Abstract
Sugar alcohols are food grade, low cost and have very high latent heat capacities. They are therefore an attractive candidate for energy storage applications. However, to be able to utilize their potential, thermal conductivity is an important constraint. Laser flash analysis (LFA 447) was used for the measurement of thermal diffusivity. Influence of sample thickness, measurement time, voltage, pulse width and choice of curve fitting model on the measured thermal diffusivity values is studied. These parameters are then carefully chosen to ensure repeatability in the diffusivity measurement of sugar alcohols. Diffusivity results obtained using these criteria for several sugar alcohols are found to be in good agreement with consecutive measurements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phase change material
Phase change materials (PCM) are substances which melt at a nearly constant temperature with the absorption of large amounts of heat. This heat goes in increasing the internal molecular energy which makes phase transition possible. This property makes these materials attractive for thermal and solar energy storage applications.
Phase transitions could be solid–liquid, liquid–gas or solid–gas, but solid–liquid transitions are the most widely used since these materials are available in wide range of temperatures suitable for large range of applications, offer high storage capacities and undergo limited volume changes during phase transition. Liquid–gas transitions also a have high energy storage capacity, but large volume changes limit their use [1]. Solid–solid PCM store energy due to change in the crystalline state of the material and are particularly attractive as they do not require containment. However, their storage capacity is far lower than other two types of phase transitions [2,3,4]. Most commonly used phase change materials include paraffin wax, salt hydrates, fatty acids and eutectics.
In order to use PCM as heat storage material, it should have melting temperature lying within the range of mean temperature of the system, high latent heat capacity, compatibility with the container, lower volume changes, stability of properties with the melt freeze cycles, non-toxic, environmentally safe, non-inflammable, cost effective and easily available.
Phase change materials (PCMs) absorb and release heat during a phase transition. This energy is in the form of latent heat. These transitions require heat to flow into and out of the material, and this is where thermal conductivity of the material comes into picture. The higher the thermal conductivity, the faster would be the phase transitions. Also, thermal conductivity is an important property to ensure cyclic stability of the material. Thermal conductivity falls for liquids, meaning it is more difficult to discharge heat from the PCM than to melt them. It therefore becomes a key property important for successful application of PCM in any area.
Review of literature relevant to the work
For ideal measurement from laser flash, it is important to have infinitely small size of the impulse, absence of radiation and convectional losses, very small thickness of sample w.r.t its diameter and homogeneous material. This model is, however, based on Fourier’s equation valid for adiabatic boundary conditions, infinitely thin sample subjected to Dirac’s impulse. It does not consider losses from the sample and the in-homogeneity in the sample as well as in the laser source [1].
For radiation losses, Cowan [2], Cape et al. [3], Heckman [4] and Clark et al. [5] worked out several correction methods. The error due to finite impulse duration was analyzed by Cape and Lehman [3] and Clark and Taylor [5]. Beedham et al. [6], MacKay et al. [7] and Taylor [8] worked on understanding the impact of surface in-homogeneity on the response of curve and found that for correct estimation of thermal diffusivity, only homogeneity of a heat source in the central part of the sample is essential.
Moskal et al. [9] found that an increase in measurement time is the primary reason for non-repeatable measurements. So an increase in sample thickness or measurement duration will increase the spread in the data. Measurement time and time between the different shots are other important parameters. For materials with good insulating properties, especially at high temperatures, a time break of 5–10 min between individual shots is recommended to obtain repeatable results. Incorrect thickness and difference in surface roughness are reasons for deviation of results from each other. The results of diffusivity measurements should not depend on the energy flashing on the front face of the sample. Further the lower is the energy used for front side of sample, the higher is the spread of the data due to the associated noise from surroundings [10]. Albers et al. [11] found that laser pulse duration and power do not influence the diffusivity values. Baseline mode should be chosen as per the kind of material and found Cowan model to be the best for most of the materials. Kim et al. [12] found that use of circular disks, using dimensionless time \(t/t_{\max }\) instead of t and using \(0.5t^{1/2}\) to \(1.5t^{1/2}\) region for specific heat measurement, gives more promising results.
Polyols are specifically interesting as phase change materials as they exist in temperature range from \(-15\,^{\circ }\)C to \(245\,^{\circ }\)C and have phase change enthalpy varying from 100–413 \(\mathrm{kJ}\, \,\mathrm{kg}^{-1}\), even higher than water!!. Since they are non-animal origin and are compatible with most of the container materials, they have obvious advantage over less expensive materials like paraffins and fatty acids [13]. Polyols have not been really studied with respect to their thermal properties though there is large literature on their application as sweeteners and excipients for drugs. Among erythritol, D-mannitol, sorbitol, isomalt, maltitol and galactitol, only erythritol and galactitol have been studied for their thermal conductivity and no such literature is found for others. Further, most of the polyols exhibit polymorphism and that makes their study even more difficult. Temperature, relative humidity, solvent type, concentration, cooling rate and various other factors can influence the forms in which they crystallize.
