Abstract
Summary
Quantitative ultrasound (QUS) has been found to be a safe and reliable method for evaluating bone mineral density (BMD). Using calcaneal QUS techniques, the current study contributes to remedying this gap in the literature by establishing normative data among children and adolescents from Colombia.
Introduction
Minimal data on BMD changes are available from populations in developing countries. BMD reference values for children and adolescents have not been published for a Latin-American population. The aim of this study was to establish a normal reference range of calcaneal broadband ultrasound attenuation (BUA) in Colombian children and adolescents with ages ranging from 9 to 17.9 years.
Methods
A sample of 1001 healthy Colombian youth (boys n = 445 and girls n = 556), children, and adolescents (9–17.9 years old) participated in the study. A calcaneus QUS parameter (BUA) was obtained for boys and girls, stratified by age group. Furthermore, height, weight, fat mass percentage, and body mass index were measured. Centile smoothed curves for the third, tenth, 25th, 50th, 75th, 90th, and 97th percentiles were calculated using the LMS method (L [curve Box–Cox], M [curve median], and S [curve coefficient of variation]).
Results
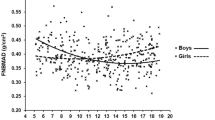
Mean (± SD) values for the participants’ anthropometric data were 12.9 ± 2.3 years of age, 45.2 ± 11.5 kg weight, 1.51 ± 0.1 m height, 19.5 ± 3.1 kg/m2 BMI, and 69.5 ± 17.1 dB/MHz BUA. Overall, all variables were significantly higher in boys except in BMI and body fat percentage. Girls generally had higher mean calcaneal BUA (dB/MHz) values than the boys, except in the age ranges 16 and 17.9, p > 0.05. In addition, the BUA (dB/MHz) increased with age throughout childhood and adolescence and reached a plateau by age 15–17.9 for girls.
Conclusions
For the first time, our results provide sex- and age-specific BUA reference values for Colombian children and adolescents aged 9–17.9 years. A more specific set of reference values is useful for clinicians and researchers and informs clinical practice to monitor bone mineral status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the demand for the measurement of bone mineral density (BMD, grams per square centimeter, bone mineral content relative to a projection area) to identify children who could be exposed to an increased risk of osteoporosis in adulthood has rapidly increased [1, 2]. According to the recommendations of the International Society for Clinical Densitometry, a combined assessment of physical measures and biochemical bone metabolic markers is useful for identifying reduced skeletal development in children [3]. Dual-energy X-ray absorptiometry (DXA) is considered a reference standard for diagnosing bone status by measuring BMD [4]. The main advantages of DXA are its short scanning times. However, the subject is exposed to ionized radiation, which varies according to the machinery and skeletal site examined [1–5]. Other disadvantages of the technique include limited portability, lack of real-time feedback, repeatability, high cost, and accessibility.
Quantitative ultrasonography (QUS), which has been used to assess bone (especially calcaneal) status for almost two decades, has proven to be widely applicable and clinically useful [6, 7]. The QUS technique is safe, easy to use, and cost-effective. Furthermore, the devices are portable (i.e., only a few minutes are needed to perform the measurements), and they are radiation-free [8]. Because radiation safety is a concern, quantitative ultrasound is especially suitable for children. Studies have demonstrated both the effectiveness and validity of broadband ultrasound attenuation (BUA) as an independent predictor of fracture risk in adults [9, 10]. In healthy children and adolescents, Mughal et al. [11] demonstrated that calcaneal BUA is significantly correlated with the total body BMD (r = 0.74, p < 0.001). Hence, QUS can be an alternative to DXA in the assessment of bone strength in children under the right clinical setting.
Osteoporosis is characterized by low bone mass and microarchitectural deterioration of bone structure resulting in increased bone fragility [12, 13]. Previous studies have reported that children with a low BMD during prepuberty maintain a low BMD in adolescence [14, 15], and perhaps even in adulthood. Because there is a close relationship between low BMD and independent predictors of fracture risk, it would be valuable to develop normative values in healthy children and adolescent populations [16]. Given the popularity and versatility of the QUS, it is surprising that there is a paucity of reference values for the test [17–22]. Only six studies attempted to develop normative data tables for BMD by reporting BUA values measured from the calcaneus, using a gel-coupled scanner (QUS-2; Metra Biosystems, Mountain View, CA) [8, 11, 23–26]. Nonetheless, there is a paucity of data in Latin-American populations. More specifically, reference values for the BUA for the Colombian population obtained with a standardized protocol such as that used by Mughal et al. [11] are lacking. Latin America is composed of developing countries, one of which is Colombia. As reflected in recent studies, children from different geographic areas have different nutritional deficits.
