Abstract
Two new species of Coltricia, C. austrosinensis and C. minima, are described from southern China on the basis of morphological characters and molecular evidence. Phylogenetic analysis based on the internal transcribed spacer (ITS) regions and nuclear large subunit (nLSU) ribosomal RNA gene regions indicated that the two new species were nested within the Coltricia clade in Hymenochaetales. Coltricia austrosinensis is characterized by centrally stipitate basidiocarps, lobed pileal margin, distinctly swollen stipe tip, cinnamon pore surface, large pores (1–3 per mm), and broadly ellipsoid basidiospores measuring 8–10 × 5.5–6.5 μm, with a distribution to date in subtropical China. Coltricia minima is characterized by tiny, centrally stipitate basidiocarps, entire pileal margin, uniform stipe, pileus bearing distinct concentric zones and pore surface dark greyish blue when fresh, small pores (3–4 per mm), narrow tramal hyphae (3.5–4 μm), and broadly ellipsoid to subglobose basidiospores measuring 6–7 × 4–5 μm, and occur in mixed tropical forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coltricia Gray, typified by C. perennis (L.) Murrill, is a special genus in Hymenochaetales (Larsson et al. 2006), as species in this genus grow mostly on the ground, while major species of Hymenochaetales are wood-inhabiting fungi. Although C. perennis is a mycorrhizal fungus (Tedersoo et al. 2007), the lifestyle of other species is not well known. Coltricia and Coltriciella Murrill resemble each other by similar growth habits and hyphal structure, but the former has smooth basidiospores, while they are ornamented in the latter (Ryvarden 1991; Dai 2010). According to the recent molecular data, the two genera are recovered either as sister clades or as a single clade. The monophyly of each genus is still unresolved (Wagner and Fischer 2002; Larsson et al. 2006; Tedersoo et al. 2007). The two genera have been studied extensively worldwide; several new taxa have been reported recently, and 54 species have been found in these two genera thus far (Burdsall 1969; Gilbertson and Ryvarden 1986; Corner 1991; Masuka and Ryvarden 1993; Rajchenberg and Wright 1998; Nunez and Ryvarden 2000; Aime et al. 2003; Niemelä 2005; Ryvarden et al. 2006; Ryvarden 2007; Gomes-Silva et al. 2009; Baltazar et al. 2010; Dai 2010; Dai et al. 2010; Dai and Li 2012; Zhou and Tedersoo 2012; Valenzuela et al. 2012; Decock 2013; Ryvarden and Melo 2014; Bian and Dai 2015).
During investigations of wood-inhabiting fungi in southern China with a special focus on Coltricia, several collections of Coltricia were found to differ from existing species and to represent two undescribed species based on morphological characters and phylogenetic analysis. Illustrated descriptions of the two new species and an identification key to the Chinese species in this genus is provided.
Materials and methods
Morphological studies
Voucher specimens are deposited at the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC). Macro-morphological descriptions were based on field notes and laboratory measurements. The microscopic routine used in this study followed Dai (2010). Drawings were made with the aid of a drawing tube. Microscopic features, measurements and drawings were made from slide preparations stained with cotton blue and Melzer’s reagent. Spores were measured from sections cut from tubes. In presenting the variation in the size of the spores, 5 % of measurements were excluded from each end of the range, and are given in parentheses. In the text, the following abbreviations are used: IKI = Melzer’s reagent, IKI– = negative in Melzer’s reagent, KOH = 5 % potassium hydroxide, CB = cotton blue, CB+ = cyanophilous, CB– = acyanophilous, L = mean spore length (arithmetic average of all spores), W = mean spore width (arithmetic average of all spores), Q = variation in the L/W ratios between the specimens studied, n (a/b) = number of spores (a) measured from given number (b) of specimens. Special color terms follow Petersen (1996).