Jesus et al. [14] studied the crystal behavior of erythritol and found that it exists in two forms, one melting at \(117\,^{\circ }\hbox {C}\) and other at \(104\,^{\circ }\hbox {C}\). Rapid cooling results in amorphous form, while slow cooling in crystalline. Also, crystalline form was found to exist in three conformations. Out of them, the conformations with \(T_\mathrm{p}\) \(119.9\,^{\circ }\hbox {C}\) and \(122.2\,^{\circ }\hbox {C}\) have equal probability of while one with \(T_\mathrm{p}\) of \(118.5\,^{\circ }\hbox {C}\) had 18% occurrence probability. Gunasekara et al. [15] estimated the thermal conductivity of erythritol as \(0.33\,\mathrm{Wm}^{-1}\mathrm{K}^{-1}\) (l, \(140\,^{\circ }\hbox {C}\)) and \(0.73\,\mathrm{Wm}^{-1}\mathrm{K}^{-1}\) (s, \(20\,^{\circ }\hbox {C}\))
The crystallization of D-mannitol may lead to the formation of different solid forms, depending on the processing conditions such as the solvent type and concentration, the temperature, or the rate of crystallization. Three pure anhydrous polymorphs observed are \(\alpha\), \(\beta\) and \(\delta\). The structure of \(\beta\)-form was solved by Berman et al. [16]. Kim et al. [17] solved the crystal structure of K-form of D-mannitol which is same as the \(\alpha\)-form in powder diffraction data. Botez et al. [18] studied the crystal structure of \(\delta\) and found that the main distinction between the \(\beta\) and \(\delta\) molecules occurs in the O2-C2-C3-O3 and O4-C4-C5-O5 torsion angles and in the hydrogen bonding patterns. Lian et al. [19] found a hydrate of mannitol to exist in low temperatures conditions by freeze-drying experiments. Results of XRD, DSC and TGA confirmed its existence, but the structure and the hydrate stoichiometry is not yet resolved. A \(\gamma\)-form is also reported in the literature by Rye and Sorum [20], but it appears to be similar to the k-form. Because of the different crystalline forms in which it exits, D-mannitol is expected to show complex thermal conductivity behavior since crystal form also affects thermal conductivity [21]. Beta form is the most stable form of D-mannitol. However, after going through a melt freeze cycle, \(\delta\)-form also starts forming after crystallization with a probability of 50% [22]. Kumaresan et al.[23] studied the thermal properties of D-mannitol and found it to have a high latent heat capacity of 326.8 \(\mathrm{k}\mathrm{J\,\,K}^{-1}\), melting temperature of \(167.8\,^{\circ }\mathrm{C}\) and a density of \(1.52\,\mathrm{g}\,\mathrm{cm}^{-3}\). Also it is stable below \(300\,^{\circ }\mathrm{C}\) after which it starts degrading. This makes it an excellent PCM for medium temperature applications. Also, this material is really cheap and available at Rs. 1000 (13.33 Euros) kg\(^{-1}\). Gil et al. [24] found that different polymorphs of mannitol are obtainable by varying their cooling rate and these forms differ in their melting ranges and the phase change enthalphies. The value of thermal conductivity as reported in the literature is 0.60 \(\mathrm{Wm}^{-1}{\rm K^{-1}}\). However, it is not clearly stated at what temperature this value is measured [33]
Siniti et al. [25] studied the crystallization behavior of sorbitol in the presence of mannitol and maltitol. Mannitol and maltitol were not found to influence sorbitol crystallization in any forms. Due to confirmational instability around C2–C3 bond, rotation occurs in the free state. Due to different conformations in solid and liquid states, crystallization becomes difficult. Study of sorbitol/water mixture also suggested different molecular conformations and polymorphism in sorbitol. Quinquenet et al. [26] studied polymorphism in sorbitol sample in relation to water content and found sorbitol to exist in many polymorphic forms. A, B, C, hydrate sorbitol and solidified melt are the different forms in which sorbitol was found to exist. Their melting temperature varies from 40 to \(100\,^{\circ }\hbox {C}\). For A, absence of band at \(940\,\mathrm{cm}^{-1}\) was observed in IR spectra, B and C were differentiated by a band at \(950\,\mathrm{cm}^{-1}\). Two unresolved bands at \(945\,\mathrm{cm}^{-1}\) were observed for hydrated form. In high sorbitol concentrations, water acts as a structure breaker and any crystallization is inhibited. Nezzal et al [27] confirmed the presence of four anhydrous forms of sorbitol apart from a 2/3 hydrate. Gamma was found to be the most stable form though it was difficult to crystallize and need very high temperatures. Alpha form was found to be the most crystalline and stable toward water absorption till 60% RH. Crystalline melt was found to be the least stable form and showed the strongest water absorption.
Isomalt is a mixture of two disaccharide alcohols derived from hydrogenation of isomaltulose. It is a nearly equimolar mixture of \(\alpha\)-D-glucopyranosyl-1-6-sorbitol (GPS) and \(\alpha\)-D-glucopyranosyl-I-6-mannitol (GPM) of which GPS crystallizes as anhydrous crystals and GPM as a dihydrate [28]. Water sorption experiments showed that isomalt is not hydroscopic below relative humidity of 85% [29]. Raudonus et al. [30] studied the crystallization of isomalt in the presence of high molecular weight compounds such as polydextrose and found \(T_\mathrm{g}\) to increase only if the content of these additives is greater than 60%. However, these additives increased the water absorption in the formed candies. At concentrations of additives when water absorbed rises to 50%, simultaneous crystallization of samples was observed apart from flow.
Capet et al. [31] found two crystalline forms of maltitol in the presence of maltotriitol. These were not the polymorphic forms of each other confirmed by IR spectra. These forms prismatic and bipyramidal were found to have the same crystal structure with different morphology.
John et al. [32] studied the thermal behavior of galactitol and found it to be a very unstable compound. It starts degrading after \(200\,^{\circ}\hbox{C}\). Their thermal diffusivity value is found to be 0.96 \(\mathrm{mm}^2\,\mathrm{s}^{-1}\) at \(20\,^{\circ }\hbox {C}\) and 0.314 \(\mathrm{mm}^2\,\mathrm{s}^{-1}\) at \(200\,^{\circ }\hbox {C}\).
Materials and methods
Materials studied
The materials tested are as follows \(Zerose^ TM\) 16952F, erythritol, \(C^*\) Mannidex-16705, D-mannitol, PharmSoribidex P 16656, sorbitol, IsoMaltidex 16500, isomalt, Maltidex CH 16385, maltitol were purchased from Cargill, Germany. Dulcitol 97% and Galactitol were purchased from Alfa Aeser, Germany. Samples were used without further purification.
Methods used
Laser flash analysis (LFA)
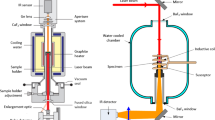
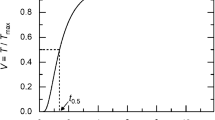
The laser flash method is a transient method for thermal diffusivity and conductivity measurement, developed by Parker, Butler, Jenkins, and Abbott of the U.S. Navy Radiological Defense Laboratory in 1961. This method relies on flashing a very short intense laser pulse on one side of the sample while measuring the transient temperature response on the other side by high-speed infrared thermometer. From the characteristic response of the temperature with time, called curve, thermal diffusivity can be calculated using Parkers formula [1]
where \(\alpha\) is the diffusivity in \(\mathrm{cm}^2\,\mathrm{s}^{-1}\), d is the samples thickness in cm and \(t^\frac{1}{2}\) is the time necessary for the signal to reach 50% of its maximum value in sec. This equation, however, holds only if the duration of the laser pulse is very small compared to \(t^{1/2}\), infinite small size of impulse (there should be no heating of the back side of the sample in the time the laser heats the front side), no heat losses from the sample and uniform energy density of the laser pulse (laser should heat all sides of sample uniformly). Table 1 details the specifications of LFA 447 used for thermal diffusivity measurements
Density measurement
For the measurement of solid density, Ultrapycnometer 1200e was used for density measurement at 20, 30 and \(40\,^{\circ }\hbox {C}\). Density at higher temperatures for solid was obtained by linear extrapolation of the data.
