Abstract
Background/Objectives:
Quantitative ultrasound (QUS) is used to measure bone quality and is known to be safe, radiation free and relatively inexpensive compared with dual-energy X-ray absorptiometry (DXA) that is considered the gold standard for bone status assessments. However, there is no consensus regarding the validity of QUS for measuring bone status. The aim of this study was to compare QUS and DXA in assessing bone status in Thai children.
Subjects/Methods:
A total of 181 Thai children (90 boys and 91 girls) aged 6 to 12 years were recruited. Bone status was measured by two different techniques in terms of the speed of sound (SOS) using QUS and bone mineral density (BMD) using DXA. Calcium intake was assessed by 24 h diet recall. Pearson’s correlation, κ-statistic and Bland and Altman analysis were used to assess the agreement between the methods.
Results:
There was no correlation between the two different techniques. Mean difference (s.d.) of the Z-scores of BMD and SOS was −0.61 (1.27) that was different from zero (P<0.05). Tertiles of Z-scores of BMD and QUS showed low agreement (κ 0.022, P=0.677) and the limits of agreement in Bland and Altman statistics were wide.
Conclusions:
Although QUS is easy and convenient to use, the SOS measurements at the radius seem not appropriate for assessing bone quality status.
Similar content being viewed by others
Introduction
For the formation of healthy and strong bones, the supply of adequate nutrients (that is, calcium and protein) along with vitamin D is important.1 In clinical practice bone quality is generally measured using radiologic techniques, and nowadays it is mostly dual-energy X-ray absorptiometry (DXA). In DXA the total body or a region of interest of the body is scanned with X-rays of two distinct energy levels and a two-dimensional picture of bone mineral density (BMD) is calculated to reflect bone status, that is, mineral deposit per square cm in the bone.2 Since the introduction of DXA in 1987, it has rapidly become the gold standard and has replaced radial X-rays for bone density.3
BMD is important and it is well known that ideally bone formation has to be build up in the early years of life to reach a peak value at age ∼30 years, after which BMD normally slowly decreases.4 A low BMD at older age is a risk factor for bone fractures. Bone fracture-related accidents are a significant contributor to morbidity and mortality at older age.5 For this reason bone measurements are important, even at younger age, to detect in an early stage a ‘lagged behind’ bone formation or at older age to detect in time a risk profile.
Unfortunately, DXA measurements are expensive and can only be done by trained professionals. Moreover, the technique exposes to radiation and is not portable.
Quantitative ultrasound (QUS) measurements claim to measure bone quality. The supplied ultrasound waves to the skin using a special transducer via a gel would travel along the bone and the speed of sound (SOS) is said to be related to bone quality.6 QUS is noninvasive (no radiation), quick and the technique is portable, and thus can be used in field studies. Initially, ultrasound measurements were done at the heel (calcaneal QUS),7 but recently new instruments have been developed and marketed that allow QUS measurements to be carried out at the radius or at the tibia that is more comfortable.
As the dietary calcium intake of Thai children is not very high,8 and the vitamin D status as measured via serum 25-hydroxyvitamin D is known to be relatively low in Thai children8 and also in adults,9 it seems justified to monitor the bone status of Thai children. Because of the limitations of DXA measurements (radiation, expensive, portability) QUS might be an alternative that could be widely used.
However, the literature reports conflicting data about the comparability of DXA and QUS, and most studies conclude that QUS needs more validation.10 The difficulty is that DXA and QUS measure different quantities (DXA: BMD; QUS: bone quality), although that should not be an issue when ranking subjects based on Z-scores.6
The aim of this study was to validate QUS measurements at radius site against DXA as the golden standard, before introducing the use of QUS at health clinics or using it on a large scale. This validation study was done in a group of 181 apparently healthy Thai children, aged 6 to 12 years and living in the Bangkok area.
Materials and methods
A total of 181 children (90 boys and 91 girls) were randomly selected from 9 schools in the Bangkok Metropolitan Area. The children took part in the South East Asia Nutrition Survey (SEANUTS).8 They were all apparently healthy and written informed consent was obtained from the parents or caretakers. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the committee on human rights related to research involving human subjects of the Faculty of Medicine Ramathibodi Hospital, Mahidol University (MURA 2010/467). The study was also registered in the Netherlands Trial Registry as NTR2462.
