Abstract
Water is one of the main components of food and its presence is related to the rate of deterioration of foods. Thus, control of the water activity (aw) is a fundamental feature of food technology. The methods currently used for determining aw of foods mostly use the relative vapor pressure of the product to quantify the aw at constant temperature. This last factor limits evaluation of the aw because the instrument used is not capable of determining the aw at temperatures lower than 25 °C and/or at negative temperatures. In this study, the potential of the biospeckle laser for aw measurement was evaluated. Contrast analysis using the biospeckle laser technique provided statistically significant differences in the concentrations and aw for lithium chloride solutions and model solutions of pectin and sucrose at temperatures of 0, 5, 10, 15, 20, and 25 °C, as well as at freezing temperatures. After calibration of the biospeckle laser with the lithium chloride solutions, aw of corn starch, oatmeal, and wheat flour was determined at different temperatures, with errors lower than 20%. This achievement provides a platform for developing protocols to measure the aw of foods at different temperatures using the biospeckle laser.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Control of the water activity (aw) of foods is a fundamental aspect of food technology and has been the subject of several studies [1,2,3,4]. Water is one of the main components present in food and is related to its deterioration. In addition to influencing microbial activity, water has a strong impact on the physico-chemical processes that affect the shelf-life of foods. Regardless of the accuracy of the analytical method, determination of the water content does not provide enough information about the stability of the product investigated. The water activity is a more precise parameter as it represents the amount of water available for degradation reactions. For products with the same aw, some are more stable than others because of interactions (both hydrophilic and hydrophobic) between water and the components of the food and the effect of soluble food molecules on the hydrogen bonds in the solvent (water) [5].
Decreasing the temperature reduces the water activity of foods [6]. A study carried out by Krispien and Rödel [7] showed that if meats and meat-based products are cooled from 25 °C to the refrigerated and frozen range, they will show reduction in the aw levels. At temperatures above the freezing point, the decrease in the aw levels is insignificant (1.5 × 10−4 K−1), whereas below this point, there is significant decrease (~ 0.008 K−1). When aw is measured at temperatures above the freezing point, there is little correlation between the temperature and the vapor pressure. Thus, it is only necessary to ensure that instrument calibration and aw measurement are carried out at the same temperature. In general, measurements are carried out at 25 °C and calibration references at this temperature are available in the literature [8].
To determine the precise water activity, certain precautions must be taken to assure the accuracy of the measurement techniques, calibration technique, temperature stability during the process, vapor pressure of equilibrium, and sample preparation. There are several techniques for determination of the aw; however, all of the current methods require standard sources for vapor pressure reference in the range of interest for equipment calibration. As such, the aw of saline solutions with concentrations in the range of 0.10–1.00 are generally used, and the applied method may be direct or indirect [9,10,11].
Technologies currently used to determine aw of foods mostly utilize the relative vapor pressure of the product to quantify aw at constant temperature [12]. Optical techniques have presented great potential for sensitive analysis of biological materials, among which, the biospeckle laser has been used in several studies involving the presence of water [13, 14]. The biospeckle laser is an optical phenomenon that occurs when a laser’s coherent light interacts with a random dispersion medium, where the photodetector receives the light that is dispersed from varied positions within the medium, forming a granular interference pattern for the observer. When this dispersed light is monitored with a digital camera, it is possible to notice that the interference varies randomly in space, producing an interference pattern in the form of grains that randomly vary in terms of shape and intensity [15, 16]. This granulated image evolves with time when there is some physical, chemical, or bio-physical activity on the illuminated surface [17,18,19]. The phenomenon’s dynamics is called “boiling” due to its appearance, where the grains disappear and reappear without any significant dislocation, and this activity is called dynamic speckle [20]. By monitoring changes in the illumination interference patterns of the laser on a surface, the light dispersion movement can occur within or outside the cells. Mostly, the level of activity is related to the degree of humidity or the water activity [14].
The biospeckle laser is a non-invasive, non-destructive, low-cost technique that has been used as a biological activity mapping and quantification technique in several areas of research [21,22,23]. Amaral et al. [24] proved that the biospeckle laser technique combined with inertia moment analysis is a non-intrusive means of detecting variations in the water activity as a function of time during the storage of powders in environments with different relative humidities.