For measuring liquid densities, a fixed mass of the solid sample was taken in the measuring cylinder and melted in the silicon oil bath. The volume of liquid was recorded at various temperatures to an accuracy of 0.1 mL. These values are then used for the calculation of the density.
Sample preparation
Samples of solid paraffin were prepared in aluminum frames designed to prepare disk of size, 12.7 mm diameter and 1 mm thickness. However, paraffin sticks too hard with the Al frame, so we switched to frames of Teflon for easy removal of the samples from the frame. However, the problem of air bubbles appearing in the disks could not be fixed when the powder sample was melted and crystallized in the frame assembly over the hot plate. The problem increased with sugar alcohols due to their high surface tension due to which they cannot spread inside the frames on melting. We therefore prepared the samples using casting and later polishing them with blade (for paraffins)/sand paper of various grades to get the required surface finish (for sugar alcohols). Isomalt, sorbitol and maltitol do not crystallize when cooled from their melt in room-temperature conditions. We therefore prepared the pellets by mechanical press under forces from 8 to 15 kN. Mass of the sample required to obtain the desired volume is calculated, and the sample was later pressed in the steel frames.
For measurement, we coated the pellets with three layers of graphite and the response is observed. If a sharp peak is observed in the beginning of the response curve, the sample is coated with more layers of graphite to ensure the material is able to absorb the energy flashing on it. This sharp peak is due to the transparency of the material to the flashing laser beam. For the measurement of liquid samples, the material (approximately 50 mL) is filled into the aluminum crucible. The lid presses the liquid or paste into a layer with a well-defined thickness of approximately 0.5 mm. The entire setup is then placed into the standard sample holder of a flash device. Prior to the test measurements, the bottom of the crucible and central area of the lid are coated with graphite to ensure a good absorption and emissivity of the surfaces irradiated by the light source.
Results and discussion
Parameters of interest in LFA
To start with, we measured certain samples of erythritol to study how the different parameters in LFA will influence the output signal and hence the values of thermal diffusivity for our samples. It was found that the parameters voltage, pulse width, duration, main amplification and delay affect the quality and the strength of the signal received. They, however, do not influence the value of the thermal diffusivity as expected.
Voltage and pulse width affect the energy flashing on the samples. Higher voltage is found appropriate for samples with low diffusivity to get measurable output signal. For higher diffusivity materials, voltage can be kept low. As per the manufacturer, we should start with a voltage of 270 V and see the output. If the signal-to-noise ratio is poor, we should go for higher voltages and if there is signal overflow, which is found to occur if the detector signal exceeds 10 V, we should decrease the voltage value. If signal response is poor even for the highest voltage, 304 V, which is possible for materials with diffusivity lower than \(0.1\,\mathrm{mm}^2\,\mathrm{s}^{-1}\), amplification factor or pulse width should be increased.
Figure 1 shows measurement of erythritol solid sample for the entire voltage range of measurement for LFA 447 and its influence on the detector signal. It is clearly seen that an increase in voltage increases the strength of the detector signal received and reduces noise. So, it is advisable to keep as high voltage as possible for better output response.
Further, the thermal diffusivity stays in the range of 0.24 to \(0.237\,\mathrm{mm}^2\,\mathrm{s}^{-1}\) for the entire voltage range of LFA. This is illustrated in Fig. 2. This means an error of 1.25% which is within the error limits of measurement. This confirms that voltage has no influence on thermal diffusivity. Ideally too, energy flashing on the front surface of the sample should not influence thermal diffusivity measurements. If it does, it shows non-uniformity in sample preparation or heat losses form the samples.
Figure 3 shows the measurements for erythritol liquid samples for the entire voltage range of LFA 447 and its influence on the detector signal. It is seen that from 304 to 247 V, the detector signal strength is falling with voltage. The highest signal response for 202 V is because of the very high amplification factor for it, i.e., 5002, while for others it was 2520. Further, it is found that 202 V shows the most noisy signal which reduced for higher voltages. This is not very evident from the figure below because we have modified the signal thickness in the plot to allow better differentiation of the different signals. Further, the value of thermal diffusivity for all the voltages was found to be \(0.082\,\mathrm{mm}^2\,\mathrm{s}^{-1}\) confirming no influence of voltage on diffusivity measurements of liquid.
Amplification factor (AF) affects the signal strength received at the detector. Its purpose is to get a measurable output signal from the detector for analysis. For low voltages, since the signal strength at the receiver end is weak, system adjusts the amplification factor to higher values to obtain a measurable response. The higher the voltage, the lower would be the amplification factor, especially when the duration is low to prevent signal overflow. However, for very low diffusivity materials, it is possible that very high amplification is required even for high input voltages like in the case of liquid erythritol. The best signal response (high signal-to-noise ratio and signal maximum in the range of 9–10 V) was obtained for 340 V, \(5002\,\times \,10\) as the amplification factor. However, for solid erythritol samples, AF of \(5002\,\times \,10\) resulted in the signal overflow for voltage as low as 202 V, because of its relatively high thermal diffusivity
Figure 4 shows the influence of amplification factor, voltage being kept constant at 304 V, and medium pulse width, and it is found that a very high signal is observed for AF of 5002, while it was comparatively low for 1260 and 155. Further, the change in detector signal output with AF is more significant at higher AFs than at lower values.
Pulse width also known as pulse duration directly affects the energy of the pulse. Larger is the pulse duration more is the pulse energy and less is the peak pulse power.
Average energy of the pulse, however, remains the same. Therefore, for the same voltage, a longer pulse width can lead to signal overflow while small pulse width results in a good response. Figure 5 shows the influence of pulse width on the output signal, voltage being kept constant and Amplification factor fixed at 1260. It is clear from Fig. 5 that energy was far too high for long and medium pulse width for solid erythritol sample and signal overflow occured.
Duration of the pulse is associated with measurement time. Duration should be so selected that \(t^{1/2}\) comes at 2/5 times of the measurement time as per the manufacturer. Parameters should be so selected that the duration is low, meaning low measurement times since larger measurement times makes repeatability of the results difficult [9]. For low thermal conductivity materials, low duration can be obtained by using high values of voltage and amplification factor.