The main aim of the FUPRECOL Study (Asociación de la Fuerza Prensil con Manifestaciones de Riesgo Cardiovascular Tempranas en Niños y Adolescentes Colombianos) is to examine relationships between physical fitness levels in children and adolescents with cardiometabolic risk factors and (un)healthy habits used to evaluate their health status. Our working hypothesis is that children and adolescents who have high physical fitness levels are healthier and enjoy a better quality of life. In addition, this study seeks to establish the general prevalence of cardiovascular risk factors (anthropometric, metabolic, and genetic markers) in the study population (children and adolescents aged 9 to 17.9 years living in Bogota, Colombia) [27]. Also examined was the potential influence of the social environment and lifestyle in developing a disease or acquiring (un)healthy habits that could eventually lead to a cardiometabolic-related disorder.
Age- and sex-specific bone reference values can help to assess skeletal development during childhood and to compare the bone health status of a child with that of the healthy population of the same age, sex, and ethnicity. Thus, deviations from a normal skeletal development can be identified in an early stage. The aim of this study was to establish a normal reference range of calcaneal BUA in Colombia children and adolescents aged 9 to 17 years.
Methods
Participants and study design
The present cross-sectional study was conducted to provide BUA values using QUS in Colombian children and adolescents in the FUPRECOL Study. Data were collected in a sample of students from a Colombian school (means ± standard deviations [SD] age 12.9 ± 2.3 years, weight 45.2 ± 11.9 kg, height 1.50 ± 0.1 m, BMI 19.4 ± 3.1 kg/m2), which is located in the Bogota capital district, in a municipality in the Cundinamarca Department, Andean region. It is located at approximately 4°35′56″N 74°04′51″W, at an elevation of approximately 2625 m (min 2500, max 3250) above sea level. Bogota is considered an urban area, with approximately 7,862,277 inhabitants [27].
Subjects
A sample of 1001 healthy Colombian youths (boys n = 445 and girls n = 556), children, and adolescents (9–17.9 years old) participated in the study. All individuals from the selected public school were invited to participate in the study. The participation rate was greater than 95 %. A convenience sample of volunteers was included and grouped by sex and age with 1-year increments (a total of nine groups). A power analysis showed that this sample size was sufficient to estimate BUA values with a precision of 10 % and a power of 90 %. The sample size was estimated at 30 participants per age-sex group. The recruitment period lasted from April 2015 to June 2015.
The Review Committee for Research on Human Subjects at the University of Rosario (Code N° CEI-ABN026-000262) approved the trial. A comprehensive verbal description of the nature and purpose of the study and its experimental risks was given to the adolescents and their parents/guardians. This information was also sent to parents/guardians by regular mail, and written informed consent was obtained from the parents and children before they participated in the study. The protocol was in accordance with the latest revision of the Declaration of Helsinki.
Procedures
A manual of operations (study rationale, test procedures, and procedures for data recording) was designed for and read by the physical education teacher involved in the FUPRECOL Study before data collection started. Instructions for the participants were provided in the manual of operations to standardize procedures. Several trained senior master students performed the measurements in a temperature-controlled environment (room temperature approximately 23–27 °C). A questionnaire was given to the parents requesting information concerning their child’s medical history. The inclusion criteria were as follows: (1) no movement restriction in the lower extremities and (2) no self-reported history of chronic disease that could affect calcium and vitamin D metabolism, metabolic bone disease, long-term medications such as corticosteroids, anticonvulsants and immunosuppressants that can affect bone metabolism, and anomalies of growth and puberty. The study was conducted in ten schools by two trained physical activity master students. With regard to the communication processes and explanations of tests, direct and simple oral language was used. When necessary, the evaluators provided visual models and examples before performing the tests.
