Abstract
The Argentine Northwest has a system of tropical and subtropical montane cloud forests on the Andes Mountains, where there is a significant diversity of Agaricales fungi yet to be discovered. Two new species from the Cedrela forest, inside the Baritú National Park, Argentina, are described in this work as Cercopemyces messii and Clitocybe cedrelae. Phylogenetic analysis was performed using the nuclear internal transcribed spacer (nrITS) region and the large subunit (nrLSU) of the nuclear ribosomal RNA. Complete taxonomic descriptions, field photographs, drawings, photographs captured with the scanning electron microscope (SEM) of the basidiospores, and comparisons of the similar and closely related species are provided. Both taxa have ornamented basidiospores that are hyaline, inamyloid, and clamp connections in all tissues. They were also found within the same area, and both presented a good number of specimens. These striking species should be considered in danger since their natural habitats are also at risk.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Argentine Northwest has a large diversity of Agaricomycetes fungi that has been broadly studied by Singer (1950a, b, 1953, 1955, 1959, 1960, 1965a, b, 1969, 1970, 1973a, b, 1975, 1976); Singer and Digilio (1951); Singer and Morello (1960); Spegazzini (1912, 1919); Wicaksono et al. (2017); Dios et al. (2017); Niveiro (2012); Niveiro et al. (2012); Niveiro et al. (2014a, b, c); Nouhra et al. (2018); Malizia et al. (2012); and Baroni et al. (2012). The majority of this macrofungal biodiversity is concentrated in the Andean Yungas, which comprise a system of tropical and subtropical montane cloud forests on the eastern slope along the Andes in South America. The Argentinean Yungas constitutes the southeast extension of the Amazonian Domain (Cabrera 1994). The weather in this region is characterized by seasonal fog and persistent cloud cover (Brown et al. 2005), and it features a high altitudinal gradient and experiences various environmental conditions, such as periods of high temperatures, drought, high humidity levels, frosts, and even snow in winter. During the months of November to February, rainfall is high, while during the rest of the year, these regions have a dry environment with low rainfall. Niveiro (2012) compiled all the macrofungal taxa for the Yungas of Argentina, recording 629 species, with a total of 125 species of Agaricales s.l. Additionally, 12 new taxa have been discovered within the genera Pouzarella Mazzer, Inocybe (Fr.) Fr., Clitocybula (Singer) Singer ex Métrod, Mycena (Pers.) Roussel, Pluteus Fr., Pholiota (Fr.) P. Kumm, and Hemimycena Singer (Niveiro et al. 2012, 2014a, b). Also, based on richness estimators, Niveiro (2012) concluded that only 23–46% of the Agaricales were recorded, and a high diversity of new taxa remained to be studied. Nouhra et al. (2018) analyzed the soil using metabarcoding, which provided 1254 OTUs for the Agaricomycetes group under consideration. Based on the number of described species within the Yungas of Argentina, they predict that many more species remain undescribed. Within the Yungas, tree diversity is high and estimated to include more than 230 species (Grau and Brown 2000; Brown et al. 2005). According to Brown et al. (2005), three main forest types can be distinguished: premontane lowland forests (400–700 m asl); lower montane forests (700–1500 m asl); and upper temperate montane forests (1500–2500 m asl). The state of preservation of the Argentine Yungas is vulnerable and insufficient to protect its biodiversity. Unsustainable logging, extensive livestock farming, and subsistence hunting, among other reasons, contribute to the degradation and loss of the ecosystem’s productive value (Brown and Malizia 2004).
An excursion to the northern province of Salta, Argentina, provided us the opportunity to document several collections of Cercopemyces and Clitocybe, within Baritú National Park. Our sampling efforts were focused on the upper temperate montane ecosystems (1500–2500 m asl). There is an area primarily composed of trees belonging to the genus Cedrela P. Browne, where several collections of the new species of Cercopemyces and Clitocybe were found. The genus Cedrela is native to Argentina (Zuloaga and Morrone 1999), and it is distributed mainly in South America, with five species in Argentina, three of which are exclusive to the northwest (C. balansae C. DC., C. lilloi C. DC., C. saltensis Zapater and del Castillo) and two from the northeast (C. fissilis Vell., C. odorata L., Zapater et al. (2004).