Molecular phylogeny
The collections of the two new species and other fungal taxa used in this study are listed in Table 1. The methods of DNA extraction and amplification followed Chen et al. (2016). CTAB rapid plant genome extraction kit (DN14; Aidlab Biotechnologies Co., Ltd, Beijing) was used to extract total genomic DNA from dried specimens of the new collections, according to the manufacturer’s instructions with some modifications. The primers ITS5 (GGA AGT AAA AGT CGT AAC AAG G, 5′ to 3′)/ITS4 (TCC TCC GCT TAT TGA TAT GC, 5′ to 3′) and LR0R (ACC CGC TGA ACT TAA GC, 5′ to 3′)/LR7 (TAC TAC CAC CAA GAT CT, 5′ to 3′) were used for PCR amplification (primer sequences used in this study were obtained from http://www.biology.duke.edu/fungi/mycolab/primers.htm). The PCR products were sequenced at the Beijing Genomics Institute, China, with the same primers. The newly generated sequences were deposited at GenBank and are listed in Table 1.
For the phylogenetic analysis, additional sequences of other taxa were selected from GenBank and are referenced in Table 1. The dataset includes 65 sequences representing 29 taxa. Fourteen taxa of Coltricia and nine taxa of Coltriciella are involved in the phylogeny. According to Wagner and Fischer (2002), the sequence of Trichaptum abietinum (Dicks.) Ryvarden obtained from GenBank (KP454016) was chosen as the outgroup. Sequences were aligned with BioEdit (Hall 1999) and ClustalX (Thompson et al. 1997). Alignment was manually adjusted to allow maximum alignment and to minimize gaps. Sequence alignment was deposited at TreeBASE (submission ID 18687, www.treebase.org).
Maximum parsimony (MP) analysis was applied to the combined dataset of ITS and nLSU sequences. The construction was performed in PAUP* version 4.0b10 (Swofford 2002). All characters were equally weighted and gaps were treated as missing data. Trees were inferred using the heuristic search option with TBR branch swapping and 1000 random sequence additions. Max-trees was set to 5000, branches of zero length were collapsed and all parsimonious trees were saved. Clade robustness was assessed using a bootstrap (BT) analysis with 1000 replicates (Felsenstein 1985). Descriptive tree statistics of tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI) were calculated for each maximum parsimonious tree (MPT) generated.
RAxML v.7.2.8 was used to construct a maximum likelihood (ML) tree with GTR+G+I model of site substitution including estimation of Gamma-distributed rate heterogeneity and a proportion of invariant sites (Stamatakis 2006). The branch support was evaluated using the bootstrapping method with 1000 replicates (Hillis and Bull 1993).
MrModeltest 2.3 (Posada and Crandall 1998; Nylander 2004) was used to determine the best-fit evolution model for each dataset for Bayesian inference (BI). BI was calculated with MrBayes 3.1.2 with a general time reversible (GTR) model of DNA substitution and a gamma distribution rate variation across sites (Ronquist and Huelsenbeck 2003). Four Markov chains were run for two runs from random starting trees for 1,500,000 generations, and trees were sampled every 100 generations. The first 375,000 generations were discarded as burn-in. A majority rule consensus tree of all remaining trees was calculated. Branches that received bootstrap support for maximum parsimony (MP), maximum likelihood (BS) and Bayesian posterior probabilities (BPP) greater than or equal to 75 % (MP and BS) and 0.95 (BPP) were considered as significantly supported.
Results
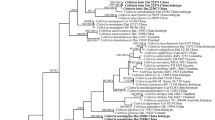
The combined dataset had an aligned length of 2035 characters, of which 912 characters are constant, 170 are variable and parsimony-uninformative, and 953 are parsimony-informative. MP analysis yielded four equally most-parsimonious trees (TL = 3590, CI = 0.553, RI = 0.788, RC = 0.436, HI = 0.447). The best model for the combined dataset estimated and applied in the Bayesian analysis was a GTR+I+G model. Bayesian and ML analysis resulted in a topology similar to that with MP analysis. Only the MP tree is provided in the Fig. 1, and the BT values (≥50 %) and BPPs (≥0.95) are shown at the nodes.