Anthropometric component
Anthropometric variables were measured by a level 2 anthropometrist certified by the International Society for the Advancement of Kinanthropometry (ISAK), in accordance with the ISAK guidelines [28], in the morning following an overnight fast, at the same time each day (7–9 a.m.). The first visit included body weight measurement to the nearest 0.05 kg, using a calibrated scale (Tanita BWB-800A®; Tanita Corp., Tokyo, Japan). Patient height was measured to the nearest 0.1 cm, using a stadiometer (SECA 220®; Seca Ltd., Hamburg, Germany), and body mass index (BMI) values were calculated. During the anthropometric measurements, students wore light clothing and were barefoot. Subsequently, for bioelectrical impedance analysis (BIA) measurements, a classical bipolar technique was used to estimate body fat (%) using a Tanita BWB-800A® (Tanita Corp., Tokyo, Japan) according to the manufacturer’s instructions.
Measuring broadband ultrasound attenuation
The reference values for calcaneal BUA in children are dependent on the commercial ultrasound bone densitometer used, and mathematical adjustments were made to the true calcaneal BUA according to Jaworski et al., who used the Achilles ultrasound densitometer (Lunar Corporation, Madison, WI, USA to measure the calcaneal BUA values in pediatric patients over the right and left heels [29]). The participant was seated with their right foot and left foot placed in the footwell, which was then was secured using two straps. Ultrasonic coupling jelly was then applied to the sides of the right and left heel, and transducers were placed on either side of the heel. Inaudible sound waves were transmitted through the calcaneum, and the BUA (in decibels per megahertz (dB/MHz)) was measured. Thus, the reference values of the BUA presented here are the result of the calculation of the values obtained for the right and left heels without considering heel dominance. For quality control and the evaluation of precision, the QUS devices were calibrated on a daily basis, using a phantom subject during the screening period. Two trained operators performed all of the measurements. The coefficient of variation (CV) for within-day measurements has previously been reported as 1.8 % for BUA. The CV for between-day measurements is 0.6 [30].
Statistical analyses
The Kolmogorov–Smirnov test was used to assess the normality of all variables. Anthropometric data and BUA values are reported as the means ± SD. We analyzed sex-group differences in the anthropometric and BUA data by one-way analysis of variance. A linear regression model and Pearson’s correlation coefficients were calculated to examine the relationships between BUA values and age, weight, height, BMI, and body fat. To provide percentile values for the sample, we analyzed the BUA (dB/MHz) data for males and females separately by the maximum penalized likelihood by using the LMS statistical method [31]. We derived smoothed centile charts with the LMS method. This procedure estimates the measurement percentiles in terms of three age-specific cubic spline curves: the L curve (Box–Cox power), M curve (median), and S curve (coefficient of variation). The appropriate number of degrees of freedom was selected on the basis of the deviance, Q-tests, and worm plots, following the suggestions of Royston and Wright [32]. The third, tenth, 25th, 50th, 75th, 90th, and 97th smoothing percentiles were chosen as age- and gender-specific reference values. For the construction of the percentile curves, data were imported into the LmsChartMaker software (V. 2.3; by Tim Cole and Huiqi Pan), and the L, M, and S curves were estimated. Except for the LMS method calculations, we used SPSS v. 21 software for Windows for the data analyses (SPSS, Chicago, Illinois, USA). For all analyses, the significance level was 0.05.
Results
Descriptive characteristics
Anthropometric characteristics and BUA (dB/MHz) of the study sample by sex are shown in Table 1. The mean values were 12.9 ± 2.3 years of age, 45.2 ± 11.5 kg weight, 1.51 ± 0.1 m height, 19.5 ± 3.1 kg/m2 BMI, and 69.5 ± 17.1 dB/MHz BUA. Overall, all variables were significantly higher in boys except in BMI and body fat (%). BUA average was similar in boys and girls (p = 0.440). In addition, the BUA (dB/MHz) increased with age throughout childhood and adolescence and reached a plateau by age 15–17.9 for girls. As shown in Fig. 1, the girls generally had higher mean calcaneal BUA values than the boys, except in the age ranges 16 and 17.9, p > 0.05.
A steady increase of BUA was seen with increasing body height in both sexes. In the 150- to 159-cm height category, the values of all parameters were significantly higher in girls than in boys (Table 2). A steady increase of BUA was seen with increasing body weight in both sexes. In the 40–49 kg group, the BUA value was significantly higher in females than in males (Table 3).