Based on existing research, it is possible to theorize that this technique may also be an efficient dynamic tool for monitoring the water activity of foods at several temperatures. Considering the trend demonstrated in recent studies on developing non-destructive, quickly-interpreted methods, the aims of this study are to correlate the images obtained using the biospeckle laser processing technique with the water activity using model solutions; to validate the biospeckle measurements against a reference method using a hygrometer, and to apply the laser biospeckle technique to analysis of the water activity in solid foods with similar sizes.
Materials and methods
Pretests and calibration of the biospeckle laser
Initially, pre-tests were performed to establish control over the factors that interfere with image recording, such as noises and variations in the lab’s power supply network. To eliminate the effect of noises, the experiment was carried out at times when fewer people were present and there was less instrumental use in the lab. A stabilizer was used to minimize the power variation problem and a weight was fixed to the support of the diode lasers for stabilization to prevent possible vibrations. Pre-tests were also carried out to choose the color of the cylindrical cup containing the test solutions, and white gave the best results in terms of the degree of differentiation among the samples.
Tests to obtain images from the biospeckle laser were carried out with the aim of reaching a speckle standard capable of differentiating saline solutions with stressed discrepancy. Saline solutions have frequently been used to calibrate equipment in several aw analysis methodologies [25], and were used also in this study to calibrate the apparatus.
Preparation of solutions
The solutions used in this work were lithium chloride (LiCl) (P.A., VETEC, Brazil), sodium chloride (NaCl) (P.A., ACS reagent, Sigma-Aldrich, Denmark), magnesium chloride (MgCl2) (P.A., VETEC, Brazil), and a model solution comprising water, sucrose (CAS [57-50-1], Dinâmica, Brazil), and citric pectin (HMP) Genu® type 105-RS (CPKelko, Brazil). LiCl solutions were prepared at concentrations of 2, 4, 6, 8, 10, and 19 mol L−1 with distilled water, providing a wide range of aw values. The MgCl2 and NaCl solutions were prepared at concentrations of 4, 8, 12, 16, 20, and 24 g of salt per 100 g of solution, with the same aim of providing a range of distinctive aw values. The model solution was prepared with 1 g of pectin per 100 g of solution in all formulations and sucrose in concentrations of 10, 20, 30, 40, 50, and 60 g of sucrose per 100 g of solution. All solutions were stored in amber glass flasks and refrigerated at 8 °C. The flasks containing the solutions were immediately closed after sampling to avoid alteration of the water activity.
Equipment assembly
The experimental apparatus, represented in Fig. 1a, consists of: (I) a diode laser (Coherent, model M202210138, Wilsonville, USA) with a wavelength of 635 nm and power of 5 mW, which projects coherent light through a lens, promoting extension of the light coverage area; (II) a digital microscope (Dino-lite, model AM7515MZT, Roanoke, USA) without a polarizing filter, used to record images with the software Speckle Tool v 1.2 [26] in the data acquisition system; and (III) an ultrathermostat bath (Nova Ética, model 521/3DE, Vargem Grande Paulista, Brazil) for stabilizing the temperature. Temperature stabilization and control were accomplished by passing cooling fluid within a stainless-steel toroid-shaped jacketed cylindrical cell (Fig. 1b). The samples were placed in cylindrical stainless steel cups painted white and coupled to the center of the jacketed cell.
The experimental setup was assembled and the images were recorded in a room with the temperature controlled at 20 °C without interference from external light. One-hundred and twenty-eight images were recorded for each sample with the software Speckle Tool v 1.2 [26] and the images obtained were analyzed using the software GNU Octave 4.0.0 by using the biospeckle laser libraries [26]. Image analysis was performed from the 21st image. The initial images were used to adjust the system luminosity. The parameters obtained with the BSLTL library for each laser-illuminated sample were the absolute value difference, average (mean), standard deviation (SD), and temporal contrast (C). During the pre-tests, the contrast (C) was found to be more sensitive to variations in the concentration of the saline solutions, and therefore, it was adopted as the standard analysis parameter.
Technique application
After reaching the steady regime by recirculating the cooling fluid used to keep the system temperature in equilibrium via heat-exchange, 2.5 mL of each solution was placed into the cells. As a preliminary step, the flasks containing the samples were immersed in the cooling fluid inside the bath to ensure thermal homogeneity between the sample and cell temperatures during the biospeckle laser analyses. The temperature of the sample in the cells was monitored with an infrared thermometer (ICEL, model TD-972, Manaus, Brazil) throughout the experiment.