The analysis revealed that the selected species of Coltricia and Coltriciella clustered together. The new collections formed two highly supported lineages (Coltricia austrosinensis MP = 100 %, BS = 100, BPP = 1.00; C. minima MP = 100 %, BS = 100, BPP = 1.00), and they nested within the Coltricia clade.
Taxonomy
Coltricia austrosinensis L.S. Bian & Y.C. Dai, sp. nov. (Figs. 2 and 3)
MycoBank no.: MB 815487
Coltricia austrosinensis is characterized by centrally stipitate basidiocarps, cinnamon pore surface, large pores (1–3 per mm), lacerate dissepiments, thick contextual hyphae (8–9 μm in diam) and broadly ellipsoid basidiospores measuring 8–10 × 5.5–6.5 μm.
Holotype. CHINA. Yunnan Province, Baoshan, Gaoligongshan Natural Reserve, on ground of mixed forest, 28 October 2012 Dai 13093 (BJFC013317).
Etymology. Austrosinensis (Lat.) referring to the locality in southern China.
Fruiting body. Basidiocarps annual, centrally stipitate, solitary, soft fibrous when fresh, becoming soft corky upon drying. Pilei more or less circular to infundibuliform, up to 4 cm in diam and 5 mm thick at centre. Pileal surface velutinate to glabrous, cinnamon to fawn when fresh, greyish brown to deep olive upon drying, with indistinct concentric zones and radially aligned lines; margin thinning out, lobed, curving down upon drying. Pore surface buff-yellow when fresh, cinnamon upon drying; pores angular, 1–3 per mm, dissepiments thin, lacerate. Context greyish brown, coriaceous, up to 1 mm thick. Tubes buff, distinctly paler than context, fragile or slightly brittle when dry, up to 4 mm long. Stipe greyish brown, corky, finely velutinate, up to 3.5 cm long, 4 mm in diam; swollen tip up to 8 mm in diam.
Hyphal structure. Hyphal system monomitic; generative hyphae simple septate; tissue darkening but otherwise unchanged in KOH.
Context. Contextual hyphae cinnamon-buff, thick-walled with a wide lumen, occasionally branched, with frequent simple septa, straight, more or less regularly arranged, 8–9 μm in diam; hyphae in the stipe golden brown, fairly thick-walled with a wide lumen, 7–9 μm in diam.
Tubes. Tramal hyphae pale yellow to buff-yellow, slightly thick-walled to thick-walled with a wide lumen, moderately branched, loosely interwoven to subparallel along the tubes, 4–6 μm in diam. Cystidia and cystidioles absent. Basidia broadly clavate, with four sterigmata and a simple septum at the base, 24–29 × 7–8 μm; basidioles similar in shape to basidia, but slightly smaller.
Spores. Basidiospores broadly ellipsoid, pale yellow, fairly thick-walled, smooth, IKI–, CB+, (7.5–) 8–10 × 5.5–6.5 (−7) μm, L = 8.96 μm, W = 5.95 μm, Q = 1.49–1.52 (n = 60/2).
Additional specimens (paratypes) examined: CHINA. Jiangxi Province, Jinggangshan, Jinggangshan Nature Reserve, on ground in mixed forest, 23 September 2008 Dai 10599 (BJFC004848). Yunnan Province, Baoshan, Gaoligongshan Natural Reserve, on ground of mixed forest, 23 October 2009 Cui 8003 (BJFC006492), 28 October 2012 Dai 13098 (BJFC013322); Shuanghe Village, on ground of mixed forest, 5 August 2014 Dai 13823 (BJFC017553).
Coltricia minima L.S. Bian & Y.C. Dai, sp. nov. (Figs. 4 and 5).