Normative values
Tables 4 show the normative values for BUA (dB/MHz) in the children and adolescents (9–17.9 years old), classified according to sex and age and expressed in percentiles from 3 to 97. The BUA values for boys were higher than those for girls (13 to 17.9 year age group). Nevertheless, the results show that the mean calcaneal BUA values increased continuously between 9 and 17 years of age and were significantly related to age, height, weight, and BMI (see Table 4).
Figure 2 shows smoothed centile curves (third, tenth, 25th, 50th, 75th, 90th, and 97th) for BUA (dB/MHz), based on sex and age. Post hoc analyses within sexes showed yearly increases in BUA for each year measured. In girls, significant increases in BUA were observed from 15 to 17.9 years.
Associations between the subjects’ basic data (age, weight, height, BMI, and body fat) for both sexes and the BUA were examined by Pearson correlations (Table 5). In both sexes, the BUA parameter correlated positively with age, weight, height, BMI, and negatively with body fat.
Finally, comparisons of the mean values for the BUA (dB/MHz) from this study are presented in Table 6.
Discussion
The main objective of this study was to establish reference values for calcaneal BUA measured by QUS in children and adolescents 9–17.9 years old. Our results show that the mean calcaneal BUA values increased continuously between 9 and 17 years of age and were significantly related to age, height, weight, and BMI. These results are consistent with many studies, which have confirmed that the QUS of the calcaneus is influenced by the growth process [8, 11, 23–26].
The calcaneus is the most common skeletal site used for ultrasonometry because only a thin soft tissue (which is relatively consistent) covers the bone, and this bone, which is more than 90 % trabecular, has a high metabolic activity, and is more responsive to age-, disease-, or therapy-induced bone alterations than cortical bone [31]. Of the substantial normative BUA publications [8, 11, 23–31], few summarize data obtained with instrumentation, procedures, or measures recommended by standardized protocols such as those used by Jaworski et al. [29] or Mughal et al. [11]. Standardization is important to allow valid comparisons within or between countries for the assessment of longitudinal or secular trends and also to reliably detect poor bone status in the clinical setting for the identification of individuals who may gain particular benefit from interventions. These studies tend to use age bands of 1 year, making it difficult to identify the age at which bone status is attained or when the most significant or largest declines in BMD occur.
Many reports have also indicated that peak bone mass is attained between 16 and 18 years of age [25, 33, 34]. The peak bone mass is the highest level of bone mass achieved as a result of normal growth, and a low value is a well-recognized risk factor for osteoporosis later in life [33]. These findings appear to suggest the attainment of peak BUA in the middle of the first and second decades of life and indicate a strength decline at an earlier age than reported in a number of previous cross-sectional studies [8, 11, 20–31].
Reference values for BUA (dB/MHz) are usually presented in a notable format or as centile curves as a function of age [8, 11, 23–26]. This is the first study to show age and sex reference values for BUA among children and adolescents from Latin America. To evaluate age-related changes across young adulthood, as well as to establish more precise age-related reference values in the pediatric population, we assessed and compared the BUA values between relatively narrow age bands. We observed that the increase in mean calcaneal BUA values was related to age, weight, height, and BMI (Tables 2 and 3). In addition, age, weight, and height were almost significantly positively associated with the BUA parameter, as has been observed in other studies [8, 11, 22–26]. Despite the known importance of peak bone mass, the age at which peak values are reached remains controversial. Some studies have indicated that peak bone mass is reached as late as 35 years of age. However, other studies have shown that more than 85 % of peak skeletal mass is accrued by the age of 18 years, making bone growth during childhood and adolescence a critical process [31, 35, 36].
However, given our results and the age range of the participants, it was impossible to determine the age at which peak bone mass was achieved. Moreover, this finding should be interpreted with caution because the present study is not longitudinal, and as such, may also reflect secular changes in bone status that have been reported internationally [23–26].