Body weight was measured accurate to 0.1 kg on a Seca digital weighing scale model 882 (SECA GmbH, Hamburg, Germany) in light indoor clothing. Height was measured barefooted to the nearest 0.1 cm using a wall-mounted microtoise (Stanley-Mabo Ltd, Besancon, France). Body mass index was calculated as weight divided by height squared (kg/m2). Overweight and obesity were defined based on the body mass index for age Z-score (BAZ) following the World Health Organization guidelines.11 Stunting was defined as height for age Z-scores (HAZ) below −2.0.11
Bone quality was measured using QUS (model 8000P, Sunlight Omnisense, Petah Tikva, Israel). Children’s weight, height, date of birth, sex and name were entered in the instrument software program. The measurement was performed at the nondominant arm at one-third distance between the radius and the olecranon, following the instructions from the manufacturer. This was done by one single operator throughout the study. The SOS was measured for at least three cycles and the mean value was used in the statistics. Low bone quality was defined at SOS Z-scores ≤−2 using the manufacturer’s reference values.
A total body scan by DXA (program encore 2008 Version 12.30, Lunar Prodigy Pro, Madison, WI, USA) was performed and the instrument software also extracted the regional (dominant forearm) area of interest. The children were picked up from school in the morning and upon arrival in Golden Jubilee Medical Center, Mahidol University, they changed to the hospital gown and all metal objects were removed from the body. Standard procedures according to the manufacturer’s instructions were followed. The results of the scan were interpreted using the pediatric Lunar software program by the hospital technician and BMD and BMDarm was used in the statistical analyses. Low BMD was defined at Z-scores ≤−2.12
A 24-h diet recall was used to assess dietary intake and the children were interviewed by well-trained staffs using measuring cups, spoons and pictorial food models to estimate eaten foods and snacks. All consumption data were converted into nutrient intakes including calcium using the INMUCAL-NV. 2.0 Computer Software Program (Institute of Nutrition, Mahidol University).
All data were analyzed using the Statistical Package for Social Science (SPSS; version 19.0 Inc., Chicago, IL, USA). Data are expressed as mean±s.d. Correlations are Pearson’s correlations. Differences between groups of subjects were tested using T-test. Z-scores of BMD and SOS were categorized into tertiles and the agreement in the 3 by 3 table was tested using κ-statistics. In addition, Bland and Altman analyses13 were performed to investigate the agreement between the BMD Z-score obtained from DXA and SOS Z-score from QUS. The limits of agreement are expressed as ±2 s.d. from the mean bias. Significance is set at P<0.05.
Results
Some characteristics of the children are given in Table 1 for boys and girls separately. No significant differences were found between sexes for the anthropometric and bone parameters. Prevalence of overnutrition (overweight and obese) was more pronounced in boys than in girls (30% in boys and 20.9% in girls). Mean BMD Z-score was slightly negative and 5 children (2 boys and 3 girls) had actual BMD Z-score values below −2.0. Mean SOS radius Z-score was positive with no differences between boys and girls and only 2 children (1 boy and 1 girl) had a SOS Z-score value below the ‘at-risk’ cutoff value of −2.0. There was a significant difference mean Z-score between SOS (0.49) and BMD (−0.12), P<0.001.
Table 2 provides the correlation coefficients of BMD Z-score and SOS Z-score with age and body composition parameters. Note that BMD correlates with various body composition parameters but SOS does not. BMD was also correlated with nutritional calcium intake. The correlation of BMD Z-score and SOS Z-score was poor (r=0.02) and not significant. In addition, the correlation between arm BMD and SOS Z-score at radius site was poor (r=−0.09) and not significant.
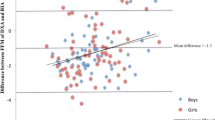
Bland and Altman analyses were applied to assess the agreement between BMD Z-score and SOS Z-score (Figure 1). The limits of agreement ranged from −3.15 to +1.93 and the mean difference between the Z-scores (−0.61±1.27) was different from zero (P<0.05).
Table 3 shows no agreement between tertiles of Z-score values for BMD and SOS respectively. Between the tertiles of SOS Z-score there was a wide overlap of BMD Z-score values, although the mean values of BMD Z-score increased in the tertiles of SOS Z-score.
The SOS Z-score of the 5 subjects with BMD Z-scores below −2.0 ranged from −1.13 to +0.98, thus none of these 5 low-BMD children would have been classified as having low bone quality based on SOS measurements. Similarly, the two children with low SOS Z-scores of −2.10 and −2.00 had BMD Z-scores of −0.20 and −0.30 respectively.
