Abstract
Three new species of Coltricia, C. fimbriata, C. lenis, and C. tenuihypha, are described from China based on both morphological and molecular data. Phylogenetic analyses based on rDNA ITS1-5.8S-ITS2 (ITS), 28S rDNA (LSU), 18S rDNA (SSU), mitochondrial 12S rDNA (mtSSU), RNA polymerase II subunits 1 (RPB1), and EF-1α (TEF1) confirmed the generic placement of the three new species. Coltricia fimbriata is characterized by centrally stipitate basidiocarps, thin and curled pileal margin with tufts of hairs, 1–3 pores per mm, and ellipsoid to broadly ellipsoid basidiospores (6.3–8.0 × 4.3–5.3 μm). Coltricia lenis is characterized by centrally stipitate basidiocarps, distinctly concentrically zonate and sulcate pileal surface, soft to spongy stipes when dry, 0.5–2 pores per mm, oblong-ellipsoid to ellipsoid basidiospores (7.0–9.3 × 4.5–5.8 μm). Coltricia tenuihypha is characterized by eccentrically to centrally basidiocarps, fan-shaped to circular pilei, 1–3 pores per mm, narrow and skeletal-alike hyphae present in the stipe, ellipsoid to broadly ellipsoid basidiospores (7.3–9.3 × 5.5–6.8 μm). An identification key to the species of Coltricia recorded in China is also provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Coltricia Gray was established in 1821 by the type species C. perennis (L.) Murrill and is a cosmopolitan genus of Hymenochaetales (Larsson et al. 2006). This genus is characterized by mostly poroid and stipitate basidiocarps, a monomitic hyphal system lacking clamp connections and colored, ellipsoid to subglobose, smooth basidiospores (Dai 2010). The complex habitat and exceedingly close morphological characteristics make this genus easy to be confused with Coltriciella Murrill which differs from Coltricia by the ornamented vs. smooth basidiospores (Ryvarden 1991), and molecular data indicated that the two genera were sister clades, forming a single lineage (Wagner and Fischer 2002; Larsson et al. 2006; Tedersoo et al. 2007; Bian and Dai 2017). Based on the above conclusions, Coltriciella was treated as a synonym of Coltricia, and all species of Coltriciella were combined in Coltricia (Wu et al. 2022).
Coltricia has been extensively studied worldwide, and about 27 new species (including 9 new species in the original Coltriciella) were reported in the last decade (Baltazar et al. 2010; Dai 2010; Baltazar and Silveira 2012; Dai and Li 2012; Valenzuela et al. 2012; Zhou and Tedersoo 2012; Decock 2013; Ryvarden and Melo 2014; Bian and Dai 2015; Bian et al. 2016; Vasco-Palacios 2016; Bian and Dai 2017; Susan et al. 2018; Bian and Dai 2020; Valenzuela et al. 2020; Vlasák et al. 2020; Wu et al. 2022). The diversity of Coltricia is being systematically studied, especially in south China (Dai et al. 2010; Wu et al. 2020; Dai et al. 2021). However, due to the absence of specialized hymenial structures, a similar morphology is shared in most Coltricia species, which make species in this genus hard to be distinguished only by morphological studies. Therefore, the diversity of Coltricia is higher than presumed.
During investigations of Coltricia in China, several collections were found and could not be assigned to any described species, also forming distinct clades in multilocus phylogenetic inferences. They are described as new species. An identification key of Coltricia observed in China is provided.
Materials and methods
Morphological studies
The studied specimens are deposited in the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC). Macromorphological descriptions were based on field notes and laboratory observations. Microscopic routine used in this study followed Dai (2010). Drawings were made with the aid of a drawing tube. Microscopic measurements were made from slide preparations stained with Cotton Blue and Melzer’s reagent. Spores were measured from section cuts from tubes. In presenting variation in basidiospore size, 5% of measurements were excluded from each end of the range and are given in parentheses. In the text, the following abbreviations were used: KOH, 5% potassium hydroxide; IKI, Melzer’s reagent; IKI–, neither amyloid nor dextrinoid; CB, Cotton Blue; CB–, acyanophilic; L, mean basidiospore length (arithmetic average of all basidiospores); W, mean basidiospore width (arithmetic average of all basidiospores); Q, variation in the ratios of L/W between specimens studied; n, number of basidiospores measured from given number of specimens. Color terms followed Kornerup and Wanscher (1978).