Based on comparisons of the mean BUA values, other studies have shown gains of 22–62 % in calcaneus BUA between childhood and late adolescence [8, 33]. There are multiple reasons for these differences. Firstly, although international comparisons of the BUA using similar methodology are lacking, varying values for calcaneal BUA in different regions and ethnicities are evident [8, 11, 23–33]. These may be accounted for by differences in anthropometric parameters such as sex, height, weight, and body composition, which vary between populations and ethnicities and are important determinants of bone status [37, 38]. Secondly, bone density is influenced by genetics, diet (particularly, calcium, vitamin D, and protein intake), and BMI. Other factors that may contribute to the rate of bone density loss include increasing age, low physical activity levels, some medications, certain medical conditions such as inflammatory bowel disease and celiac disease, smoking, and alcohol consumption [39].
The majority of the published bone status reference values were determined in children from high-income countries in Europe [11, 26] and Asia/Oceania [8, 23–25]. However, there is a scarcity of reference values for children using harmonized BMD measures in Latin America and other low- and middle-income countries (LMICs) that are undergoing a nutritional transition [40]. This makes it impossible to evaluate secular trends within these regions. Colombia is in the midst of a nutritional transition that mirrors the changes occurring elsewhere in the world, such as an increase in the overweight/obese population and a general decrease in the chronically undernourished population [41]. Previously, in pediatric populations, we have reported that children living in rural areas and in geographic regions with lower economic and structural development generally have lower serum concentrations of micronutrients than children from urban areas [42–44]. Likewise, in the dietary study, we conducted in Villamor et al. [45], only 13.4 % of the protein in the diets of children was derived from eggs and milk, with 40 % of protein from meat. Colombia is a country that is geographically, climatically, and ethnically diverse. Clearly, these differences could affect the food supply, dietary practices, and, consequently, the micronutrient intake. Indigenous and other ethnic groups of Colombia are very diverse, which leads to different dietary patterns and climates. Colombia’s rapid economic development and modernization has led to the “Westernization” of the Colombian diet, as characterized by an increase in the absolute number of calories, saturated fat, and fast food consumed and a decrease in the consumption of legumes, fruit, and cereals [27, 42–44]. These dietary patterns may partially explain our results, given the effect of diet on bone metabolism in Colombian children and adolescents.
Considering the present BUA reference values, the third age-specific percentile in 9- to 17.9-year-old boys (range 32.6–55.6 dB/MHz) and girls (range 32.6–55.2 dB/MHz) may be considered as a warning threshold for low BMD and reduced skeletal development. The pathological meaning of these values needs to be further evaluated.
It should be highlighted that only Sani et al. [46] investigated BUA values in children with morphologic changes associated with bone loss in thalassemia disorders. The mean BUA values obtained were 53.9 ± 1.1 dB/MHz in twelve 10- to 13-year-old children and 56.0 ± 3.7 dB/MHz in fourteen 14- to 17-year-old adolescents with osteopenia. In conjunction with their findings, our reference values for 9.0–17. 9 year-old girls in the third age-specific BUA percentile (range 32.6–55.2 dB/MHz) are close to this pathological value for osteopenia.
To obtain reference values that did not stem from disturbed skeletal development due to diseases or medical treatments that directly affect bone metabolism, children and adolescents with these conditions were excluded. However, fat mass may also have a pathophysiological effect on bone metabolism. In this study, we found an inverse association between BUA values and body fat in schoolchildren (r = −0.257, p = 0.01). This is further supported by Eliakim et al. [47] and Correa-Rodriguez et al. [48], who showed reduced QUS measures in obese children compared to non-obese children. Other studies such as Bredella et al. [49] show that adipose tissue is a negative predictor of the micro-architecture of the bone’s mechanical properties in obese men. Similar findings were described by Pollock et al. [50], who conclude that overweight combined with metabolic anomalies is closely associated with a lower bone mineral density in adolescents. While it does not appear that current nutritional status modulates the association between BUA and QUS values, studies with a more comprehensive evaluation of current nutritional status are needed to more robustly evaluate this hypothesis.