MycoBank no.: MB 815488
Coltricia minima is characterized by tiny, centrally stipitate basidiocarps (less than 1.5 cm in diam), dark greyish blue to deep olive pore surface, small pores (3–4 per mm), narrow tramal hyphae (3.5–4 μm) and broadly ellipsoid to subglobose basidiospores measuring 6–7 × 4–5 μm.
Holotype. CHINA. Hainan Province, Qiongzhong County, Limushan Natural Reserve, on ground of tropical forest, 30 May 2015 Dai 15206 (BJFC019317).
Etymology. Minima (Lat.) referring to the tiny fruiting body.
Fruiting body. Basidiocarps annual, centrally stipitate, solitary, soft fibrous when fresh, becoming soft corky upon drying. Pilei tiny, more or less circular to infundibuliform, up to 1.3 cm in diam and 2 mm thick at centre. Pileal surface velutinate to glabrous, concentric zonate, cinnamon-buff to clay-buff when fresh, clay-buff to orange-brown and concentric zones becoming indistinct upon drying; margin thin, entire, curving down upon drying. Pore surface dark greyish blue when fresh, clay-buff to deep olive when dry; pores angular, 3–4 per mm; dissepiments thin, slightly lacerate. Context dark brown, soft corky, up to 0.3 mm thick. Tubes concolourous with the pore surface, fragile, up to 1.7 mm thick. Stipe clay-buff when fresh, greyish brown to deep olive when dry, corky, finely velutinate, up to 1.7 cm long, 2 mm in diam, more or less uniform.
Hyphal structure. Hyphal system monomitic, generative hyphae simple septate, IKI–, CB–; tissue darkening but otherwise unchanged in KOH.
Context. Contextual hyphae cinnamon-buff to cinnamon, slightly thick-walled with a wide lumen, occasionally branched, frequently simple septate, straight, loosely interwoven, 4.5–6.5 μm in diam; hyphae in stipe similar to context, parallel along the stipe, rarely branched, 4–6 μm in diam.
Tubes. Tramal hyphae cinnamon-buff to yellowish brown, slightly thick-walled with a wide lumen, moderately branched, frequently simple septate, more or less straight, loosely interwoven to subparallel along the tubes, 3.5–4 μm in diam. Cystidia and cystidioles absent, basidia more or less barrel-shaped, thin-walled, with four sterigmata and a basal simple septum, 16.5–18 × 7 μm, basidioles in shape similar to basidia, but slightly smaller.
Spores. Basidiospores broadly ellipsoid to subglobose, yellowish, thick-walled, smooth, IKI–, CB+, 6–7 (−8) × 4–5 (−5.5) μm, L = 6.49 μm, W = 4.70 μm, Q = 1.36–1.40 (n = 60/2).
Additional specimens (paratypes) examined: CHINA. Hainan Province, Qiongzhong County, Limushan Natural Reserve, on ground of tropical forest, 30 May 2015 Dai 15203 (BJFC019314), Dai 15221 (BJFC019332), Dai15222 (BJFC019333).
Discussion
Coltricia austrosinensis has centrally stipitate basidiocarps, large angular pores and smooth hyphae; these features are shared by C. abieticola Y.C. Dai, C. crassa Y.C. Dai, and C. focicola (Berk. & M.A. Curtis) Murrill. However, C. abieticola has duplex stipes, smaller basidiospores measuring 7–8 × 5.7–6.5 μm, and grows on Abies in boreal forest (Dai 2010). C. crassa differs from C. austrosinensis by its large and thick basidiocarps (up to 6 cm in diam and 2 cm thick at centre) and the presence of tortuous dendrohyphidia-like hyphae (Dai 2010); C. focicola can be distinguished by cylindrical to oblong-ellipsoid basidiospores measuring 8–11 × 4–5 μm (Ryvarden and Melo 2014). Phylogenetically, C. austrosinensis grouped with C. minor Y.C. Dai, C. australica L.W. Zhou, Tedersoo, & Y.C. Dai and Coltriciella globosa L.S. Bian & Y.C. Dai, and formed a moderately supported lineage (Fig. 1). Coltricia minor, however, can be distinguished by tiny laterally stipitate basidiocarps and oblong-ellipsoid basidiospores measuring 5.5–6.8 × 3.5–4 μm (Dai et al. 2010); C. australica differs by its smaller pores (3–4 mm per mm), entire dissepiments and smaller basidiospores measuring 6–7.3 × 4.4–5.2 μm (Zhou and Tedersoo 2012); Coltriciella globosa differs from C. austrosinensis by the presence of cystidioles and finely verrucose basidiospores (Bian and Dai 2015).