Increase in temperature also affects the signal strength received as output. This especially becomes important at higher temperatures. At temperatures above \(120\,^{\circ }\hbox{C}\), increase in temperature by 20–25 °C can increase the output signal by 0.1–1.5 V so the parameters should be carefully chosen to prevent signal overflow especially for higher temperatures and high input voltages.
Thermal behavior of erythritol
Erythritol (\(\mathrm{C}_4\mathrm{H}_{10}\mathrm{O}_4\)) with melt temperature of \(121.5\,^{\circ }\hbox {C}\) was tested for its thermal diffusivity in solid and liquid states. Figure 6 shows the influence of graphite coating on the detector signal.
For solid sample measurement, we used voltage of 270 V, medium pulse width, AF of 623 and duration in the range 8500–9500 ms. The results at each temperature are the values averaged for five shots. The response of curve fitting with Cowan and Radiation model is shown in Fig. 7. Based on the evaluation, Radiation model was used as the model for the calculation of the data.
It was found that diffusivity of solid erythritol depends strongly on temperature and a decrease in diffusivity with temperature was observed. Table 2 details the diffusivity values for erythritol solid samples at different temperature for different sample thicknesses. This decrease in diffusivity increased with increasing temperatures. The diffusivity was found to be really high for sample with thickness 1.222 mm. The reason could also be the higher sample thickness [1]. This could also be due to the different crystalline conformation of the sample [14]. The average standard deviation around a temperature excluding the results for sample 4 is 2.1% which is quite a promising result. The results are shown in Fig. 8.
For liquid samples, three layer analysis was used (Three layer because the liquid is placed in a crucible and covered with a lid forming three layers). For measurements in liquid state, we prepared pellets and melted them in sample holder using the Infrared lamp. For liquid measurement, we used 304 V, medium pulse width, and AF of 2520 till \(160\,^{\circ }\mathrm{C}\) and 1260 for \(170\,^{\circ }\mathrm{C}\). The duration stayed in the range of 6000–8000 ms.
A minor increase in diffusivity with temperature was observed. The results are shown in Fig. 9. Table 3 details the diffusivity obtained at different temperatures. The value of diffusivity for \(130\,^{\circ }\mathrm{C}\) matches closely with the value of solid erythritol, confirming that the sample was not able to melt in the device at this temperature which results in such high diffusivity values. Measured samples at higher temperatures have very close diffusivity values with error less than 3%. Heat loss pulse width correction method was used for the calculation of the data.
Using the diffusivity, density and heat capacity, thermal conductivity was calculated and the results are shown in Fig. 10. Heating capacity values used are those for the heating cycle. The value of density in solids till \(110\,^{\circ }\hbox {C}\) is obtained by extrapolation of the data. Heat capacity, density and thermal conductivity in solid state was found to increase with temperature while they decreased for liquids. Thermal diffusivity decreased with temperature for solid and increased for liquid. The values of thermal conductivity that we obtained for solid (\(0.7671\,\mathrm{Wm}^{-1}\mathrm{K}^{-1}\) at \(20\,^{\circ }\hbox {C}\)) closely matches with the values reported in literature (\(0.733\,\mathrm{Wm}^{-1}\,\mathrm{K}^{-1}\) at \(20\,^{\circ }\hbox {C}\)). However, the values we obtained for liquid (\(0.437\,\mathrm{Wm}^{-1}\,\mathrm{K}^{-1}\) at \(140\,^{\circ }\hbox {C}\)) are quite higher than the reported results (\(0.33\,\mathrm{Wm}^{-1}\mathrm{K}^{-1}\)at 140 °C).
Thermal behavior of D-mannitol
D-mannitol (\(\mathrm{C}_6\mathrm{H}_{14}\mathrm{O}_6\)) is an acyclic sugar alcohol (polyol) that, unlike its optical isomer, L-mannitol, is naturally produced by several plants and animals. D-mannitol distinguishes itself from other polyols by its strong tendency to crystallize from frozen aqueous solutions. It has a melt temperature of \(165\,^{\circ }\hbox {C}\) and degrades very fast at high temperatures in oxygen environment.
The difference in the detector signal response for the two cases is shown in Fig. 11. The signal also shows how the Cowan model would evaluate the signal respone.
Measurement of diffusivity of solid D-mannitol sample were performed at 270 V, medium pulse width, AF was kept constant at 623 and duration at 7792 ms. A linear decrease in thermal diffusivity with temperature was observed. The thermal diffusivity behavior as a function of temperature is shown in Fig. 12. Table 4 lists the diffusivity results obtained for different measurement conditions. Standard deviation of the data around a temperature is about 8%.
For samples with low thickness, diffusivity values decreased. The signal response for low and high thickness sample is shown in Fig. 13.
The behavior of lower diffusivity for lower thickness agrees with the findings of Parker et al. [1] which says that for thermal diffusivity greater than \(0.2\,\mathrm{mm}^2\,\mathrm{s}^{-1}\), greater thickness of the sample should be used since samples of lower thickness (1 mm) results in low values of diffusivity. This discrepancy could also be due to the different crystal structure of the sample. Since literature suggests that \(\beta\) is the most stable form of D-mannitol which results when it is cooled from its melt. However, when the material goes through melt cool cycles several times, another of its form \(\delta\) also starts forming and has the equal probability of occurence [22]. Another reason for this behavior could be the finite pulse width effect which would increase for thin samples at high temperatures. The deviation of data excluding the results of sample with thickness around 1.041 mm is around 4% which is an acceptable deviation. The detector response for the low value of diffusivity and high value is shown in figure below. There is apparently no change in the signal behavior which hints at the fact that finite pulse width effect is not the reason for this discrepancy.
For liquid samples, we found difficulty in getting repeatable measurements. Since at high temperatures, radiation losses increases, signal optimization becomes difficult. Further, if there isn’t perfect vacuum in the system, degradation of the samples is also possible. Figure 14 shows the two kind of detector response signals obtained for the liquid mannitol samples when kept for different durations in the sample holder.