The findings in this study must be interpreted with caution because the present study is not longitudinal and therefore may also reflect secular changes in bone status, as has been reported internationally [8, 11, 23–25]. In our study, pubertal staging using the method of Tanner was not performed. The unknown pubertal status of the subjects is a limitation of this study. A second limitation of this study is the cross-sectional design. Longitudinal data of individuals give more accurate reference data. The third limitation of the existing research was that no measurements were taken of other components of bone health, such as diet (particularly, calcium, vitamin D, and protein intake), birth weight, and weight and physical activity levels, which may contribute to bone accretion [49]. Accordingly, the third to 25th percentile curves obtained in this study could be used as cut-off points, below which the BUA level can be considered inadequate. Liu et al. [49] showed that subjects who presented BUA values in the lowest two quartiles in the sample distribution at admission were at increased risk of being considered nutritionally-at-risk. Additionally, Liu et al. [49] reported that the QUS values of Chinese males and females peaked after 20 years, and the values of youths less than 20 years of age can reach more than 90 % of the peak value.
The correct interpretation of calcaneal BUA data requires comparing the score obtained in a particular person with normative values for the general population of the same sex and age. Therefore, additional work is needed to more fully characterize BUA values within the Colombian population and to identify population-specific cut-off points for health/adequate bone status and other components of bone metabolism. These values may ideally be combined with evaluations of markers or the nutritional status, which is vital to the success of bone health. Several studies have highlighted the importance of considering more than one bone metabolic marker, i.e., at least one bone formation and resorption marker each to assess reduced bone turnover or decreased skeletal development. Despite these limitations, the present study presents BUA data measured at the Achilles tendon for healthy Colombian children and adolescents, which could serve as reference values for evaluating the bone status of young people.
In summary, the QUS tool may be used to measure bone status, or more correctly, to stratify patients according to their risk of fracture. The pediatric CUBA is a relatively low cost and portable system with a scan time of less than 3 min. None of the studies included in this assessment reported any adverse events associated with the use of QUS. However, the results may vary according to the QUS parameters and type of device used and the skeletal site tested. Jaworski et al. [29] found that calcaneal BUA values discriminated healthy children from those known to have osteoporosis (mean total body BMD [SD] score −3.3 [0.9]).
In conclusion, this is the first paper to provide sex- and age-specific reference values of BUA in Colombian children and adolescents aged 9–17.9 years, based on a large and apparently healthy cross-sectional study. These reference values provide a foundation for the evaluation of bone growth and metabolism in children and adolescents and for monitoring bone mineral status.
References
Baroncelli GI (2008) Quantitative ultrasound methods to assess bone mineral status in children: technical characteristics, performance, and clinical application. Pediatr Res 63(3):220–228
Henwood MJ, Binkovitz L (2009) Update on pediatric bone health. J Am Osteopath Assoc 109(1):5–12
Boyce AM, Gafni RI (2011) Approach to the child with fractures. J Clin Endocrinol Metab 96:1943–1952
Bonnick SL (2009) Bone densitometry in clinical practice: application and interpretation. Humana Press, Totowa
Njeh CF, Fuerst T, Hans D, Blake GM, Genant HK (1999) Radiation exposure in bone mineral density assessment. Appl Radiat Isot 50:215–236
Gluer CC (1997) Quantitative ultrasound techniques for the assessment of osteoporosis: expert agreement on current status. J Bone Miner Res 12:1280–1288
Genant HK, Engelke K, Fuerst T, Gluer C-C, Grampp S, Harris ST et al (1996) Noninvasive assessment of bone mineral and structure: state of the art. J Bone Miner Res 11:707–730