The genus Cercopemyces T.J. Baroni, Kropp and V.S. Evenson was proposed by Baroni et al. (2014) and is supported as a monophyletic group based on nLSU and ITS sequences. Our phylogenetic analysis results are consistent with those of Baroni et al. (2014). It is characterized by its medium to large, solid basidiomata, with whitish tones, amanitoid appearance, absence of hymenial cystidia, and ornamented spores. Cercopemyces forms a sister group with Ripartitella Singer and Cystodermella Harmaja, but is differentiated from both by several characteristics; Ripartitella typically presents smaller basidiomata generally associated with wood rather than growing directly on the ground, and it possesses encrusted pleurocystidia. Cystodermella is differentiated by having smooth spores and spherocytes in the pileipellis. Currently, only three species of Cercopemyces are known, and this new description is an important discovery that supports the monophyly of the genus.
Clitocybe (Fr.) Staude is a genus in the family Clitocybaceae Vizzini et al. and comprising approximately 500 species (CABI Bioscience & Landcare Research 2024). Molecular phylogenetic analyses (Matheny et al. 2006; Alvarado et al. 2015) have demonstrated that the genus is placed within the Clitocybeae tribe, along with Collybia (Fr.) Staude and Lepista (Fr.) W.G. Sm. This group remains taxonomically unresolved, with discrepancies between taxonomy and phylogeny (Alvarado et al. 2015). Recent multi-locus phylogenetic and phylogenomic analyses of Clitocybaceae revealed the presence of six generic clades: Clitocybe, Collybia, Dendrocollybia, Lepista, Pseudolyophyllum, and Singerocybe (He et al. 2023) and placed several Lepista species into Collybia. He and Yang (2024) recommended conserving the name Clitocybe based in a conserved type.
The genus Clitocybe is characterized by its gregarious to clustered basidiomata, a white spore print, small ellipsoidal and inamyloid spores, hyphae with clamp connections, absence of cystidia, and a poorly differentiated pileipellis (Singer 1986). Due to the limited morphological characteristics and variations caused by the environment, species identification remains difficult (Bigelow and Smith 1969), requiring molecular phylogenetic analyses to resolve their taxonomic positions.
In this study, two new species, Cercopemyces messii and Clitocybe cedrelae, were described from specimens collected in the Andean Yungas during a survey using both phylogenetic analyses and detailed morphological examinations.
Material and methods
Specimens studied
The specimens for this research were collected within Baritú National Park, located in Salta, Argentina, during the initial months of 2023. All the collections were found in a forest composed of trees of the genus Cedrela, locally known as the “Cedral” site. They were meticulously documented and preserved using the established methodologies for Agaricales (Largent 1986). The collections were deposited in the mycological herbarium of the Departamento de Biodiversidad y Biología Experimental of the Facultad de Ciencias Exactas y Naturales, UBA (BAFC).
Morphological studies
For the macroscopic descriptions, colors were noted following Kornerup and Wanscher (1987). Microscopic description followed the standard method used for Agaricales (Lechner 2021). Longitudinal radial sections were made on the surface of the pileus to observe the pileipellis and transverse perpendicular sections on the gills to describe basidia, cystidia, and spores and longitudinal sections on the stem to characterize caulocystidia. Different media and stains were used to facilitate the observation of structures, ornamentations, and reactions on the fresh basidiomata: 3% KOH, 1% aqueous phloxine, and Melzer’s reagent. All microscopic work was conducted using a bright field Leica DM750 microscope, and images were captured using a Leica iCC50 W integrated camera. All observed structures were measured, and the following symbols were employed: Q for the ratio of length to width of spores; Qe for the mean of Q values; N for the number of spores measured; Me for the average size of spores. For the presented basidiospore photographs, a scanning electron microscope FE SEM SUPRA 40 Carl Zeiss AG was used.
DNA extraction, PCR conditions, and sequencing
Genomic DNA was extracted from all collections with NaOH (Steiner et al. 1995), and the PCR amplification was performed with the primer pairs ITS4–ITS5 (White et al. 1990) and LR0R-LR5 (Vilgalys and Hester 1990), for the nuclear ITS and partial LSU regions, respectively. The amplification program used for ITS was an initial denaturation at 95 °C for 2 min, followed by 40 cycles at 95 °C for 30 s, 55 °C for 45 s, 72 °C for 1 min, and a final extension at 72 °C for 15 min; and for nLSU, it was an initial denaturation at 95 °C for 3 min, followed by 40 cycles at 95 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 15 min. PCR products were checked through 1% agarose gel electrophoresis and processed by the services of Macrogen Inc. (South Korea) for sequencing. All the sequences obtained were manually reviewed with BioEdit v7.2.5 (Hall 1999) and checked with a Blast search in the GenBank to compare with the existing sequence data. New sequences were deposited in the GenBank database.