Molecular phylogeny
The collections and sequences of the three new species and other fungal taxa used in this study are listed in Table 1. Twenty-seven new sequences were generated for this study and deposited to GenBank. The methods of DNA extraction and amplification in this study followed Chen et al. (2016). The CTAB rapid plant genome extraction kit-DN14 (Aidlab Biotechnologies Co., Ltd, Beijing) was used to extract total genomic DNA from dried specimens according to the manufacturer’s instructions. The primers ITS5/ITS4 for rDNA ITS1-5.8S-ITS2 (ITS), LR0R/LR7 for 28S rDNA (LSU), NS1/NS4 for 18S rDNA (SSU), MS1/MS2 for mitochondrial 12S rDNA (mtSSU), RPB1-Af /RPB1-Cr for RNA polymerase II subunit 1 (RPB1), and EF1-983F/EF1-1567F for EF-1α (TEF1) were used for PCR amplifications (Vilgalys and Hester 1990; White et al. 1990; Matheny 2005). The PCR products were sequenced in Beijing Genomics Institute, China, with the same primers. The newly generated sequences were deposited at GenBank.
For the phylogenetic analyses, the combined dataset of ITS, LSU, SSU, mtSSU, RPB1, and TEF1 included 78 sequences representing 41 taxa, in which 39 taxa of Coltricia are involved. Fomitiporella chinensis (Pilát) Y.C. Dai, X.H. Ji & Vlasák and Inonotus griseus L.W. Zhou were chosen as the outgroup. Sequences were aligned with MAFFT (Katoh and Toh 2008), BioEdit (Hall 1999) and Clustal X (Thompson et al. 1997). Sequence alignments were deposited at TreeBASE (submission ID 29082, www.treebase.org). The best-fit evolutionary model was estimated using MrModeltest 2.3 (Posada and Crandall 1998) as GTR + I + G for the combined dataset.
Maximum parsimony (MP) analyses were applied to the combined dataset. The construction was performed in PAUP* version 4.0b10 (Swofford 2002). All characters were equally weighted, and gaps were treated as missing data. Trees were inferred using the heuristic search option with TBR branch swapping and 1000 random sequence additions. Max-trees was set to 5000, branches of zero length were collapsed, and all parsimonious trees were saved. Clade robustness was assessed by a bootstrap (BT) analysis with 1000 replicates (Felsenstein 1985). Descriptive tree statistics, i.e., tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI), were calculated for each Maximum Parsimonious Tree (MPT) generated.
RAxML v.7.2.8 was used to construct maximum likelihood (ML) trees with the GTR+I+G model of site substitution including estimation of Gamma-distributed rate heterogeneity and a proportion of invariant sites (Stamatakis 2006). The branch support was evaluated with a bootstrapping method of 1000 replicates (Hillis and Bull 1993).
BI analyses were calculated with MrBayes3.1.2 with a general time reversible (GTR) model of DNA substitution and a gamma distribution rate variation across sites (Ronquist and Huelsenbeck 2003). Four Markov chains were run for 2 runs from random starting trees for 500000 generations, and trees were sampled every 100 generations. The first 25% of sampled trees were set as burn-in. A majority rule consensus tree of all remaining trees was calculated. BS (bootstrap support for MP and ML) values and BPPs (Bayesian posterior probabilities for BI) simultaneously not less than 75 % and 0.95, respectively, are shown at the nodes.
Results
Phylogenetic inference
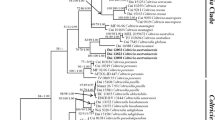
The combined dataset had an aligned length of 6245 characters, of which 3461 characters are constant, 406 are variable and parsimony uninformative, and 2378 are parsimony informative. MP analysis yielded four equally most-parsimonious trees (TL = 10232, CI = 0.444, RI = 0.722, RC = 0.321, HI = 0.556). Bayesian analysis and ML analysis resulted in a similar topology to MP analysis. The MP tree is provided in Fig. 1.
The phylogeny analysis revealed that species of Coltricia formed three clades, and the newly sampled species nested within the three clades, respectively. The sampled specimens of Coltricia lenis and C. tenuihypha formed well-supported lineages respectively (C. lenis 100% in MP, 100% in ML, 1 in BI; C. tenuihypha 100% in MP, 100% in ML, 1 in BI). C. fimbriata grouped with C. hamata and formed a well-supported lineage with C. lateralis, C. rigida and C. velutina (95% in MP, 96% in ML, 1 in BI).
According to the phylogenetic analyses, the three new species are distinctive from the other sampled species.
Taxonomy
Coltricia fimbriata L.S. Bian, M. Zhou & J. Yu, sp. nov. Fig. 2A, Fig. 3
MycoBank no.: MB 842181
Diagnosis: this species is characterized by centrally stipitate basidiocarps, thin and curled pileal margin with tufts of hairs, 1–3 pores per mm, 5–9 μm wide contextual hyphae, 3–7 μm wide tramal hyphae and ellipsoid to broadly ellipsoid basidiospores, 6.3–8.0 × 4.3–5.3 μm (average).
Holotype: CHINA. Zhejiang Province, Hangzhou, Huanggongwang Nature Reserve, on ground of angiosperm forest, 2 June 2021, Dai 22300.
