].
Storage stability of the nanoemulsion
The β-CE nanoemulsions were stored at room temperature for 25 d to investigate the storage stability. The Z-average droplet size and PDI of the storaged nanoemulsions are shown in Fig. 4. Both Z-average droplet size and PDI of the storaged nanoemulsions did not show significantly difference (p > 0.05) before stored at 15 d. After 15 d of storage, the Z-average droplet size and PDI of β-CE nanoemulsion increased seriously. The Z-average droplet size was increased from about 30 nm to about 280 nm, but no layer was observed in β-CE nanoemulsions. These findings were contrasted with previous reports by Dickinson that casein-based emulsions were stored at room temperature for longer than 2 weeks was found depletion flocculation [35].
The physical stability of β-CE nanoemulsion in digestion model
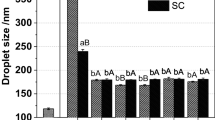
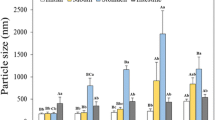
The zeta-potential of the particle in β-CE nanoemulsion was measured after exposure to gastrointestional conditions (Fig. 5b). The casein-coated lipid droplets had relatively high negative charges at the initial stage, which can be attributed to the fact that the pH of the aqueous phase (pH 6.8) was well above their isoelectric points. After exposure to the simulated mouth phase, the negative charge on the lipid droplets was little reduced. This result may be explained by the increase in ionic strength caused by mineral ions in the simulated saliva. In addition, charged mucin molecules in the saliva was absorbed to the droplet surface and altered charge characteristics. The magnitude of the negative charge on the droplets decreased drastically in β-CE nanoemulsion after exposure to the simulated stomach conditions (Fig. 5b). This result was in agreement with some previous studies using protein-based emulsions [36, 37]. The magnitude of the negative charge on the droplets decreased suggested that there might have been some protein displacement and adsorption of negative charged substances which caused a change in surface charge [36]. Otherwise, under acid condition, H+ combination with COO− become neutral (COOH) and NH2 become positively charged (NH3+). Thus, the negative charge density on the surface decreased [28]. At the end of the incubation period in the small intestine, the zeta-potential showed difference from initial, mouth and stomach digestion models. In the simulated intestinal phase, the initial emulsifiers had been displaced by anionic bile salts and phospholipids in the simulated intestinal fluids [38]. In addition, anionic free fatty acids released from the lipid digestion may absorb to the oil–water interface and therefore contribute a negative charge on the droplets. Under neutral condition, small anionic ions (such as OH−) in water may also adsorb to oil–water interface and contribute to negative charge [39].
The microstructure and stability of β-CE nanoemulsion
Casein-based nanoemulsion tends to contain relatively small droplets that sustain stable to aggregation prior to digestion. The Z-average droplet size and microstructures are shown in Figs. 5a and 6, respectively. Simulated saliva was added to the β-CE nanoemulsion only caused a slight increase in their Z-average droplet sizes (Fig. 5a). This result suggested that it only promoted a limited amount droplet aggregation. Confocal microscopy images indicated that some flocculation had occurred in the nanoemulsion (Fig. 6). Simulated saliva contains mucin, which is a charged glycoprotein that can promote droplet flocculation.
β-CE nanoemulsion became highly aggregated when exposed to gastric condition (Figs. 5a, 6), Which may be due to a number of physicochemical phenomenon, First, there is a reduction in pH and increase in ionic strength in the simulated stomach, which may have altered electrostatic interactions between droplets. Indeed, there was a reduction in magnitude of the zeta-potential of the droplets in the gastric phase (Fig. 5b), which would have resulted in a reduction in the electrostatic repulsion between the droplets and caused the droplet flocculation. Second, the simulated gastric fluid contains pepsin, which is a digestive enzyme that can hydrolyze protein. Hydrolysis of the proteins adsorbed to the fat droplet surfaces may have reduced their stability to aggregation, thereby promoting droplet flocculation and aggregation.
After incubation in simulated small intestinal fluids, the β-CE nanoemulsion exhibited an increase in Z-average droplet size. Confocal microscopy images indicated that some flocculation had occurred in the nanoemulsion (Fig. 6). The result showed that there was a reduction in the physical stability of the β-CE nanoemulsion upon exposure to small intestinal conditions. The result may be due to the fact that corn oil released to long chain fatty acid and monoacylglycerols by lipolysis in the presence of adsorbed to the lipid droplets, which may also decrease the coalescence stability [7].
Free fatty acid release
The free fatty acid (FFA) release during simulated small intestinal digestion under a pH-stat methodology offers an opportunity to investigate the rate and extent of nanoemulsion lipolysis. Accordingly, the FFA release profiles obtained during the lipolysis are presented in Fig. 7. As can be seen, the extent of the FFA release increased quickly at lipolysis time from 0 to 60 min, this result may be attributing to the increase of available lipase to surface area of the droplets. Additionally, the caseinate adsorption layer is porous to bile salt adsorption and bile salts may also displace casein facilitating increased lipase action. Mun at al [40]. reported that casein emulsions were more rapidly hydrolyzed because of high surface porosity of caseinate emulsions and unhindered accessibility to lipase. However,the amount of free fatty acid release in the current study was lower than previous study reported by Qian et al. [7]. The difference may be explained by a relatively high fat content was used in this study, which is known to decrease the rate and extent of lipid digestion.
Conclusion
The findings of this study show that β-CE nanoemulsions are stable under conditions of alkaline pH, heat and ion strength. The microstructure and stability of β-CE nanoemulsion in various digestion models show physical differences in terms of Z-average droplet size and zeta-potential. The extent of the FFA release is related to surface area of the droplets. Overall, this study has important implication for the design of effective delivery systems for encapsulation of carotenoids and other lipophilic bioactive components by controlling emulsion stability.