Goh SY, Aragon JM, Lee YS, Loke KY (2011) Normative data for quantitative calcaneal ultrasound in Asian children. Ann Acad Med Singap 40(2):74–79
Khaw KT, Reeve J, Luben R, Bingham S, Welch A, Wareham N et al (2004) Prediction of total and hip fracture risk in men and women by quantitative ultrasound of the calcaneus: Epic-Norfolk prospective population study. Lancet 363:197–202
Bauer DC, Glucer CC, Cauley JA, Vogt TM, Ensrud KE, Genant HK et al (1997) Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 157:629–634
Mughal MZ, Langton CM, Utretch G, Morrison J, Specker BL (1996) Comparison between broad-band ultrasound attenuation of the calcaneum and total body bone mineral density in children. Acta Paediatr 85:663–665
Saraff V, Hoegler W (2015) Endocrinology and adolescence: osteoporosis in children: diagnosis and management. Eur J Endocrinol 173(6):R185–197
Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, Makitie O, Munns CF, Shaw N (2014) Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. J Clin Densitom 17:275–280
Foley S, Quinn S, Jones G (2009) Tracking of bone mass from childhood to adolescence and factors that predict deviation from tracking. Bone 44:752–757
Herrmann D, Intemann T, Lauria F, Mårild S, Molnár D, Moreno LA, Sioen I, Tornaritis M, Veidebaum T, Pigeot I, Ahrens W, IDEFICS consortium (2014) Reference values of bone stiffness index and C-terminal telopeptide in healthy European children. Int J Obes (Lond) 38(Suppl 2):S76–S85
Marcelli C (2007) Osteoporosis in children and adolescents. Presse Med 36(7–8):1078–1083
van den Bergh JP, Noordam C, Ozyilmaz A, Hermus AR, Smals AG (2000) Otten BJ Calcaneal ultrasound imaging in healthy children and adolescents: relation of the ultrasound parameters BUA and SOS to age, body weight, height, foot dimensions and pubertal stage. Osteoporos Int 11:967–976
Sawyer A, Moore S, Fielding KT, Nix DA, Kiratli J, Bachrach LK (2001) Calcaneus ultrasound measurements in a convenience sample of healthy youth. J Clin Densitom 4:111–120
Mughal MZ, Ward K, Qayyum N, Langton CM (1997) Assessment of bone status using the contact ultrasound bone analyser. Arch Dis Child 76:535–536
Wunsche K, Wunsche B, Fahnrich H, Mentzel HJ, Vogt S, Abendroth K, Kaiser WA (2000) Ultrasound bone densitometry of the os calcis in children and adolescents. Calcif Tissue Int 67:349–355
Zhu ZQ, Liu W, Xu CL, Han SM, Zu SY, Zhu GJ (2007) Ultrasound bone densitometry of the calcaneus in healthy Chinese children and adolescents. Osteoporos Int 18:533–541
Rauchenzauner M, Schmid A, Heinz-Erian P, Kapelari K, Falkensammer G, Griesmacher A et al (2007) Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab 92:443–449
Lin YC, Tu SH, Pan WH (2007) Bone mass status of school-aged children in Taiwan assessed by quantitative ultrasound: the Nutrition and Health Survey in Taiwan Elementary School Children (NAHSIT Children 2001–2002). Asia Pac J Clin Nutr 16:585–593
Tuzun S, Karacan I, Akarirmak U, Kasapcopur O, Arisoy N (2003) Evaluation of bone with quantitative ultrasound in healthy Turkish children. Turk J Pediatr 45:240–244
Zhu ZQ, Liu W, Xu CL, Han SM, Zu SY, Zhu GJ (2007) Ultrasound bone densitometry of the calcaneus in healthy Chinese children andadolescents. Osteoporos Int 18(4):533–541
Lavado-Garcia JM, Calderon-Garcia JF, Moran JM, Canal-Macias ML, Rodriguez-Dominguez T, Pedrera-Zamorano JD (2012) Bone mass of Spanish school children: impact of anthropometric, dietary and body composition factors. J Bone Miner Metab 30(2):193–201
Ramírez-Vélez R, Rodrigues-Bezerra D, Correa-Bautista JE, Izquierdo M, Lobelo F (2015) Reliability of health-related physical fitness tests among Colombian children and adolescents: the FUPRECOL study. PLoS One 10(10):e0140875
Marfell-Jones M, Olds T, Stewart A (2006) International standards for anthropometric assessment Potchefstroom. ISAK, South Africa
Jaworski M, Lebiedowski M, Lorenc RS, Trempe J (1995) Ultrasound bone measurement in pediatric subjects. Calcif Tissue Int 56(5):368–371
Vignolo M, Brignone A, Mascagni A, Ravera G, Biasotti B, Aicardi G (2003) Influence of age, sex, and growth variables on phalangeal quantitative ultrasound measures: a study in healthy children and adolescents. Calcif Tissue Int 72:681–688