Etymology: fimbriata (Lat.): referring to the species having velutinate and lacerate pileal margin.
Basidiocarps: annual, centrally stipitate, solitary, soft and without odor or taste when fresh, becoming soft corky when dry. Pilei more or less circular, flat, thin, up to 1.5 cm in diam and 1 mm thick at center. Pileal surface greyish brown to deep olive when dry, hirsute in the center, radially aligned fine hair extending to the margin, with indistinct concentric zones; margin thin, curled, velutinate and lacerate with tufts of hairs when dry. Pore surface cream to honey-yellow upon drying; pores angular, 1–3 per mm; dissepiments thin, entire, sterile margin distinct. Context deep olive, leathery, up to 0.5 mm thick. Tubes cream, distinctly paler than context, fragile or slightly brittle when dry, up to 0.5 mm long. Stipe fuscous, corky, finely velutinate to smooth, up to 2 cm long, 1.2 mm in diam, swollen near the base.
Hyphal structure: hyphal system monomitic; generative hyphae simple septate; tissue darkening but otherwise unchanged in KOH.
Context: contextual hyphae yellowish brown to honey-yellow, thick-walled with a wide lumen, occasionally branched, frequently simple septate, straight, more or less regularly arranged, 5–9 μm in diam; hyphae in stipe similar to those in context, parallel along the stipe, occasionally branched, 3–6 μm in diam.
Tubes: tramal hyphae cinnamon-buff to fawn, thin- to slightly thick-walled with a wide lumen, moderately branched, frequently simple septate, straight, subparallel along the tubes, 3–7 μm in diam. Cystidia and cystidioles absent, basidia barrel-shaped, thin-walled, with four sterigmata and a simple septum at the base, 15–18 × 8–10 μm; basidioles similar like basidia in shape, but slightly smaller.
Spores: basidiospores ellipsoid to broadly ellipsoid, buff-yellow, thick-walled, smooth, IKI–, CB–, 6.3–8.0(–8.5) × 4.3–5.3(–5.5) μm, L = 7.08 μm, W = 4.80 μm, Q = 1.48 (n=30/1).
Habitat: on ground of angiosperm forest, known only from the type locality in southern China.
Coltricia lenis L.S. Bian, M. Zhou & J. Yu, sp. nov. Fig. 2B, Fig. 4
MycoBank no.: MB 842182
Diagnosis: this species is characterized by centrally stipitate basidiocarps, distinctly concentrically zonate and sulcate pileal surface, soft to spongy stipes when dry, 0.5–2 pores per mm, oblong-ellipsoid to ellipsoid basidiospores, 7.0–9.3 × 4.5–5.8 μm (average).
Holotype: CHINA. Fujian Province, Yongtai County, Tianmen Moutain National Forest Park, on ground of angiosperm forest, 5 June 2021, Dai 22374.
Etymology: lenis (Lat.): referring to the species having soft stipes when dry.
Basidiocarps: Annual, centrally stipitate, solitary, soft and without odor or taste when fresh, becoming soft and corky when dry. Pilei more or less circular, flat to infundibuliform, up to 3 cm in diam and 2 mm thick at center. Pileal surface fawn to orange-brown, velutinate to glabrous, distinctly concentrically zonate and sulcate; bristles erect in the center; magin thin and obtuse, curving down upon drying. Pore surface curry-yellow to honey-yellow upon drying; pores angular, 0.5–2 per mm; dissepiments thin, entire. Context fawn to orange-brown, leathery, up to 0.5 mm thick. Tubes curry-yellow, distinctly paler than context, fragile or slightly brittle when dry, up to 1.5 mm long. Stipe reddish brown, soft to spongy, irregular when dry, finely velutinate, up to 3.5 cm, 4 mm in diam, swollen near the base.
Hyphal structure: hyphal system monomitic; generative hyphae simple septate; tissue darkening but otherwise unchanged in KOH.
Context: contextual hyphae cinnamon-buff to reddish brown, thick-walled with a wide lumen, unbranched, frequently simple septate, straight, more or less regularly arranged, 8–13 μm in diam; hyphae in stipe similar to those in context, parallel along the stipe, moderately branched, 10–16 μm in diam.
Tubes: tramal hyphae buff-yellow to yellowish brown, thin- to slightly thick-walled with a wide lumen, moderately branched, frequently simple septate, straight, subparallel along the tubes, 6–12 μm in diam. Cystidia and cystidioles absent, basidia barrel-shaped, thin-walled, with four sterigmata and a simple septum at the base, 17–20 × 10–12 μm; basidioles similar like basidia in shape, but slightly smaller.
Spores: basidiospores oblong-ellipsoid to ellipsoid, yellowish, thick-walled, smooth, IKI–, CB–, (6.8–)7.0–9.3(–9.5) × 4.5–5.8(–6.3) μm, L = 8.28 μm, W = 5.13 μm, Q = 1.56–1.63 (n=60/2).