Discussion
In this study, 181 apparently healthy children aged 6–12 years and living in Bangkok were randomly recruited from the SEANUTS study population.8 They can be regarded as representative for Bangkok children of that age. The number of overweight/obese children was 46 (25.4%), 15 (8.3%) children were thin and 8 (4.4%) children were stunted. These numbers in the sample are similar to what can be expected from the prevalence in the general Thai children population.8 The low calcium intake was also found in the general Thai children population.8
The BMD Z-score showed positive correlations with weight, height, body mass index and calcium intake, whereas the QUS Z-score showed no such positive correlations. It is to be expected that heavier children (higher weight, higher body mass index) have stronger bones and thus a higher BMD. The positive correlation with calcium intake is also to be expected, although it could be argued that a single 24-h diet recall does not necessarily reflect the usual calcium intake.
The aim of the current study was to compare SOS radius Z-score values with BMD Z-score values. The Bland and Altman13 plot in Figure 1 shows no agreement between the two techniques. Not only are the limits of agreement very wide, but the data points are also very scattered and in fact there are children with a very high positive Z-score for BMD but a very low Z-score for QUS and vice versa. The same pattern is also seen in Table 3, where in fact the 9 cells have all about the same number of children, resulting in a κ of 0.022 (P=0.677). The mean bias in Z-score (Figure 1) of −0.61±1.27 could be because of differences in the characteristics in the reference populations that were used for BMD and QUS. For QUS the reference population was an Asian (mainly Korean) population (personal communication with the BeamMed Ltd manufacturer). For DXA the normative database is based on children of the Caucasian race.3 It might also be that the cutoff point of −2.0 for QUS Z-score is too low to detect children with low BMD, and thus the method with the criteria given by the manufacturer is not sensitive enough.14 Such a mean bias is not necessarily a drawback as cutoff points can be adapted.14 The wide limits of agreement are the main reason of the lack of comparability.
Of the 181 children, 5 children had BMD Z-scores below −2.0, but in none of them their low BMD would have been detected by QUS. The lowest observed QUS Z-score in these 5 children was −1.13. Interestingly, these 5 children with low BMD had all negative BAZ values (mean −1.5, ranging from −0.06 to −2.15), and thus were all relatively thin. In addition, their HAZ was negative and 3 of the 5 children could be classified as stunted (HAZ ≤−2) (mean −1.4, ranging from −0.16 to −2.09).11 Thus, there seems to be a clear pattern between BMD and nutritional status parameters, something that is to be expected.14 However, QUS did not show any relationship with either BAZ or HAZ (see also Table 2). Of the 181 children, 2 had a QUS Z-score ≤−2.0 but had a normal BMD. One child was obese and the other had a BAZ score of −1.05. Their HAZ values were −0.57 and 0.64, and thus there is no clear pattern with BAZ or HAZ. The finding in this study is similar to other comparative studies between QUS and DXA at lumbar spine site in healthy children,15 children with hemophilia16 and patients with thalassemia.17 In addition, the SEANUTS study in Malaysia recently reported that QUS and DXA are only weakly correlated and that the use of QUS resulted in a high proportion of falsely classified children with low bone status.18
QUS is said to provide information on bone strength in three dimensions, and it can be argued that it might probably not be comparable with BMD, which is a two-dimensional measure, and thus it may not identify the same status of children. However, in previous studies QUS measured at the calcaneus site showed acceptable correlations with BMD.19, 20 It might be that soft tissue overlaying the radius site is negatively affecting the QUS measurement. At the calcaneal site there is less soft tissue that could interfere with the ultrasound measurements. Another possibility is that the heel bone as a weight-bearing bone reflects the total BMD better than the radius that is obviously not weight bearing. Furthermore, the calcaneal validations studies were done in adults, not in children.
Conclusions
Despite the promise of QUS to be an easy to use, inexpensive and safe method to assess bone status in clinical and population settings, the results of the current study in Thai children confirms other published literature that the two methods are not comparable and that QUS seems unable to detect children at risk for low BMD in a general population.
References
Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for Calcium and Vitamin D. National Academy Press: Washington, DC, 2010.
Pietrobelli A, Formica C, Wang Z-M, Heymsfield SB . Dual energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol 1996; 271: E941–E951.
National Osteoporosis Society Bone Densitometry Techniques II: Single and Dual Energy X-ray Absorptiometry (cited 10 February 2015). Available from: https://www.nos.org.uk/document.doc?id=654.
Watkins J . Structure and Function of the Musculoskeletal System, 2nd edn. Human Kinetics: Champaign, IL, USA, 2010.