Cole TJ, Green PJ (1992) Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 11:1305–1319
Royston P, Wright EM (2000) Goodness-of-fit statistics for age-specific reference intervals. Stat Med 19:2943–2962
Bachrach LK (2001) Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab 12(1):22–28
Rivas-Ruiz R, Méndez-Sánchez L, Castelán-Martínez OD, Clark P, Tamayo J, Talavera JO et al (2015) Comparison of international reference values for bone speed of sound in pediatric populations: meta-analysis. J Clin Densitom. doi:10.1016/j.jocd.2015.04.005
Bonjour JP, Theintz G, Buchs B, Slosman D, Rizzoli R (1991) Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab 73:555–563
Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, Andon MB, Smith KT, Heaney RP (1994) Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. J Clin Invest 93:799–808
Lloyd T, Petit MA, Lin HM, Beck TJ (2004) Lifestyle factors and the development of bone mass and bone strength in young women. J Pediatr 144(6):776–782
Chiu GR, Araujo AB, Travison TG, Hall SA, McKinlay JB (2009) Relative contributions of multiple determinants to bonemineral density in men. Osteoporos Int 20(12):2035–2047
Lim LS, Hoeksema LJ, Sherin K, ACPM Prevention Practice Committee (2009) Screening for osteoporosis in the adult U.S. population: ACPM position statement on preventive practice. Am J Prev Med 36(4):366–375
Parra DC, Iannotti L, Gomez LF, Pachón H, Haire-Joshu D, Sarmiento OL, Kuhlmann AS, Brownson RC (2015) The nutrition transition in Colombia over a decade: novel household classification system of anthropometricmeasures. Arch Public Health 73(1):12
Sarmiento OL, Parra DC, González SA, González-Casanova I, Forero AY, Garcia J (2014) The dual burden of malnutrition in Colombia. Am J Clin Nutr 100(6):1628S–1635S
Martínez-Torres J, Meneses-Echavéz JF, Ramírez-Vélez R (2014) Prevalence and demographic factors associated with vitamin A deficiency in Colombian children aged 12–59 months. Endocrinol Nutr 61(9):460–466
Martínez-Torres J, Ramírez-Vélez R (2014) Zinc deficiency and associated factors in colombian children; results from the 2010 national nutrition survey; a cross sectional study. Nutr Hosp 29(4):832–837
Ramírez-Vélez R, Matínez-Torres J, Meneses-Echavez JF (2014) Prevalence and demographic factors associated with ferritin deficiency in Colombian children, 2010. Rev Peru Med Exp Salud Publica 31(2):237–242
Villamor E, Mora-Plazas M, Forero Y, Lopez-Arana S, Baylin A (2008) Vitamin B-12 status is associated with socioeconomic level and adherence, to an animal food dietary pattern in Colombian school children. J Nutr 138:1391–1398
Sani FM, Sarji SA, Bilgen M (2011) Quantitative ultrasound measurement of the calcaneus in Southeast Asian children with thalassemia: comparison with dual-energy X-ray absorptiometry. J Ultrasound Med 30(7):883–894
Eliakim A, Nemet D, Wolach B (2001) Quantitative ultrasound measurements of bone strength in obese children and adolescents. J Pediatr Endocrinol Metab 14:159–164
Correa-Rodríguez M, Rueda-Medina B, González-Jiménez E, Navarro-Pérez CF, Schmidt-RioValle J (2014) The levels of bone mineralization are influenced by body composition in children and adolescents. Nutr Hosp 30(4):763–768
Liu W, Xu CL, Zhu ZQ, Wang W, Han SM, Zu SY, Zhu GJ (2006) Characteristics of calcaneus quantitative ultrasound normative data in Chinese mainland men and women. Osteoporos Int 17:1216–1224
Pollock NK, Bernard PJ, Gutin B, Davis CL, Zhu H, Dong YMD (2011) Adolescent obesity, bone mass, and cardiometabolic risk factors. J Pediatr 158:727–734
Acknowledgments
The FUPRECOL Study received funding from the Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnología “Francisco José de Caldas” COLCIENCIAS [Contract N° 671-2014 Code 122265743978]. The authors are grateful to the Bogota District Education Secretary for the data used in this research. The authors would also like thank the Bogota District students, teachers, schools, and staff, who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Ramírez-Vélez, R., Ojeda-Pardo, M.L., Correa-Bautista, J.E. et al. Normative data for calcaneal broadband ultrasound attenuation among children and adolescents from Colombia: the FUPRECOL Study. Arch Osteoporos 11, 2 (2016). https://doi.org/10.1007/s11657-015-0253-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-015-0253-0