Habitat: on ground of angiosperm forest, known only from the type locality in southern China.
Additional material (paratype) examined: CHINA. Fujian Province, Yongtai County, Tianmen Moutain National Forest Park, on ground of angiosperm forest, 5 June 2021, Dai 22373.
Coltricia tenuihypha L.S. Bian, M. Zhou & J. Yu, sp. nov. Fig. 2C, Fig. 5
MycoBank no.: MB 842183
Diagnosis: this species is characterized by eccentrically to centrally basidiocarps, fan-shaped to circular pilei, lacerate pileal margin, 1–3 pores per mm, narrow and skeletal-alike hyphae present in the stipe, ellipsoid to broadly ellipsoid basidiospores, 7.3–9.3 × 5.5–6.8 μm (average).
Holotype: CHINA. Yunnan Provice, Jianchuan County, Jizu Moutain Nature Reserve, on ground of angiosperm forest, 1 Sep 2021, Dai 22684.
Etymology: tenuihypha (Lat.): referring to the species having narrow hyphae in the stipe.
Basidiocarps: annual, eccentrically to centrally stipitate, solitary, soft and without odor or taste when fresh, becoming soft corky when dry. Pilei fan-shaped to more or less circular, flat to infundibuliform, up to 2.5 cm in diam and 1.5 mm thick at center. Pileal surface greyish brown to deep olive when dry, hirsute in the center with bristles erected, velutinate, with indistinct concentric zones; margin thin, lacerate, curving down upon drying. Pore surface greyish brown to fuscous upon drying; pores angular, 1–3 per mm; dissepiments thin, lacerate, sterile margin distinct. Context fawn, leathery, up to 0.3 mm thick. Tubes bluish grey to honey-yellow, distinctly paler than context, fragile or slightly brittle when dry, up to 1.2 mm long. Stipe greyish brown to deep olive, corky, smooth, up to 4 cm long, 1.5 mm in diam, sometimes branched, swollen near the base.
Hyphal structure: hyphal system monomitic to pseudodimitic; generative hyphae simple septate; tissue darkening but otherwise unchanged in KOH.
Context: contextual hyphae yellowish brown to greyish brown, thick-walled with a wide lumen, rarely branched, frequently simple septate, straight, more or less regularly arranged, 6–9 μm in diam; hyphae in stipe thick-walled with a narrow lumen, rarely septate and branched, skeletal-alike, sometimes sclerified, distinctly narrower than those in context, loosely interwoven, 2.5–5 μm in diam.
Tubes: tramal hyphae cinnamon-buff to yellowish brown, thin- to slightly thick-walled with a wide lumen, rarely branched, frequently simple septate, more or less straight, subparallel along the tubes, 5–8 μm in diam. Cystidia and cystidioles absent; basidia barrel-shaped, thin-walled, with four sterigmata and a simple septum at the base, 15–20 × 8–10 μm; basidioles similar like basidia in shape, but slightly smaller.
Spores: basidiospores ellipsoid to broadly ellipsoid, yellowish, thick-walled, smooth, IKI–, CB–, (7.0–)7.3–9.3(–9.5) × 5.5–6.8(–7.0) μm, L = 8.20 μm, W = 5.87 μm, Q = 1.32–1.39 (n=60/2).
Habitat: on ground of angiosperm forest, known only from the type locality in Southwest China.
Additional material (paratype) examined: CHINA. Yunnan Provice, Jianchuan County, Jizu Moutain Nature Reserve, on ground of angiosperm forest, 1 Sep 2021, Dai 22684.
Discussion
Coltricia fimbriata has centrally stipitate basidiocarps, large pores, and ellipsoid to broadly ellipsoid basidiospores, similar to C. pyrophila (Wakef.) Ryvarden and C. wenshanensis L.S. Bian & Y.C. Dai. However, Coltricia pyrophila can be distinguished from C. fimbriata by large basidiocarps (up to 8.5 cm in diam), decurrent pores and distinctly smaller basidiospores (4–5.5 × 3–3.5 μm vs. 6.3–8.0 × 4.3–5.3 μm; Ryvarden 1972). Coltricia wenshanensis has larger basidiocarps (up to 5 cm in diam) with distinctly concentrically zonate and sulcate, wider basidiospores (7.5–8 × 6–7 μm vs. 6.3–8.0 × 4.3–5.3 μm; Bian and Dai 2017). In addition, C. fimbriata can be also distinguished by the hairy pileal margin. This feature is shared by C. barbata Ryvarden & de Meijer and C. velutina Baltazar & Gibertoni. But the latter two species have smaller pores (4–9 per mm and 5–7 per mm, respectively, vs. 1–3 per mm in C. fimbriata; Ryvarden and de Meijer 2002; Baltazar et al. 2010). Coltricia fimbriata is closely related to C. hamata (Romell) Ryvarden in the phylogenetic analysis (Fig. 1). However, C. hamata has large (3–8 cm in diam) and rigid basidiocarps, setal hyphae in lower context and bigger basidiospores (8–10 × 5.5–6.5 μm vs. 6.3–8.0 × 4.3–5.3 μm; Ryvarden 1974).