Zittermann A, Gummert JF, Börgemann J . Vitamin D deficiency and mortality. Curr Opin Nutr Metab Care 2009; 12: 634–639.
Prins SH, Jorgensen HL, Jorgensen LV, Hassager C . The role of quantitative ultrasound in the assessment of bone: review. Clin Physiol 1998; 18: 3–17.
Krieg MA, Barkman R, Gannelli S, Stewart A, Bauer DC, Barquero LD et al. Quantitative ultrasound in the management of osteoporosis: the 2007 ISCD official position. J Clin Densitom 2008; 11: 163–187.
Rojroongwasilkul N, Kijboonchoo K, Wimonpeerapattana W, Purttiponthanee S, Yamborisut U, Boonpraderm A et al. SEANUTS: the nutritional status and dietary intake of 0.5-12-year-old Thai children. Br J Nutr 2013; 110: S36–S44.
Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B . Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC Public Health 2011; 11: 853.
EI-Desouki MI, Sherafzal MS, Othman SA . Comparison of bone mineral density with dual energy x-ray absorptiometry, quantitative ultrasound and single energy x-ray absorptiometry. Saudi Med J 2005; 26: 1346–1350.
De Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J . Development of a WHO growth reference for school-aged-children and adolescents. Bull World Health Organ 2007; 85: 660–667.
Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD pediatric official position. J Clin Densitom 2008; 11: 43–58.
Bland JM, Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 116: 307–310.
Du X, Greenfield H, Fraser DR, Ge K, Zheng W, Huang L et al. Low body weight and its association with bone health and pubertal maturation in Chinese girls. Eur J Clin Nutr 2003; 57: 693–700.
Williams JE, Wilson CM, Biassoni L, Suri R, Fewtrell MS . Dual energy X-ray absorptiometry and quantitative ultrasound are not interchangeable in diagnosing abnormal bones. Arch Dis Child 2012; 97: 822–824.
Christoforidis A, Economou M, Papadopoulou E, Kazantzidou E, Gompakis N, Athanassion-Metaxa M . Bone status of children with hemophilia a assessed with quantitative ultrasound sonography (QUS) and dual energy X-ray absorptiometry (DXA). J Pediatr Hematol Oncol 2010; 32: e259–e263.
Christoforidis A, Perifanis V, Papadopoulou E, Dimitriadou M, Kazantzidou E, Vlachaki E et al. Poor correlations between measurements of bone quality by qualitative ultrasound sonography and dual energy X-ray absorptiometry in patients with β-thalassaemia major. Eur J Haematol 2008; 82: 15–21.
Chong KH, Poh BK, Aini JN, Azmi KN, Deurenberg P . Radial quantitative ultrasound and dual energy X-ray absorptiometry: inter-method agreement for bone status assessment in children. Biomed Res Int 2015; 2015: 1–7.
Fielding KT, Nix DA, Bachrach LK . Comparison of calcaneus ultrasound and dual X-ray absorptiometry in children at risk of osteopenia. J Clin Densitom 2003; 6: 7–15.
Jones G, Boon P . Which bone mass measures discriminate adolescents who have fractured from those who have not? Osteoporis Int 2008; 19: 251–255.
Acknowledgements
We are grateful to the children and their parents for their participation in this study. We also thank all staff at the Institute of Nutrition, Mahidol University, especially Kriyot Sudsa-ard, Rittirong Unjana and Petcharat Kunaphan for their help during the DXA measurements. Special thanks to the technicians of Golden Jubilee Medical Center, Mahidol University who were involved in the DXA measurements. FrieslandCampina, The Netherlands, sponsored the study.
Author contributions
WS and PD were responsible for the data analyses and reporting. WT, KK, NR and WW were closely involved in the data collection and NR, IK and PD were involved in the design of the study. All authors contributed to the manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Srichan, W., Thasanasuwan, W., Kijboonchoo, K. et al. Bone status measured by quantitative ultrasound: a comparison with DXA in Thai children. Eur J Clin Nutr 70, 894–897 (2016). https://doi.org/10.1038/ejcn.2015.180
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2015.180
- Springer Nature Limited
This article is cited by
-
Factors associated with bone health status of Malaysian pre-adolescent children in the PREBONE-Kids Study
BMC Pediatrics (2021)
-
Validity of quantitative ultrasound and bioelectrical impedance analysis for measuring bone density and body composition in children
European Journal of Clinical Nutrition (2021)