The sample solutions were prepared in triplicate and their water activities were measured using a dew point hygrometer (Aqualab Decagon Devices, model 3TE, Washington, USA). The aw of the solutions determined by using the dew point hygrometer were compared with the water values reported by Rouweler [27] and the values obtained by applying the theoretical equations.
The water activities of corn starch, oatmeal, and wheat flour were measured using the dew point hygrometer and were subsequently compared with the adjusted contrast values to determine the errors by applying Eq. (1).
Statistical analyses
The relationships between the contrast and the water activity and the concentration and the water activity were analyzed using the software Origin 8.0 (Origin Lab Inc., Northampton, MA), which uses regression methods for data adjustment. The results obtained for the LiCl solution and the model solution were evaluated by variance analysis (ANOVA) and by applying the average test (Scott-Knott, p < 0.05) using the statistical package Statistical Analysis System 9.1.2 (SAS Institute Inc., Cary, USA, 2008).
Results and discussion
Device calibration
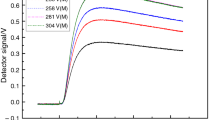
The variables adjusted to record the images established after the pre-test were the laser distance, lens distance, and digital microscope distance, considered from a reference plane parallel to the sample surface (Fig. 2). LiCl was selected for verifying the applicable zoom because LiCl presents a broad range of aw values based on the concentration. Under these conditions, strong distinctions could be made between the contrasts analyzed from solutions with concentrations of 2 and 19 mol L−1 at 25 °C, where aw values were respectively 0.931 and 0.124. The differences observed at the respective applied zooms (×80, ×160, and ×230) are summarized in Fig. 3 and Table 1.
Based on the contrasts of the 2 and 19 mol L−1 solutions, ×230 zoom was the most effective for differentiating both solutions (Table 1); therefore, this zoom was applied in the experiment. After amplification standards were established, the NaCl, MgCl2, and LiCl solutions were analyzed. Table 2 presents the values of the contrasts for the saline solutions. Using the contrast values as a function of the aw determined at 20 °C, the LiCl solutions were chosen as a standard for application in this study to achieve greater contrast (C) differentiation.
Correlation between C, concentration, and a w for the solutions
LiCl solution
Figure 4 demonstrates the linear correlation between the concentration and aw of the LiCl solutions at 0, 5, 10, 15, 20, and 25 °C. Similar behaviors were observed because the aws of these solutions are constant at the temperatures studied and can be represented by linear equations as a function of the concentration. Figure 4 also shows the linear relationship between the contrast and the concentrations at all temperatures evaluated, and these correlations are also described by linear equations for each temperature.
For the LiCl solutions, the water activity declined with increasing concentration due to the lesser availability of free water in the environment [25]. Figure 4 also shows that the contrast values declined at higher LiCl concentrations at a given temperature. This behavior was a general trend: the lower the contrast value (C), the lower the water activity (aw) because the environment contains less free water to interfere with the speckle pattern. Kurenda et al. [28] reported a decrease in the biospeckle activity when a biological sample was refrigerated in the typical temperature range for post-harvest storage. However, at temperatures lower than 10 °C, there were situations in which the activities of the speckle patterns were thought to result from Brownian movement and noises, rather than being related to the biological activity of the test samples. Table 3 shows the correlation between the average values of the contrast, the temperature, and concentration.
The data in Table 3 show that for the LiCl solutions with concentrations of 6, 8, and 10 mol L−1, the contrast values showed no significant variation (p > 0.05) as a function of the temperature (0–25 °C), which is similar to the water activity behavior in foods in the same temperature range. In general, at other concentrations (2, 4, and 19 mol L−1), significant differences were observed with variation of the temperature.
For all solutions presented in Table 3, the contrast variation for different concentrations at the same temperature showed significant differences (p < 0.05) among the samples. At temperatures of 0, 5, 10, and 15 °C, the average contrast values decreased with increasing LiCl concentration, and at 20 and 25 °C, the same behavior was observed, except for the solutions with concentrations of 4 and 6 mol L−1. The aw values of the LiCl solutions as a function of the concentration are represented by a single equation (Eq. 2) with a correlation coefficient of R2 = 0.89:
where aw is the water activity and [x] is the concentration of the LiCl solution (mol L−1). The equations representing the contrast (C) as a function of the concentration of the LiCl solution for each temperature are presented in Table 4. Considering that the variables aw (Eq. 2) and C (Eq. 3–8) are linearly correlated to the concentration of the LiCl solutions [x], it is possible to establish a relation describing aw as a function of C by fixing the values for [x], resulting in the equations presented in Table 5 and Fig. 5. These equations allow the determination of aw from a direct reading of C using the biospeckle laser. Figure 5 shows that under the operational conditions specified for the biospeckle laser, contrast values in the range of 0.65–0.93 corresponding to water activity values of 0–1.0 represent the limit for the use of this instrument for lithium chloride.