Coltricia lenis resembles C. fragilissima (Mont.) Ryvarden and C. permollis Baltazar et Gibertoni in having centrally stipitate basidiocarps, zonate pileal surface, large pores, and thick stipes. However, C. fragilissima has large basidiocarps (8.5 cm in diam, 3 cm thick at the base), solid stipes, and distinctly smaller basidiospores (4–5.5 × 3–3.5 μm vs. 7.0–9.3 × 4.5–5.8 μm; Ryvarden 1982). Coltricia permollis differs from C. lenis by bigger pores (0.5–1.5 mm in diam), bulbous stipes tapering toward the apex (base up to 1.5 cm in diam), and longer basidiospores (8–9.5 × 5.5–6.5 μm vs. 7.0–9.3 × 4.5–5.8 μm; Baltazar et al. 2010). In the phylogenetic analysis, Coltricia lenis and C. wenshanensis L.S. Bian & Y.C. Dai formed a well-supported lineage (100% in MP, 100% in ML, 1 in BI) and distinctly differed from other species (Fig. 1). However, C. wenshanensis has larger basidiocarps (up to 5 cm in diam and 5.5 mm thick at center), corky stipes, and wider basidiospores (7.5–8 × 6–7 μm vs. 7.0–9.3 × 4.5–5.8 μm; Bian and Dai 2017).
Coltricia tenuihypha resembles C. austrosinensis L.S. Bian & Y.C. Dai and C. progressus Corner ex Y.C. Dai & Hai J. Li in having eccentrically to centrally stipitate basidiocarps, large spores, velutinate pilei, and ellipsoid to broadly ellipsoid basidiospores. However, C. austrosinensis has thicker basidiocarps (5 mm thick at center) and longer basidiospores (8.2–9.8 × 5.5–6.5 μm vs. 7.3–9.3 × 5.5–6.8 μm; Bian et al. 2016). Coltricia progressus has imbricate basidiocarps, thicker context (up to 11 mm thick), and slightly longer basidiospores (8–9.8 × 5–6.5 μm vs. 7.3–9.3 × 5.5–6.8 μm; Dai and Li 2012). Actually, C. tenuihypha can be readily recognized by the branched stipes and distinctly narrow skeletal-alike hyphae in the stipe. Coltricia hirtipes Corner also has branched stipes, but it can be distinguished by smaller pores (4 per mm vs. 1–3 per mm), wider stipe hyphae (up to 8 μm in diam), and smaller basidiospores (5.2–6.2 × 4.7–5.5 μm vs. 7.3–9.3 × 5.5–6.8 μm; Dai and Li 2012). Skeletal-alike hyphae are also present in C. rigida L.S. Bian & Y.C. Dai, but the latter has laterally stipitate and woody-hard basidiocarps, smaller pores (7–8 per mm vs. 1–3 per mm), subglobose to globose basidiospores (6–7 × 5–6.5 μm) and skeletal-alike hyphae are also present in pilei. According to the phylogenetic analysis, the sampled specimens of C. tenuihypha formed a well-supported lineage which is distinctive from the other sampled species.