References
J.J.M. Castenmiller, C.E. West, Annu. Rev. Nutr. 18, 19 (1998)
M.L. Failla, T. Huo, S.K. Thakkar, In 10th Asian Congress of Nutrition. (HEC Press: Taipei, 2007), pp. 200–203
H. Gerster, Int. J. Vitam. Nutr. Res. 63, 93 (1993)
J. von Lintig, Annu. Rev. Nutr. 30, 35 (2010)
C. Qian, E.A. Decker, H. Xiao, D.J. McClements, Food Chem. 135, 1440 (2012)
C. Qian, E.A. Decker, H. Xiao, D.J. McClements, Food Chem. 132, 1221 (2012)
L.K. Mao, D.X. Xu, F. Yang, Y.X. Gao, J. Zhao, Food Technol. Biotech. 47, 336 (2009)
E.G. Donhowe, F.P. Flores, W.L. Kerr, L. Wicker, F.B. Kong, LWT-Food Sci. Technol. 57, 42 (2014)
M.J. Sáiz-Abajo, C. González-Ferrero, A. Moreno-Ruiz, A. Romo-Hualde, C.J. González-Navarro, Food Chem. 138, 1581 (2013)
C. Solans, P. Izquierdo, J. Nolla, N. Azemar, M.J. Garcia-Celma, Curr. Opin. Colloid 10, 102 (2005)
O. Sonneville-Aubrun, J.T. Simonner, F. L’Alloret, Adv. Colloid Interface Sci. 108–09, 145 (2004)
P. Borel, Clin. Chem. Lab. Med. 41, 979 (2003)
S.K. Thakkar, B. Maziya-Dixon, A.G.O. Dixon, M.L. Failla, J. Nutr. 137, 2229 (2007)
K.H. van Het Hof, C.E. West, J.A. Weststrate, J.G. Hautvast, J. Nutr. 130, 503 (2000)
V. Tyssandier, B. Lyan, P. Borel, BBA-Mol. Cell Biol. 153, 285 (2001)
Y.D. Livney, Curr. Opin. Colloid Interface Sci. 15, 73 (2010)
J. Yi, Y. Li, F. Zhong, Food Hydrocolloids 35, 19 (2014)
P. Grolier, S. Agoudavi, V. Azaisbraesco, Nutr. Res. 15, 1507 (1995)
A.M. Nik, A.J. Wright, M. Corredig, J. Am. Oil Chem. Soc. 88, 1397 (2011)
R.S. Parker, Eur. J. Clin. Nutr. 51, 86 (1997)
H.S. Ribeiro, J.M.M. Guerrero, K. Briviba, G. Rechkemmer, H.P. Schuchmann, H. Schubert, J. Agric. Food Chem. 54, 9366 (2006)l
D.J. McClements, Y. Li, Adv. Colloid Interface Sci. 159, 213 (2010)
L. Salvia-Trujillo, C. Qian, O. Martin-Belloso, D.J. McClements, Food Chem. 141, 1472 (2013)
L. Salvia-Trujillo, C. Qian, O. Martin-Belloso, D.J. McClements, Food Chem. 139, 878 (2013)
Y.G. Chang, D.J. McClements, Food Hydrocolloids 61, 92 (2016)
M. Espinal-Ruiz, F. Parada-Alfonso, L.P. Restrepo-Sanchez, C.E. Narvaez-Cuenca, D.J. McClements, Food Funct. 5, 3083 (2014)
Y. Li, D.J. McClements, J. Agric. Food Chem. 58, 8085 (2010)
J. Ji, J.P. Zhang, J.S. Chen, Y.L. Wang, N. Dong, C.L. Hu, H.P. Chen, G. Li, X. Pan, C.B. Wu, Food Hydrocolloids 51, 156 (2015)
A. Kulmyrzaev, R. Chanamai, D.J. McClements, Food Res. Int. 33, 15 (2000)
T. Nobuhara, K. Matsumiya, Y. Nambu, A. Nakamura, N. Fujii, Y. Matsumura, Food Hydrocolloids 34, 39 (2014)
J. Surh, E. Decker, D.J. McClements, Food Hydrocolloids 20, 607 (2006)
D.T. Piorkowski, D.J. McClements, Food Hydrocolloids 42, 5 (2014)
E.L. Parkinson, E. Dickinson, Colloids Surf. B 39, 23 (2004)
M. Srinivasan, H. Singh, P.A. Munro, Food Hydrocolloids 16, 153 (2002)
E. Dickinson, Int. Dairy J. 9, 305 (1999)
S. Marze, A. Meynier, M. Anton, Food Funct. 4, 231 (2013)
C.Y. Qiu, M.M. Zhao, E.A. Decker, D.J. McClements, Food Chem. 175, 249 (2015)
S.J. Hur, D.J. McClements, Food Chem. 114, 253 (2009)
J.P. Hsu, A. Nacu, J. Colloid Interface Sci. 259, 374 (2003)
S. Mun, E.A. Decker, D.J. McClements, Food Res. Int. 40, 770 (2007)
Acknowledgements
This work was supported by China Postdoctoral Science Foundation Grant (2012M520756; 2014T70360), from Chinese Postdoctoral Science Foundation Commission. The Fundamental Research Funds for the Central Universities (Grants No. HIT. NSRIF. 2014094).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Research involving in human or animal participants
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Zhang, Y., He, S., Li, Y. et al. The physical stability and digestibility of β-carotene in oil-in-water sodium caseinate nanoemulsion. Food Measure 11, 864–871 (2017). https://doi.org/10.1007/s11694-016-9457-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-016-9457-2