Model solutions of pectin and sucrose
The contrast values for model solutions of pectin and sucrose were also recorded at temperatures of 0, 5, 10, 15, 20, and 25 °C. The contrast values declined with a decrease in the water activity at all temperatures. The correlation between the contrast and the sucrose concentration with pectin added to the model solution at temperatures of 0–25 °C is presented in Table 6.
Table 7 presents the relationship between the average contrast values of the model solutions and the temperature and concentrations. Except for the model solution with a concentration of 10 g sucrose per 100 g of solution, the contrast did not present a significant variation (p > 0.05) as a function of the temperature.
Analysis of the variation the concentration at the same temperature for all samples showed a significant difference in the contrast values (p < 0.05). In general, the average contrast values decreased as the concentration increased, and such behavior was similar to that presented by the LiCl solutions. Therefore, numeric contrast analysis employing the biospeckle laser technique is applicable for direct analysis where there are concentration variations, and can be used directly to determine the water activity of foods.
LiCl solutions at freezing temperatures
For the LiCl solution with a concentration of 19 mol L−1, the contrast values obtained using the biospeckle laser were evaluated at temperatures from − 15 to 25 °C at intervals of 5 °C and the correlations with the aw at negative temperatures was verified. The test was only carried out with the most concentrated LiCl solution because it remained unfrozen even at negative temperature and no ice was formed on the sample surface, where the freezing process would cause interference with the contrast results. Figure 6 shows the contrast behavior of the LiCl solution as a function of the temperature. The instrument could detect changes in the water mobility and the contrast values obtained via the biospeckle laser technique, showing an exponential decrease with temperature reduction at very low values.
Application to solid foods
The correlation between the contrast and the aw of the LiCl solutions was evaluated at each of the stated temperatures. The values of the aw as a function of the C value for three solid foods (corn starch, oatmeal, and wheat flour) with the similar particle sizes (Fig. 7) were calculated using the equations in Table 5 for each temperature.
Figure 8a shows the values of the contrast, the aw values measured at 25 °C, the aw values calculated from the contrast using the equations presented in Table 5, and the errors obtained when these two values were compared for oatmeal at all evaluated temperatures. For oatmeal at 5 °C, the average value of C was 0.807 and the calculated error was 1.6%; at 0 °C, the value was C = 0.715 and with an error equal to 75.5%. Based on this result, a variation of ΔC = 0.092 causes a variation in the error of 73.9% and proves the sensitivity of the instrument when used for determination of the aw using the values of C.
The results obtained with the adjustments of the apparatus demonstrate that the water activities for a solid food group could be quantitatively analyzed based on the contrast using the biospeckle laser, with < 20% deviation from the real values and over a wide range of temperatures. The water activity of corn starch, oatmeal, and wheat flour was determined in the temperature range of 5–25 °C (Fig. 8b). Extremely high errors were observed for all foods at 0 °C, where condensation of water and formation of ice on the surfaces of the solid specimens possibly cause interference in determining the contrast using the biospeckle laser.
Conclusions
The results obtained in this study demonstrate that it is possible, by calibrating the biospeckle laser, to obtain significant contrast variation correlations for different concentrations of a solution over a broad temperature range. The biospeckle laser technique for contrast analysis provides a tool for differentiating the water activity in solution.
By adjusting linear equations it was possible to estimate the water activity of corn starch, oatmeal, and wheat flour at different temperatures, with errors of < 20%. The shape, state, viscosity, and mainly environmental conditions are recognized as interfering factors in the contrast analysis technique; with systematic control of these factors, the biospeckle laser can be used as a direct reading tool for determining variations in the concentration of solutions and monitoring the water activity of food.