Key to species of Coltricia in China
1 Basidiospores smooth...........................................................2
1* Basidiospores finely ornamented or verrucose.................27
2 Hymenophore more or less concentrically lamellate.................................................. C. montagnei (Fr.) Murrill
2* Hymenophore poroid.........................................................3
3 Basidiocarps pendent........C. tsugicola Y.C. Dai & B.K. Cui
3* Basidiocarps erect...............................................................4
4 Hyphae in stipe distinctly narrower than those in context, skeletal-alike hyphae present.........................................................................................C. tenuihypha L.S. Bian, M. Zhou & J. Yu
4* Hyphae in stipe similar to those in context, skeletal-alike hyphae absent..........................................................................5
5 Basidiocarps laterally stipitate..............................................6
5* Basidiocarps eccentrically to centrally stipitate..................9
6 Mature pilei < 0.5 cm in diam...............................................................................................C. minor Y.C. Dai
6* Mature pilei > 0.5 cm in diam.............................................7
7 Basidiocarps woody-hard; basidiospores subglobose to globose, < 7 μm long.....................C. rigida L.S. Bian & Y.C. Dai
7* Basidiocarps soft to corky; basidiospores ellipsoid to broadly ellipsoid, > 7 μm long................................................8
8 Basidiocarps up to 1 cm thick; basidiospores 8–10 × 6–7 μm..................................................C. duportii (Pat.) Ryvarden
8* Basidiocarps up to 2 mm thick; basidiospores 7–8 × 5.2–6 μm........................................C. lateralis L.S. Bian & Y.C. Dai
9 Stipe duplex; on fallen trunk of Abies......................................................................................C. abieticola Y.C. Dai
9* Stipe homogeneous; on ground of mixed forests..............10
10 Pores 0.5–3 per mm..........................................................11
10* Pores 3–6 per mm...........................................................20
11 Hyphae strongly verrucose...............................................12
11* Hyphae smooth..............................................................13
12 Basidiocarps up to 1 cm in diam, pores 2–3 per mm; basidiospores 7.5–9 × 4.8–5 μm............................................................................C. verrucata Aime, T.W. Henkel & Ryvarden
12* Basidiocarps up to 2.5 cm in diam, pores 0.5–2 per mm; basidiospores 7.8–9 × 6–7 μm.......................................................C. subverrucata L.S. Bian & Y.C. Dai
13 Context thick, up to 12 mm thick; dendrohyphidia-like hyphae present............................................C. crassa Y.C. Dai
13* Context thin, up to 1 mm thick; dendrohyphidia-like hyphae absent............................................................................14
14 Basidiocarps eccentrically stipitate, with unpleasant odor......................................................C. macropora Y.C. Dai
14* Basidiocarps centrally stipitate, without odor......................15
15 Stipe soft to spongy when dry...........................................................C. lenis L.S. Bian, M. Zhou & J. Yu
15* Stipe corky when dry......................................................16
16 Basidiospores < 5.5 μm wide...........................................17
16* Basidiospores > 5.5 μm wide.........................................18
17 Pileal margin without hairs; basidiospores oblong-ellipsoid, 8–11 × 4–5 μm.......................................................................................C. focicola (Berk. & M.A. Curtis) Murrill
17* Pileal margin with tufts of hairs; basidiospores ellipsoid to broadly ellipsoid, 6.3–8 × 4.3–5.3 μm......................................................C. fimbriata L.S. Bian, M. Zhou & J. Yu
18 Contextual hyphae > 10 μm wide...................................................C. subcinnamomea L.S. Bian & Y.C. Dai
18* Contextual hyphae < 10 μm wide..................................19
19 Stipe more or less uniform; basidiospores 7.5–8.2 × 6–6.8 μm...............................C. wenshanensis L.S. Bian & Y.C. Dai
19* Stipe up to 8 mm diam at the base; basidiospores 8–10 × 5.5–6.5 μm..................C. austrosinensis L.S. Bian & Y.C. Dai
20 Stipe bearing numerous spines...........C. strigosipes Corner
20* Stipe smooth or velutinate..............................................21
21 Growing in gymnosperm forests; basidiospores ellipsoid...............................................C. perennis (L.) Murrill
21* Growing in angiosperm forests; basidiospores broadly ellipsoid to globose................................................................22
22 Tramal hyphae 3–4 μm in diam........................................23
22* Tramal hyphae 4–9 in diam............................................24
23 Mature pilei > 1.5 cm in diam; basidiospores 7.8–9 × 5.2–6 μm......................................C. sinoperennis Y.C. Dai & F. Wu
23* Mature pilei < 1.5 cm in diam; basidiospores 6–7 × 4–5 μm.........................................C. minima L.S. Bian & Y.C. Dai
24 Basidiospores < 4 μm wide.....................................................................C. pyrophila (Wakef.) Ryvarden
24* Basidiospores > 4 μm wide............................................25
25 Pileal surface hyphae dichotomously branched........................................................................................C. weii Y.C. Dai
25* Pileal surface hyphae unbranched..................................26
26 Mature pilei > 2 cm in diam; basidiospores 7–8 × 5.5–6.5 μm............................................C. cinnamomea (Jacq.) Murrill
26* Mature pilei < 2 cm in diam; basidiospores 5.5–6.2 × 4.5–5.8 μm..................................C. velutina Baltazar & Gibertoni
27 Basidiocarps resupinate to effused-reflexed.....................28
27* Basidiocarps stipitate......................................................29
28 Hyphae at dissepiment edge mostly moniliform; cystidioles present.........................................................................C. subglobosa (Y.C. Dai) Y.C. Dai & F. Wu
28* Hyphae at dissepiment edge uniform; cystidioles absent..............................................................................................C. baoshanensis (Y.C. Dai & B.K. Cui) Y.C. Dai & F. Wu
29 Basidiocarps pendent.......................................................30
29* Basidiocarps erect...........................................................31
30 Basidiospores 6–9 × 4–5.5 μm.................................................................C. dependens (Berk. & M.A. Curtis) Imazeki
30* Basidiospores 9–11.8 × 5–6.2 μm......................................C. pseudodependens (L.S. Bian & Y.C. Dai) Y.C. Dai & F. Wu
31 Basidiocarps laterally stipitate, pilei fan-shaped....................................................................C. pusilla Imazeki & Kobayasi
31* Basidiocarps centrally stipitate, pilei more or less circular...................................................................................32
32 Basidiospores ellipsoid.....................................................33
32* Basidiospores naviculate or subglobose to globose...........................................................................................................34
33 Pores 3–4 per mm; basidiospores 6.5–7.8 × 4.8–5.5 μm...........................C. subpicta (Lloyd) Imazeki & Kobayasi
33* Pores 1–2 per mm; basidiospores 8.5–10.2 × 5–6 μm........................................C. oblectabilis (Lloyd) Ryvarden
34 Basidiospores naviculate, 8–11 × 5–6.2 μm.................................................................................................C. naviculiformis (Y.C. Dai & Niemelä) Y.C. Dai & F. Wu
34* Basidiospores subglobose to globose, 6–7 × 5.8–7 μm......................................................................................C. globosa (L.S. Bian & Y.C. Dai) Y.C. Dai & F. Wu
Data availability
The sequence data generated in this study are deposited in NCBI GenBank.