Coltricia minima is morphologically similar to C. cinnamomea (Jacq.) Murrill, C. pyrophila (Wakef.) Ryvarden, and C. weii Y.C. Dai, by sharing centrally stipitate basidiospores, small pores and broadly ellipsoid basidiospores. However, C. cinnamomea has larger fruiting bodies (pileus up to 12 cm in diam) and larger basidiospores measuring 6.9–8.1 × 5.5–6.4 μm (Niemelä 2005; Ryvarden and Melo 2014); C. pyrophila has a swollen stipe tip and smaller basidiospores measuring 4.7–5.8 × 3.4–4 μm (Dai 2010); C. weii has larger fruiting bodies (pileus up to 3 cm in diam) and dichotomously branched pileal hyphae (Dai et al. 2010). Phylogenetically, C. minima grouped with C. strigosipes Corner and C. verrucata Aime, T.W. Henkel & Ryvarden, and formed a highly supported lineage (Fig. 1). However, C. strigosipes differs from C. minima by its larger fruiting bodies (pileus up to 2.8 cm) and densely strigose stipes (Corner 1991; Dai 2010); C. verrucata differs from C. minima by bearing erect bristles at the pileal surface, thicker tramal hyphae (7–11 μm in diam) and strongly verrucose hyphae (Aime et al. 2003; Dai 2010). Coltricia minor also has tiny basidiocarps, but it can be distinguished by the lateral stipe, thicker tramal hyphae (7–11 μm in diam) and smaller ellipsoid basidiospores measuring 5.5–6.8 × 3.5–4 μm (Dai et al. 2010).
Key to species of Coltricia in China
-
1 Hymenophore more or less concentrically lamellate . . . . . . . . . . .. . . .. . . .. . . .. . . .. . C. montagnei
-
1 Hymenophore poroid . . . . . . . . . . .. . 2
-
2 Basidiocarps pendent . . . . . . . . . . .. . C. tsugicola
-
2 Basidiocarps erect . . . . . . . . . . .. . 3
-
3 Basidiocarps laterally stipitate . . . . . . . . . . .. . 4
-
3 Basidiocarps eccentrically to centrally stipitate . . . . . . . . . . .. . 5
-
4 Mature pilei < 0.5 cm in diam; basidiospores 5.5–6.8 × 3.5–4 μm . . . . . . . . . . .. . C. minor
-
4 Mature pilei > 0.5 cm in diam; basidiospores 9–10.3 × 5.7–6.8 μm . . . . . . . . . . .. . C. duportii
-
5 Stipe duplex; on fallen trunk of Abies . . . . . . . . . . .. . C. abieticola
-
5 Stipe homogeneous; on ground . . . . . . . . . . .. . 6
-
6 Pores 0.5–3 per mm . . . . . . . . . . .. . 7
-
6 Pores 3–6 per mm . . . . . . . . . . .. . 11
-
7 Pileal surface bearing erect bristle; hyphae strongly pruinate . . . . . . . . . . .. . C. verrucata
-
7 Pileal surface velutinate to smooth; hyphae smooth . . . . . . . . . . .. . 8
-
8 Context thick, up to 12 mm thick, dendrohyphidia-like hyphae present . . . . . . . . . . .. . C. crassa
-
8 Context thin, up to 1 mm thick, dendrohyphidia-like hyphae absent . . . . . . . . . . .. . 9
-
9 Basidiocarps eccentrically stipitate, with unpleasant odour when fresh and dry . . . . . . . . . . .. . C. macropora
-
9 Basidiocarps centrally stipitate, without odour consistently . . . . . . . . . . .. . 10
-
10 Basidiospores oblong-ellipsoid, < 5.5 μm wide . . . . . . . . . . .. . C. focicola
-