On the left side of Fig. 14 shows the detector signal for which the measurement duration was 6000 ms. This signal on evaluation resulted in high values. Figure on the right shows response for samples for which the measurement duration was around 14,000 ms, more than twice than for other samples. This signal resulted in lower values. Out of the five samples tested, only three samples resulted in measurable results. Figure 15 shows the measurement results for diffusivity of liquid D-mannitol as a function of temperature. Table 5 lists the diffusivity values obtained for different measurement conditions
The reason for significantly high values at \(180\,^{\circ }\mathrm{C}\) is that the solid pellets were not able to melt uniformly in the sample holder at this temperature. Auto-adjustment for samples 1 and 3 took values of AF, 2520 for \(180\,^{\circ }\mathrm{C}\) and 1260 for higher temperatures while duration stayed between 6000 and 7500 ms. The voltage used for measurement was 270 V , medium pulse width. However, for sample 2, signal optimization took AF for \(180\,^{\circ }\mathrm{C}\) as 5002 and after 2 shots to 2520. The AF for other temperatures kept varying between 2520 and 1260. Also, the duration of measurement for this sample varied between 13,000 and 15,000 ms which is more than twice for other samples. More time means more errors since losses from radiation and conventional sources increase especially if the measurements are made at higher temperatures. This could be a reason for remarkably different values for this test. So the values for samples 1 and 3 are more trustworthy than for sample 2.
Using the diffusivity, density and heat capacity, thermal conductivity was calculated and the results are shown in Fig. 16. Heat capacity values are those for the heating cycle. The values of density in solid are the extrapolated data of the Pycnometer. For liquid density, no melting was observed for 180 and \(190\,^{\circ }\hbox {C}\) in the oil bath. The volume till \(210\,^{\circ }\hbox {C}\) remain constant. After \(210\,^{\circ }\hbox {C}\), fuming of the oil was observed. So the density value obtained at \(200\,^{\circ }\hbox {C}\) is used for measurement of thermal conductivity for all temperatures in liquid state. For thermal diffusivity, the results for sample with thickness 1.041 mm is not considered.
Heat capacity and thermal conductivity was found to increase with temperature for both solids and liquids. Density was found to remain constant in liquid state for volume measured to an accuracy of 0.1 mL. Thermal diffusivity decreased with temperature for solid and increased for liquid. The values of thermal conductivity as reported in literature is \(0.60\,\mathrm{Wm}^{-1}\mathrm{K}^{-1}\). However, it is not clearly stated at which temperature this value is measured [33]
Thermal behavior of sorbitol
D-sorbitol (\(\mathrm{C}_6\mathrm{H}_{14}\mathrm{O}_6\))is an linear sugar alcohol obtained by hydrogenation of D-glucose with a melt temperature of \(96\,^{\circ}\hbox{C}.\) It is highly soluble in water as well as in polar solvents. In the solid state, it exhibits a monotropic complex polymorphism. There are several polymorphs of sorbitol with different melting points as discussed in review above.
The measurements were performed at 304 V, medium pulse width, AF varied from 1260 at lower temperature to 623 at higher temperatures while duration stayed in the range 13,000–17,000 ms. The results of diffusivity observed for various forces as a function of temperature is shown in Fig. 17.
Cracking in the graphite layers was observed for pellets prepared under all the forces under which the pellets are pressed. However, the extend of cracking was least for pellets prepared with 15 kN force. Apparently, force seems to be influencing the observed diffusivity with the diffusivity rising with the force used for pressing the pellets. However, the standard deviation around a temperature for pellets prepared with 8 kN force is 8.24, 18% for 10 kN and 11.4% for 15 kN. This made it difficult to comment if this behavior is indeed trustworthy. The cracking of graphite layers could possibly be the result of absorbed water in the pellets which on being heated might be expanding causing the cracking of the adjacent graphite layer. This could be the reason for the spread of the data. Another reason for different values for different forces could be the different porosity of the samples pressed under different forces. Reason for higher spread for highest force could be because of the polymorphism since elimination of water while pressing it under large force made the samples more amorphous and sorbitol exhibits complex polymorphism in amorphous state [27].
To get better results, we dried the sorbitol pellets at a temperature of \(80\,^{\circ }\hbox {C}\) in atmospheric pressure conditions for around a days and the results were found to improve. The samples were measured at voltage of 304 V, medium pulse width, AF of 1260 and duration in the range 12,000–13,000 ms. The correction results for Cowan and Radiation model is shown in Fig. 18. Based on the results, we choose Radiation model for the evaluation of the data.
Figure 19 shows the results for thermal diffusivity of solid sorbitol sample as a function of temperature. The results for each temperature are the values averaged for three shots. It was found that diffusivity of solid sorbitol depends on temperature and a decrease in diffusivity with temperature was observed. This decrease in diffusivity increased with increasing temperatures. Further, it seems that high thickness will result in high values. The values are detailed in Table 6. The higher deviation for the sample with highest thickness could also be due to the different amorphous form of the sample. The deviation of data around a temperature is around 9%. Excluding the measurement result for sample with thickness 1.203 mm, the deviation is less than 3% which is an acceptable error.
For liquid, we melted the pellets in the sample holder using Infrared lamp. All the samples were tested at 304 V, long pulse width, AF of 2520 and duration stayed between 8000 and 13,000 ms. The obtained detector response is as shown below. For evaluation of the signal, we limited the duration between 2000 and 3000 ms since the signal after this is usually the result of noise and losses from the samples.
For liquid, two kinds of value were obtained as the measurement result, one lower and other higher. Out of the six samples tested, three resulted in lower end values with deviation around 2%, while other three resulted in higher end values with deviation of about 1%. The diffusivity values obtained are as shown in Fig. 20.
Values of thermal diffusivity obtained are tabulated in Table 7. The deviation for the whole range is around 9%. The reason for this difference could be the different moisture content in the different pellets. To test whether the moisture content was influencing the signal, we melted the pellets on the hot plate and heat it for sometime above 100 °C to ensure that all the water is eliminated. However, the signal failed to optimize for this case and we could not obtain a measurable signal. We therefore sticked to the technique of melting the sample within the sample holder in the device itself.
Further, sorbitol exhibits different conformations in the liquid state. Rotation around C2–C3 bond occurs on melting which prevents the crystallization of the melt. Rotation around C3–C4 bond is also observed in the literature. Though these different conformations might be evenly distributed, they could be a reason for different behaviors too. An increase in diffusivity with the temperature was observed for all the samples in the entire measurement range for liquids.