References
Baltazar JM, Silveira RMB (2012) A new name for a Coltricia (Basidiomycota) from India. Mycotaxon 119:385–389. https://doi.org/10.5248/119.385
Baltazar JM, Ryvarden L, Gibertoni TB (2010) The genus Coltricia in Brazil: new records and two new species. Mycologia 102:1253–1262. https://doi.org/10.3852/09-227
Bian LS, Dai YC (2015) Coltriciella globosa and C. pseudodependens spp. nov. (Hymenochaetales) from southern China based on morphological and molecular characters. Mycoscience 56:190–197. https://doi.org/10.1016/j.myc.2014.06.001
Bian LS, Dai YC (2017) Morphological and molecular evidence for three new species of Coltricia (Hymenochaetaceae, Basidiomycota) from southern China. Mycologia 109:64–74. https://doi.org/10.1080/00275514.2017.1286571
Bian LS, Dai YC (2020) Molecular phylogeny and morphology reveal two new species of Coltricia (Hymenochaetaceae Basidiomycota) from China. Mycol Prog 19:657–666. https://doi.org/10.1007/s11557-020-01583-7
Bian LS, Wu F, Dai YC (2016) Two new species of Coltricia (Hymenochaetaceae, Basidiomycota) from southern China based on the evidence from morphology and DNA sequence data. Mycol Prog 15:27. https://doi.org/10.1007/s11557-016-1173-0
Chen JJ, Cui BK, Dai YC (2016) Global diversity and molecular systematics of Wrightoporia s.l. (Russulales, Basidiomycota). Persoonia 37:21–36. https://doi.org/10.3767/003158516x689666
Dai YC (2010) Hymenochaetaceae (Basidiomycota) in China. Fungal Divers 45:131–343. https://doi.org/10.1007/s13225-010-0066-9
Dai YC, Li HJ (2012) Type studies on Coltricia and Coltriciella described by E. J. H. Corner from Southeast Asia. Mycoscience 53:337–346. https://doi.org/10.1007/s10267-011-0174-8
Dai YC, Yuan HS, Cui BK (2010) Coltricia (Basidiomycota, Hymenochaetaceae) in China. Sydowia 62:11–21
Dai YC, Yang ZL, Cui BK, Wu G, Yuan HS, Zhou LW, He SH, Ge ZW, Wu F, Wei YL, Yuan Y, Si J (2021) Diversity and systematics of the important macrofungi in Chinese forests. Mycosystema 40:770–805. https://doi.org/10.13346/j.mycosystema.210036
Decock C (2013) Coltricia oboensis sp. nov. from the high elevation cloud forest of São Tomé. Cryptogamie Mycol 34:175–181. https://doi.org/10.7872/crym.v34.iss2.2013.175
Felsenstein J (1985) Confidence intervals on phylogenetics: an approach using bootstrap. Evolution 39:783–791
Hall TA (1999) Bioedit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium Series 41:95–98
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192. https://doi.org/10.2307/2992540
Katoh K, Toh H (2008) Improved accuracy of multiple ncRNA alignment by incorporating structural information into a MAFFT-based framework. BMC Bioinformatics 9:212. https://doi.org/10.1186/1471-2105-9-212
Kornerup A, Wanscher JH (1978) Methuen handbook of colour, 3rd edn. Eyre Methuen, London
Larsson K-H, Parmasto E, Fischer M, Langer E, Nakasone KK, Redhead SA (2006) Hymenochaetales: a molecular phylogeny for the hymenochaetoid clade. Mycologia 98:926–936. https://doi.org/10.1080/15572536.2006.11832622
Matheny PB (2005) Improving phylogenetic inference of mushrooms with RPBl and RPB2 nucleotide sequences. Mol Phylogenet Evol 35:1–20. https://doi.org/10.1016/j.ympev.2004.11.014
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818. https://doi.org/10.1093/bioinformatics/14.9.817
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Ryvarden L (1972) A critical checklist of the Polyporaceae in tropical East Africa. Norwegian Journal of Botany 19:229–238
Ryvarden L (1974) Type-studies in the Polyporaceae 3. Species described by L. Romell. Svensk Botanisk Tidskrift 68:273–284
Ryvarden L (1982) Type-studies in the Polyporaceae 11. Species described by J.F. Montagne. either alone or with other authors. Nord J Bot 2:75–84