Phylogenetic analyses
Two datasets were constructed for each species using the internal transcribed spacers including the 5.8S gene (ITS1-5.8S-ITS2) and the large subunit 28S gene (LSU). An additional concatenated analysis comprising the ITS–nLSU regions was also constructed. The selection of sequences was based on Baroni et al. (2014); Hakizimana et al. (2023); Carbone et al. (2020); Zhang et al. (2019); Saar et al. (2009); Varga et al. (2019); Vizzini et al. (2011); Alvarado et al. (2015); Putra et al. (2022); Sjökvist et al. (2012); Wang et al. (2019); Hartley et al. (2009); He et al. (2023); and He and Yang (2024) and NCBI Blast best hits. Members of Tricholomataceae (Tricholoma ligusticum M. Carbone, Boccardo and Calledda and Lepista nuda (Bull.) Cooke), Agaricaceae (Lepiota cristata (Bolton) P. Kumm. and Smithiomyces mexicanus (Murril) Singer), and Pseudoclitocybaceae (Musumecia bettlachensis Vizzini and Contu), which remain clearly outside the Cercopemyces and Lepista clade, were selected for the outgroup (Baroni et al 2014; Wang et al. 2019). The species, location, and GenBank accession numbers for the sequences used in the datasets are presented in Tables 1 and 2. All alignments were performed using the Muscle tool (Edgar 2004) as implemented in MegaX, and then manually optimized in BioEdit v7.2.5 (Hall 1999).
Best maximum likelihood (ML) tree was obtained with MegaX, after testing for the best evolutionary model for each region. Support values were obtained using 1000 bootstrap iterations. Gaps and missing data were completely deleted. The obtained topologies were then visualized in FigTree version 1.4.0 (Rambaut 2009), and the alignments were submitted to TreeBase under the submission ID: 30888.
Taxonomy
For taxonomic treatment, names and synonyms were cited and consulted according to the ICNafp (Turland et al. 2018) and Index Fungorum—Authors of Fungal Names (CABI Bioscience and Landcare Research 2020). Herbarium acronyms were cited following Thiers (2012), and reference descriptions were consulted (Singer 1950a; Bigelow and Smith 1969; Franco-Molano 1993; Baroni et al. 2014; Heluta et al. 2019; Wright and Albertó 2002). New taxa were submitted to MycoBank database.
Results
Molecular phylogeny
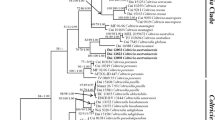
The maximum likelihood (ML) analysis of the nLSU dataset of Cercopemyces and related species (Fig. 1) indicates that the collections of Cercopemyces messii form a highly supported monophyletic group within the Cercopemyces clade, with Ripartitella as sister clade and Cystodermella as sister to both. The final alignment used for the nLSU dataset consisted of 26 sequences of 616 bp in length, with 531 of these sites being conserved and 85 being variable. The topology obtained is consistent with the work of Saar et al. (2009), Baroni et al. (2014), Varga et al. (2019), Zhang et al. (2019), Carbone et al. (2020), and Hakizimana et al. (2023).
The ML analysis of the combined nITS and nLSU dataset of Clitocybe, Lepista and related species (Fig. 2) places the three collections of Clitocybe cedrelae within the Lepista clade with high BS values. Currently, this clade is better positioned within the genus Clitocybe (He et al. 2023; He and Yang 2024). These collections form a monophyletic clade with Lepista sordida and L. tarda as the closest related species to C. cedrelae. These species are found within the violet Lepista clade with L. nuda and L. personata. The final alignment consisted of 43 sequences of 1528 bp in length, with 1221 of these sites being conserved and 298 being variable. The topology obtained is consistent with the work of Hartley et al. (2009), Vizzini et al. (2011), Alvarado et al. (2015), and Wang et al. (2019).
Taxonomy
Cercopemyces messii J.M. Suárez, A.P. Martínez, B.E. Lechner & J. Aliaga, sp. nov. (Fig. 3).
MycoBank: MB851202.
GenBank: rDNA LSU: OR896541.
Diagnosis: Cercopemyces messii is easily distinguishable by its light coloration, medium to large size, pileus convex to campanulate, white with pale orange over the disc, covered by verrucose squamules over the disc, closely spaced lamellae, and large scales on the stipe, basidiospores (5.4)5.7–6.8(7.6) × (2.6)2.9–3.6(4.1) µm, hyaline, oblong, with small verrucose ornamentation, basidia 4-sterigmate, clavate, 19.6–26.2 × 4.6–5.7 µm, with granular content, pileipellis a cutis with transitions to a trichodermium in areas where scales occur, stipitipellis a cutis with hyaline interwoven cylindrical hyphae, 3.9–4.4 µm wide, with scale elements, hyphae of scale elements clavate, cylindric, rarely ventricose, some with H- or Y-shaped, 10.9–16.3 µm wide, sometimes with the apex slightly capitate.