Using the diffusivity, density and heat capacity, thermal conductivity was calculated and the results are shown in Fig. 21. Heat capacity values used are those obtained for the heating of the powder sample since the diffusivity was tested for powder. For values around \(80\,^{\circ }\hbox {C}\), the data at lower temperatures was extrapolated to get the \(C_\mathrm{p}\) values. For thermal diffusivity, results of 1.203 mm thickness sample is not considered during averaging. Heat capacity and thermal conductivity were found to increase with temperature for both solids and liquids. Density was found to remain constant in liquid state for volume measured to an accuracy of 0.1 mL, while a linear increase with temperature was observed for solid. Thermal diffusivity decreased with temperature for solid and increased for liquid. There is no reported data on the thermal conductivity of sorbitol to the best of my knowledge.
Thermal behavior of isomalt
IsoMaltidex 16,500 is pure crystalline isomalt with properties of low hygroscopicity and non-stickiness. Its glass transition is above the room temperature, and so it cannot crystallize in room-temperature conditions. However, it was found to solidify well in the room-temperature conditions. It melts between 145 and \(150\,^{\circ }\hbox {C}\). It is stable till a temperature of \(160\,^{\circ }\hbox {C}\) as per the manufacturer after which it starts decomposing/degrading.
Solid samples of isomalt were prepared using mechanical press under a pressure of 15 kN. They were tested for their thermal diffusivity at voltage of 270 V, medium pulse width and AF of 623 in the duration range 13,000–16,000 ms. The detector response obtained is as shown below. Figure 22 also shows the influence of the correction method on the calculation result.
Based on the evaluation of detector signal, we chose Cowan model for calculation of the data. The results at each temperature are the values averaged for three shots. Figure 23 shows the influence of temperature and thickness on observed diffusivity. The values are tabulated in Table 8. It was found that diffusivity of solid isomalt depends strongly on temperature and a linear decrease in diffusivity with temperature was observed. Also, no clear correction was found between the sample thickness and the observed diffusivity. Deviation of the data around a temperature is around 3% which is an acceptable error.
With isomalt liquid, we melted the solid pellets over the hot plate keeping the lid on it while melting. This was done because lid was found to tilt after every measurement when pellets were melted in the sample holder in the device. Browning of the samples after the test run was observed after every test performed till \(200\,^{\circ }\hbox {C}\). The test run took around 1 hr 30 min time. However, no browning was observed when solid samples were heated on a hot plate till \(220\,^{\circ }\hbox {C}\) meaning degradation of isomalt occurs around 190–\(200\,^{\circ }\hbox {C}\) if left for long time otherwise it shows stable behavior. Also, it doesn’t crystallize on melting and stickiness was observed. However, it solidifies in room-temperature conditions when left for sometime as opposed to sorbitol which was found to undergo glass transition and no solidification was possible.
Liquid isomalt was tested as a function of temperature at 270 V, medium pulse width and AF of 1260 while duration stayed in the range 8000–9000 ms. The detector signal obtained and the diffusivity response are shown in Fig. 24. The measured values are tabulated in Table 9. Three samples were tested, and they differed in diffusivity values by less than 3% which is within our error limits. Observed thermal diffusivity was not found to be influenced by temperature. Further, the browned liquid sample was tested again to see whether degradation affects the thermal behavior of the sample and it retraced its behavior, confirming no influence of degradation on thermal conductivity.
Using the diffusivity, density and heat capacity, thermal conductivity was calculated and the results are as shown in Fig. 25. Heat capacity values used are those obtained for the heating of the powder sample since the diffusivity was tested for powder. Heat capacity and density was found to increase with temperature for solids. Also a very strong influence of temperature on density of liquid isomalt samples was observed. Thermal conductivity decreased with temperature for both solid and liquid, while thermal diffusivity decreased for solid and remained constant for liquid. There is no reported data on the thermal conductivity of isomalt to the best of my knowledge.
Thermal behavior of maltitol
Maltitol (\(\mathrm{C}_{11}\mathrm{H}_{24}\mathrm{O}_{11}\)) is obtained by catalytic hydrogenation of maltose syrups and exits as an amorphous hygroscopic solid. It has a melt temperature of \(150\,^{\circ }\hbox {C}\). Samples solidify on melting and did not vitrify like sorbitol samples. However, no crystallization was observed in room-temperature conditions.
Solid maltitol pellets were also prepared by pressing the powder using mechanical press under a force of 15 kN. However, the pellet preparation is also possible using the aluminum frames since it solidifies well in ambient temperature conditions. Solid samples were tested for their thermal diffusivity at voltage of 270 V, medium pulse width, AF of 315 while duration in the range 11,000–12,000 ms. A very good detector signal was observed. The influence of correction method on the signal evaluation is shown in Fig. 26.
The results at each temperature are the values averaged for three shots. It was found that diffusivity of solid maltitol depends strongly on temperature and a sharp decrease in diffusivity with temperature was observed. Also, sample thickness was found to influence the measured results and a linear increase in diffusivity with thickness is apparent. However, it is also to be noticed that in certain range of values, the influence of thickness on the diffusivity is negligible, For this compound, samples with thickness from 1.225 to 1.252 mm resulted in nearly same values. Deviation of the data around a temperature is around 5% which is without our error limits. Figure 27 shows the result for the diffusivity of solid maltitol as a function of temperature. The observed diffusivity values are tabulated in Table 10.
For liquid samples, we melted the pellets in the sample holder on the hot plate keeping the lid on it so as to make sure there is no tilting of the lid during the test and it fits well in position. Sample was allowed to solidify before placing in the sample holder in the LFA. An increase in diffusivity with temperature was observed. Three samples were tested, and they differed in diffusivity values by less than 3% which is within our error limits. Samples were tested at 270 V, medium pulse width, AF of 1260 while duration stayed around 8000 ms. Heat loss pulse width correction method is used for the calculations. The detector response and the thermal diffusivity behavior are shown in Fig. 28. The measured diffusivity values are tabulated in Table 11.
Using the diffusivity, density and heat capacity, thermal conductivity was calculated and the results are as shown in Fig. 29. Heat capacity values used are those obtained for the heating of the powder sample since the diffusivity was tested for powder. Heat capacity and density were found to increase with temperature for solids, while density decreased for liquids. Thermal conductivity showed nonlinear behavior with temperature, while thermal diffusivity decreased with temperature for both solid and liquids. There is no reported data on the thermal conductivity of maltitol to the best of my knowledge.
Thermal behavior of galactitol
galactitol (\(\mathrm{C}_{6}\mathrm{H}_{14}\mathrm{O}_{6}\)) is a reduction product of galactose. The samples crystallize well in room-temperature conditions, and so the pellets were prepared in aluminum frames as discussed before.