10 Basidiospores broadly ellipsoid to subglobose, > 5.5 μm wide . . . . . . . . . . .. . C. austrosinensis
-
11 Stipe bearing plenty of spines . . . . . . . . . . .. . C. strigosipes
-
11 Stipe smooth or velutinate . . . . . . . . . . .. . 12
-
12 Growth in gymnosperm forests; basidiospores ellipsoid . . . . . . . . . . .. . C. perennis
-
12 Growth in angiosperm forests; basidiospores broadly ellipsoid to globose . . . . . . . . . . .. . 13
-
13 Tramal hyphae 3–4 μm in diam . . . . . . . . . . .. . 14
-
13 Tramal hyphae 4–9 in diam . . . . . . . . . . .. . 15
-
14 Mature pilei > 1.5 cm in diam; basidiospores > 7.5 μm long . . . . . . . . . . .. . C. subperennis
-
14 Mature pilei < 1.5 cm in diam; basidiospores < 7.5 μm long . . . . . . . . . . .. . C. minima
-
15 Basidiospores < 4 μm wide . . . . . . . . . . .. . C. pyrophila
-
15 Basidiospores > 4 μm wide . . . . . . . . . . .. . 16
-
16 Pileal surface hyphae unbranched . . . . . . . . . . .. . C. cinnamomea
-
16 Pileal surface hyphae dichotomously branched . . . . . . . . . . .. . C. weii
References
Aime MC, Henkel TW, Ryvarden L (2003) Studies in neotropical polypores 15: new and interesting species from Guyana. Mycologia 95:614–619
Baltazar JM, Ryvarden L, Gibertoni TB (2010) The genus Coltricia in Brazil: new records and two new species. Mycologia 102:1253–1262. doi:10.3852/09-227
Bian LS, Dai YC (2015) Coltriciella globosa and C. pseudodependens spp. nov. (Hymenochaetales) from southern China based on morphological and molecular characters. Mycoscience 56:190–197. doi:10.1016/j.myc.2014.06.001
Burdsall HH (1969) A new polypore from the Eastern United States. Mycologia 61:647–651. doi:10.2307/3757257
Chen JJ, Cui BK, Dai YC (2016) Global diversity and molecular systematics of Wrightoporia s.l. (Russulales, Basidiomycota). Persoonia 37:21–36. doi:10.3767/003158516X689666
Corner EJH (1991) Ad Polyporaceas 7. Nova Hedwigia 101:1–175
Dai YC (2010) Hymenochaetaceae (Basidiomycota) in China. Fungal Divers 45:131–343. doi:10.1007/s13225-010-0066-9
Dai YC, Li HJ (2012) Type studies on Coltricia and Coltriciella described by E. J. H. Corner from Southeast Asia. Mycoscience 53:337–346. doi:10.1007/s10267-011-0174-8
Dai YC, Yuan HS, Cui BK (2010) Coltricia (Basidiomycota, Hymenochaetaceae) in China. Sydowia 62:11–21
Decock C (2013) Coltricia oboensis sp. nov. from the high elevation cloud forest of São Tomé. Cryptog Mycol 34:175–181. doi:10.7872/crym.v34.iss2.2013.175
Felsenstein J (1985) Confidence intervals on phylogenetics: an approach using bootstrap. Evolution 39:783–791. doi:10.2307/2408678
Gilbertson RL, Ryvarden L (1986) North American polypores 1. Abortiporus–Lindtneria. Fungiflora, Oslo
Gomes-Silva AC, Ryvarden L, Gibertoni TB (2009) New and interesting species of Hymenochaetaceae from the Brazilian Amazonia. Mycol Prog 8:273–279. doi:10.1007/s11557-009-0606-4
Hall TA (1999) Bioedit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biodivers 42:182–192. doi:10.1093/sysbio/42.2.182