References
M. Karel, Stability of low and intermediate moisture foods, in Freeze-Drying and Advanced Food Technology, ed. by S. A. Goldlith, L. Rey, W. W Rothmayr (Academic Press, London, 1975), pp. 177–202
P.J. Fellows, Tecnologia do processamento de alimentos: princípios e prática, 2 edn. (Artmed, Porto Alegre, 2006), p. 602
A.A. Gabriel, Food Chem. 108(3), 1106–1113 (2008)
S. Damodaran, K.L. Parkin, O.R. Fennema, Química de alimentos de Fennema, 4th edn. (Artmed, Porto Alegre, 2010), p. 900
M. Mathlouthi, Food Control 12(7), 409–417 (2001)
T.P. Labuza, A. Kaanane, J.Y. Chen, J. Food Sci. 50(2), 385–392 (2008)
K. Krispien, W. Rödel, Fleischwirtschaft 56, 709–714 (1976)
W. Rödel, Water activity and its measurement in food, in Instrumentation and Sensors of the Food Industry, 2nd edn., ed. by E. Kress-Rogers, C.J.B Brimelow (CRC Press, New York, 2001), pp. 453–474
L. Greenspan, J. Res. Natl. Bur. Stand. 81A(1), pp. 89–96 (1977)
S.L. Resnik, G. Favetto, J. Chirife, C.F. Fontan, J. Food Sci. 49(2), 510–513 (1984)
L.N. Bell, T.P. Labuza, Moisture Sorption: Practical Aspects of Isotherm Measurement and Use, 2nd edn. (American Association of Cereal Chemists, St. Paul, 2000), p 122
A. Stolarska, H. Garbalińska, Heat Mass Transf. 53(5), 1603–1617 (2016)
J.A. Alves, R.A. Braga, E.V.B. Vilas Boas, Postharvest Biol. Technol. 86, 381–386 (2013)
R.R. Cardoso, A.G. Costa, C.M.B. Nobre, R.A. Braga, Opt. Commun. 284(8), 2131–2136 (2011)
D.A. Boas, A.K. Dunn, J. Biomed. Opt. 15, 011109 (2010)
H.J. Rabal, R.A. Braga, Dynamic Laser Speckle and Applications (CRC Press, Boca Raton, 2008), p. 282
R. Arizaga, M. Trivi, H. Rabal, Opt. Laser Technol. 31(2), 163–169 (1999)
R.A. Braga, L. Dupuy, M. Pasqual, R.R. Cardoso, Eur. Biophys. J. 38(5), 679–686 (2009)
R.A. Braga, F.P. Rivera., J. Moreira, A Practical Guide to Biospecle Laser Analysis: Theory and Software (Ed. UFLA, Lavras, 2016), p. 158
E. Todorovich, A.L.D. Pra, L.I. Passoni, M. Vázquez, E. Cozzolino, F. Ferrara, G. Bioul, J. Real-Time Image Process. 11(3), 535–545 (2013)
A. Oulamara, G. Tribillon, J. Duvernoy, J. Mod. Opt. 36(2), 165–179 (1989)
M. Szymanska-Chargot, A. Adamiak, A. Zdunek, Sci. Hortic. 145, 23–28 (2012)
K.M. Ribeiro, B. Barreto, M. Pasqual, P.J. White, R.A. Braga, L.X. Dupuy, Ann. Bot. 113(3), 555–563 (2014)
I.C. Amaral, J.V. Resende, R.A. Braga Júnior, R.R. Lima, Dry. Technol 35, 55–65 (2016)
G.V. Barbosa-Canovas, A.J. Fontana, S.J. Schmidt, T.P. Labuza, Water Activity in Foods: Fundamentals and Applications (Blackwell Publishing, Iowa, 2007), p. 440
BSLTL - Bio-Speckle Laser Tool Library (2017), http://www.nongnu.org/bsltl/. Accessed 24 July 2017
J. Rouweler, Water activity aw calculations (2.1) of aqueous solutions and of solids mixtures.xlsx - Raoult-Norrish-Ross, Samapunto-Favetto-Pitzer, and Labuza-Chirife-Baeza (HAS University of Applied Sciences, 2016). https://doi.org/10.13140/RG.2.1.1049.9608
A. Kurenda, A. Adamiak, A. Zdunek, Postharvest Biol. Technol. 67, 118–123 (2012)
Acknowledgements
The authors wish to thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG - Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Brazil), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - Brazil) for financial support for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Silva, S.H., Lago, A.M.T., Rivera, F.P. et al. Measurement of water activities of foods at different temperatures using biospeckle laser. Food Measure 12, 2230–2239 (2018). https://doi.org/10.1007/s11694-018-9839-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9839-8