The pellets were tested for its thermal behavior in the temperature range 120 to \(160\,^{\circ }\mathrm{C}\), tested at a voltage of 270 V, medium pulse width, AF of 315 and duration in the range 9000–10,000 ms. The detector response obtained and the basis of selection of the correction model are shown in Fig. 30. Based on the result of evaluation of detector signal, Cowan model was used for the calculation of the data.
The results at each temperature are the values averaged for three shots. The diffusivity values are shown in Fig. 31. The values are tabulated in Table 12. It was found that diffusivity of solid galactitol depends strongly on temperature and a decrease in diffusivity with temperature was observed. No clear correlation can be drawn between samples thickness and the observed diffusivity. However, it is clear that samples with very high thickness resulted in quite high diffusivity values. The results of the measurement are as shown below.
For the measurement of liquid samples, pellets were melted in the sample holder on the hot plate keeping the lid on it to ensure there is not tilting of the lid during the test run. Sample was solidified before placing it in the sample holder in LFA. Browning of the samples were observed after the measurements confirming the degradation of the samples at high temperature. All the samples were measured at 304 V, medium pulse width, AF of 1260 for lower temperatures, AF of 623 for higher temperatures while duration stayed in the range of 8000–9000 ms. The results are as shown in Fig. 32. The measured values are tabulated in Table 13. The deviation of the data around a temperature is less than 2% which is a promising result. An increase in diffusivity was observed with increasing temperature. The obtained detector signal and the diffusivity values are as shown in Fig. 32.
Using the diffusivity, density and heat capacity, thermal conductivity was calculated and the results are as shown below. Heat capacity used is the value for the heating part of the cycle. Density and heat capacity were found to increase with temperature for solid samples. For liquid density, we could only measure till \(210\,^{\circ }\hbox {C}\) since silicon oil bath was found to start fuming after this temperature. Therefore, for thermal conductivity measurement in liquid state, value of density at \(210\,^{\circ }\hbox {C}\) is used for all the temperatures. Thermal conductivity was found to increase with temperature for both solids and liquids, while thermal diffusivity decreased for solids with temperature.
Thermal diffusivity of galactitol as reported in literature is 0.96 \(\mathrm{mm}^2\,\mathrm{s}^{-1}\) at \(20\,^{\circ }\hbox {C}\) which means a thermal conductivity of \(2.027\,\mathrm{Wm}^{-1}\mathrm{K}^{-1}\) using the value of density and heat capacity we have, while our calculations on extrapolation would result in \(\lambda\) of \(2.313\,\mathrm{Wm}^{-1}\mathrm{K}^{-1}\) at \(20\,^{\circ }\hbox {C}\). This deviation is acceptable. They observed a diffusivity of 0.314 \(\mathrm{mm}^2\,\mathrm{s}^{-1}\) at \(200\,^{\circ }\hbox {C}\) which is far higher than the values we obtained. Figure 33 shows the observed results.
Conclusions
In the present work, we have tested the thermal properties of some common sugar alcohols using Netzsch LFA 447. Testing of erythritol samples under different LFA parameters showed that LFA parameters did not influence the observed results. This is expected since diffusivity is an intrinsic material property and should not be influenced by measurement conditions. Voltage and pulse width was found to increase the energy falling on the front face of the sample. These parameters should be kept high to get a finer detector response curve. However, very high energy for very low conductivity material may cause instantaneous melting of the front face of the sample. But, higher energy would eliminate the possibility of the surrounding noise signal to influence the detector result. The idea is to choose the values so as to keep the noise minimum. Duration was found to influence the liquid calculations. We limited duration between 2000 and 3000 ms for data evaluation since literature and manual suggested that for liquids, signal after this duration is the result of losses from the samples. Delay should be kept high for higher thickness and high temperature measurements to allow maximum time for the sample to come to equilibrium. Amplification factor influences the signal received from the back side of the sample. It is best to choose the set of parameters so as to keep the measurement time small. Higher duration resulted in erroneous results.
Some sugar alcohols failed to absorb the laser energy flashing on the front face when coated with three graphite layers. To fix this, we coated them with more graphite. However, it may be possible that too much graphite may cause an increase in apparent thermal diffusivity of the material due to the really high thermal conductivity of graphite.
Sugar alcohols test results for diffusivity showed that values were very high for solid samples compared to liquid. This puts an important limitation on its use as PCM since low thermal conductivity in liquid state will make their solidification difficult. Sample thickness was found to influence the measurement results with the diffusivity linearly rising with rising thickness except for galactitol and isomalt samples for which no clear correction was observed. This behavior was also observed for paraffin samples tested. In a range of thickness, the diffusivity wasn’t found to be influenced by thickness and it is best to consider these values as the diffusivity result. Too high thickness increases the possibility of radiation losses, especially at higher temperatures, while too low thickness makes finite pulse width effect more noticeable, especially for high conductivity materials. Choice of optimum thickness is therefore important. Further, since sugar alcohols exhibit complex polymorphism, it is best to prepare all the samples in one day to make sure that measurement conditions are same. Further, it is also advisable to test the samples before measurement for their IR spectra.
For sugar alcohols which did not solidify, pellets were prepared by pressing the powder. However, maltitol and isomalt showed comparatively very high density in their solidified form compared to the powder. They may also exhibit different thermal behaviors in the same case. Also this would closely represent the materials as they would be used in real time systems. This further needs to be studied. An influence of pressing force (forced applied to press the powder samples for forming pellets) on diffusivity was also observed. However, it is hard to say it with confirmation since the data were very spread around a temperature for higher forces (as much as 18%)
For solid density, due to the limitations of the measuring system, density values at 20, 30 and \(40\,^{\circ }\hbox {C}\) were extrapolated to obtain solid densities at higher temperatures (till \(160\,^{\circ }\hbox {C}\) for galactitol) which showed a linearly rising trend. This values may not represent the actual behavior since values should ideally decrease with temperature.
In nutshell, sugar alcohols seems to have very interesting properties with respect to their application as a phase change material. However, its thermal behavior is challenging to evaluate due to its complex polymorphism and hydroscopic properties.
References
Parker WJ, Jenkins RJ, Butler CP, Abbott GL. Thermal diffusivity measurements using the flash technique. J Appl Phys. 1961;32:1679.
Cowan RD. Pulse method of measuring thermal diffusivity at high temperatures. J Appl Phys. 1963;34:926.