Larsson K-H, Parmasto E, Fischer M, Langer E, Nakasone KK, Redhead SA (2006) Hymenochaetales: a molecular phylogeny for the hymenochaetoid clade. Mycologia 98:926–936. doi:10.3852/mycologia.98.6.926
Masuka A, Ryvarden L (1993) Two new polypores from Malawi. Mycologia Helvetica 5:143–148
Niemelä T (2005) Polypores, lignicolous fungi. Norrlinia 13:1–320
Nunez M, Ryvarden L (2000) East Asian polypores 1. Ganodermataceae and Hymenochaetaceae. Synopsis Fungorum 13:1–168
Nylander JAA (2004) MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University
Petersen JH (1996) Farvekort. The Danish mycological society’s colour-chart. Foreningen til Svampekundskabens Fremme, Greve
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818. doi:10.1093/bioinformatics/14.9.817
Rajchenberg M, Wright JE (1998) Two interesting polypore species (Hymenochaetales) from Argentina. Folia Cryptog Estonica 33:119–122
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180
Ryvarden L (1991) Genera of polypores. Nomenclature and taxonomy. Synopsis Fungorum 5:1–363
Ryvarden L (2007) Studies in Neotropical polypores 23. New and interesting wood-inhabiting fungi from Belize. Synopsis Fungorum 23:32–50
Ryvarden L, Melo I (2014) Poroid fungi of Europe. Synopsis Fungorum 31:1–455
Ryvarden L, Krisai-Greilhuber I, Hausknecht A (2006) Coltricia grandispora and Tyromyces vitellinus, two new polypores. Österreichische Zeitschrift für Pilzkunde 15:143–147
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi:10.1093/bioinformatics/btl446
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sinauer Associates, Sunderland
Tedersoo L, Suvi T, Beaver K, Saar I (2007) Ectomycorrhizas of Coltricia and Coltriciella (Hymenochaetales, Basidiomycota) on Caesalpiniaceae, Dipterocarpaceae and Myrtaceae in Seychelles. Mycol Prog 6:101–107. doi:10.1007/s11557-007-0530-4
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882. doi:10.1093/nar/25.24.4876
Valenzuela R, Raymundo T, Cifuentes J, Esqueda M, Amalfi M, Decock C (2012) Coltriciella sonorensis sp. nov. (Basidiomycota, Hymenochaetales) from Mexico: evidence from morphology and DNA sequence data. Mycol Prog 11:181–189. doi:10.1007/s11557-011-0740-7
Wagner T, Fischer M (2002) Proceedings towards a natural classification of the worldwide taxa Phellinus s.l. and Inonotus s.l., and phylogenetic relationships of allied genera. Mycologia 94:998–1016. doi:10.2307/3761866
Zhou LW, Tedersoo L (2012) Coltricia australica sp. nov. (Hymenochaetales, Basidiomycota) from Australia. Mycotaxon 122:123–128. doi:10.5248/122.123
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Project No. 31530002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Zhu-Liang Yang
Rights and permissions
About this article
Cite this article
Bian, LS., Wu, F. & Dai, YC. Two new species of Coltricia (Hymenochaetaceae, Basidiomycota) from southern China based on evidence from morphology and DNA sequence data. Mycol Progress 15, 27 (2016). https://doi.org/10.1007/s11557-016-1173-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-016-1173-0