Ryvarden L (1991) Genera of polypores. Nomenclature and taxonomy. Synop Fungorum 5:1–363
Ryvarden L, de Meijer AAR (2002) Studies in neotropical polypores 14. New species from the state of Paraná, Brazol. Synop Fungorum 15:34–69
Ryvarden L, Melo I (2014) Poroid fungi of Europe. Synop Fungorum 31:1–455
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Susan D, Retnowati A, Sukarno N (2018) Coltriciella minuscula sp. nov., a new species of poroid fungus on Pinus merkusii from an Indonesian tropical forest. Mycoscience 59:49–53. https://doi.org/10.1016/j.myc.2017.08.005
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sinauer Associates, Sunderland, Massachusetts
Tedersoo L, Suvi T, Beaver K, Saar I (2007) Ectomycorrhizas of Coltricia and Coltriciella (Hymenochaetales, Basidiomycota) on Caesalpiniaceae, Dipterocarpaceae and Myrtaceae in Seychelles. Mycol Prog 6:101–107. https://doi.org/10.1007/s11557-007-0530-4
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882. https://doi.org/10.1093/nar/25.24.4876
Valenzuela R, Raymundo T, Cifuentes J, Esqueda M, Amalfi M, Decock C (2012) Coltriciella sonorensis sp. nov. (Basidiomycota, Hymenochaetales) from Mexico: evidence from morphology and DNA sequence data. Mycol Prog 11:181–189. https://doi.org/10.1007/s11557-011-0740-7
Valenzuela R, Raymundo T, Decock C, Lara-Díaz BN, Luna-Vega I, García-Sandoval R (2020) Coltriciella multipileata (Agaricomycetes, Hymenochaetaceae), a new species from Mexico, related to ectomycorrhizal lineages. Phytotaxa 475:79–90. https://doi.org/10.11646/phytotaxa.475.2.2
Vasco-Palacios AM (2016) Ectomycorrhizal fungi in Amazonian tropical forests in Colombia. Panamericana Formas e Impresos S.A, Colombia
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
Vlasák J, Vlasák J Jr, Ryvarden L (2020) Studies in Neotropical polypores 46 Some new and noteworthy polypores from Costa Rica. Synop Fungorum 42:30–33
Wagner T, Fischer M (2002) Proceedings toward a natural classification of the worldwide taxa Phellinus s.l. and Inonotus s.l., and phylogenetic relationships of allied genera. Mycologia 94:998–1016. https://doi.org/10.2307/3761866
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: a guide to methods and applications. Academic Press, San Diego
Wu F, Yuan HS, Zhou LW, Yuan Y, Cui BK, Dai YC (2020) Polypore diversity in South China. Mycosystema 39:653–682. https://doi.org/10.13346/j.mycosystema.200087
Wu F, Zhou LW, Vlasák J, Dai YC (2022) Global diversity and systematics of Hymenochaetaceae with poroid hymenophore. Fungal Divers. https://doi.org/10.1007/s13225-021-00496-4
Zhou LW, Tedersoo L (2012) Coltricia australica sp. nov. (Hymenochaetales, Basidiomycota) from Australia. Mycotaxon 122:123–128. https://doi.org/10.5248/122.123
Funding
The research was supported by the National Natural Science Foundation of China (Project No. 31800018).
Author information
Authors and Affiliations
Contributions
The study conception and design: Lu-Sen Bian and Jian Yu. Specimens collecting: Lu-Sen Bian, Meng Zhou, and Jian Yu. Morphological studies: Lu-Sen Bian. Phylogenetic analyses: Meng Zhou. Discussion: Lu-Sen Bian and Jian Yu. The first draft: Lu-Sen Bian. Review and editing: Jian Yu. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Yu-Cheng Dai
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bian, LS., Zhou, M. & Yu, J. Three new Coltricia (Hymenochaetaceae, Basidiomycota) species from China based on morphological characters and molecular evidence. Mycol Progress 21, 45 (2022). https://doi.org/10.1007/s11557-022-01792-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-022-01792-2