Cape JA, Lehman GW. Temperature and finite pulse-time effects in the flash method for measuring thermal diffusivity. J Appl Phys. 1963;34:1909.
Heckman RC. Finite pulse-time and heat-loss effects in pulse thermal diffusivity measurements. J Appl Phys. 1973;44:1455–60.
Clark LM III, Taylor RE. Radiation loss in the flash method for thermal diffusivity. J Appl Phys. 1975;46:714.
Beedham K, Dalrymple IP. The measurement of thermal diffusivity by the flash method. An investigation into errors arising from the boundary conditions. Rev Int Hautes Temp Refract. 1970;7:178–283.
McKay JA, Schriempf JT. Corrections for nonuniform surface heating errors in flash method thermal diffusivity measurements. J Appl Phys. 1976;47:1668–71.
Taylor RE, Clark LM III. Finite pulse time effect in flash diffusivity method. High Temp High Press. 1974;6:65–72.
Moskal G, Cwajna J. Analysis of influence of measurement conditions on repeatable results of thermal diffusivity of ceramic moulds designed for manufacturing the superalloys castings, IOP Conf. series: materials science and engineering; 2010. vol 15, p. 012064. https://doi.org/10.1088/1757-899X/15/1/012064
dos Santosa WN, Mummery P, Wallwork A. Polym Test. Thermal diffusivity of polymers by the laser flash technique. 2005;24:628–34.
Albers APF, Restivo TAG, Pagano L, Baldo JB. Effect of testing conditions on the laser Flash thermal diffusivity measurements of ceramics. Thermochim Acta. 2001;370:111–8.
Kim SK, Kim YJ. Improvement of specific heat measurement by the flash method. Thermochim Acta. 2007;455:30–3.
Gunasekara SN, Pan R, Chiu JN, Martin V. Polyols as phase change materials for surplus thermal energy storage. Appl Energy. 2016;162:1439–52.
Lopes Jesus AJ, Nunesb SCC, Ramos Silva c M, Matos Bejac A, Redinha JS. Erythritol: crystal growth from the melt. Int J Pharm. 2010;388:129–35.
Gunasekara SN, Pan R, Chiu JN, Martin V. Polyols as phase change materials for low-grade excess heat storage. Energy Procedia. 2014;61:664–9.
Berman HM, Jeffrey GA, Rosenstein RD. The crystal structures of the a8 and b forms of D-mannitol. Acta Crystallogr Sect B Struct Crystallogr Cryst Chem. 1968;24:442–9.
Kim HS, Jeffrey GA, Rosenstein RD. Crystal structure of K form of D-mannitol. Acta Crsyt. 1968;24:1449.
Botez CE, Stephens PW, Nunes C, Suryanarayanan R. Crystal structure of anhydrous D-mannitol. Int Cent Diffr Data. 2003;. doi: 10.1154/1.1582460.
Yu L, Milton N, Groleau EG, Mishra DS, Vansickle RE. Existence of a mannitol hydrate during freeze-drying and practical implications. J Pharm Sci. 1999;88:196–8.
Rye A, Sorum H. Acta Chem Scand. 1952;6:1128.
Chen H, Ginzburg VV, Yang J, Yang Y, Liua W, Huang Y, Libo D, Chen B. Thermal conductivity of polymer-based composites: fundamentals and applications. Prog Polym Sci. 2016. https://doi.org/10.1016/j.progpolymsci.2016.03.001.
Barreneche C, Gil A, Sheth F, Fernandez AI, Cabeza Luisa F. Effect of D-mannitol polymorphism in its thermal energy storage capacity when it is used as PCM. Sol Energy. 2013;94:344–51.
Kumaresan G, Velraj R, Iniyan S. Thermal analysis for D-mannitol for use as phase change material for thermal energy storage. J Chem Sci. 2011;11:3044–8.
Gil A, Barreneche C, Moreno P, Sole C, Ins Fernndez A, Cabeza Luisa F. Thermal behaviour of D-mannitol when used as PCM: comparison of results obtained by DSC and in a thermal energy storage unit at pilot plant scale. Appl Energy. 2013;11:1107–13.
Siniti M, Jabraneb S, Leatoffe JM. Study of the respective binary phase diagrams of sorbitol with mannitol, maltitol and water. Thermochim Acta. 1999;325:171–80.
Quinquenet S, Ollivon M, Grabielle-Madelmontont C. Polymorphism of hydrated sorbitol. Thermochim Acta. 1988;125:125–40.
Nezzal A, Aerts L, Verspaille M, Henderickx G, Redl Andreas. Polymorphism of sorbitol. J Cryst Growth. 2009;311:3863–70.
Cammenga HK, Zielasko B. Thermal behaviour of Isomalt. Thermochim Acta. 1996;271:149–53.
Bolhuis GK, Engelhart JJP, Eissens AC. Compaction properties of Isomalt. Eur J Pharm Biopharm. 2009;72:621–5.
Raudonus J, Bernard J, Januen H, Kowalczyk J, Carle R. Effect of oligomeric or polymeric additives on glass transition, viscosity and crystallization of amorphous Isomalt. Food Res Int. 2000;33:41–51.
Capet F, Comini S, Odou G, Looten P, Descamps M. Orientated growth of crystalline anhydrous maltitol (4-O-a-D-glucopyranosyl-D-glucitol). Carbohydr Res. 2004;339:1225–31.
John G, Konig-Haagen A, Kingondu CK, Bruggemann D, Nkhonjera L. Galactitol as phase change material for latent heat storage of solar cookers: investigating thermal behavior in bulk cycling. Sol Energy. 2015;119:415–21.
Xu T, Chen Q, Huang G, Zhang Z, Gao X, Lu S. Preparation and thermal energy storage properties of D-Mannitol/ expanded graphite composite phase change material. Solar Energy Materi Solar Cells. 2016;155:141–6.
Acknowledgements
The author is extremely thankful to Dipl.Georg Hagelstein, my supervisor, during the work at Fraunhofer Institute for Solar Energy Systems (ISE), Germany, for providing guidance and support at various stages of work and the Division for Thermal Systems and buildings, Fraunhofer ISE, for providing the necessary facilities for making the work possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agarwal, T. Influence of measurement parameters of laser flash analysis on the observed thermal diffusivity and the choice of parameters to get repeatable measurements. J Therm Anal Calorim 134, 1183–1203 (2018). https://doi.org/10.1007/s10973-018-7468-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7468-4