Etymology: The proposed name is as a tribute to the illustrious Lionel Andrés Messi Cuccittini, whose remarkable talent has brought immense joy and pride to the people of Argentina.
Holotype: Argentina, Salta, Parque Nacional Baritú, Bosque El Cedral, 1700 m., 03 Mar. 2023, 22°27′39.24″ S 64°44′31.56″ W, leg. J.M. Suárez, A.P. Martínez & J. Aliaga (BAFC53456).
Description: Basidiomata (Fig. 3) medium-sized to large. Pileus 40–170 mm broad, convex to campanulate, at first white (1A1), with a pale orange over the disc (5A3), then turning pale yellow (1A3) to grayish yellow (2B3); surface dry, covered by verrucose squamules of 1–2 mm broad over the disc, and with white (1A1) more delicate floccose scales arranged radially, becoming glabrescent; curved margin with traces of veil. Flesh white (1A1) when fresh, becoming pale yellow (1A3) when dried, up to 20 mm broad. Lamella white (1A1), sinuate, narrow, with lamellulae of different sizes. Stipe white (1A1) 100–180 × 12–25 mm, cylindric, central, solid, occasionally widening towards the base with a small bulb, surface dry, glabrous over the apex, covered with large pale orange (5A3) floccose scales down to the base, sometimes upper scales adhering in a collar-like ring, and become small towards the base; stipe flesh turns light orange (5A4) from bruising. All basidiomata turns pale yellow (1A3) to light orange (5A4) when dried. Mild fungoid odor. Taste not recorded.
Basidiospores (Fig. 4a) (5.4)5.7–6.8(7.6) × (2.6)2.9–3.6(4.1) µm, Me = 6.2 × 3.2 µm, Q = (1.4)1.7–2.3, Qe = 1.9, N = 31; hyaline, oblong, inamyloid, with small verrucose ornamentation decreasing in size towards the apiculus (Figs. 4a and 5a, b), barely observable with Melzer’s reagent. Basidia (Fig. 4b) 4-sterigmate, clavate, 19.6–26.2 × 4.6–5.7 µm, with granular content, sterigmata up to 2.5 µm long. Hymenial cystidia absent. Lamellar trama hyaline composed of parallel, cylindric to inflated hyphae, 25.9–69.1 × 5.6–13.8 µm, branched in the center, some with H- or Y-shaped patterns. Pileipellis (Fig. 4c), a cutis with transitions to a trichodermium in areas where scales occur, composed of cylindric hyphae, hyaline to yellowish, 6.8–11.5 µm wide, hyphae of scale elements clavate, cylindric, rarely ventricose, some with H or Y-shaped (Fig. 4d), 10.9–16.3 µm wide, sometimes with the apex slightly capitate. Stipitipellis, a cutis with hyaline interwoven cylindrical hyphae, 3.9–4.4 µm wide, with scale elements producing trichodermial hyphae, 22.9–44.5 × 4.8–11.7 µm. Clamp connections present in all tissues.
Habit and habitat: solitary to gregarious, abundant, growing in groups on the ground, among leaf litter, near the base under Cedrela spp. (Meliaceae). This species has been collected only in the Baritú National Park, Salta, within a forest mainly composed of long-lived Cedrela specimens.
Commentary: Cercopemyces messii is easily distinguishable by its light coloration, medium to large size, closely spaced lamellae, and large scales on the stipe. Within the genus, only three species are known, which are reported as infrequent and included in the red lists that encompass endangered taxa (Franco-Molano 1993; Baroni et al. 2014; Heluta et al. 2019). Cercopemyces crocodilinus T.J. Baroni, Kropp & V.S. Evenson is a similar species distinguished by its smaller basidiomata, the presence of a bulb at the base of the stipe, larger scales on the pileus, smaller and more globose spores, 4.8–6.4 × 3.5–5.5 µm, Qe = 1.37 (Baroni et al. 2014). Cercopemyces ponderosus (A.H. Sm. & Singer) T.J. Baroni, Kropp & V.S. Evenson is a rare species from the eastern USA and France, also differentiated by its smaller spores, 3.6–4 (5) × 2.7–3 µm (Franco-Molano 1993). Cercopemyces rickenii (Bohus) Dima & L. Nagy also presents smaller spores with lighter ornamentation, 4.6–5.8 × 2.8–3.7 µm (Heluta et al. 2019). Singer (1986) reported C. rickenii as edible, although there is limited precise information available regarding its edibility. The distribution of the Cercopemyces species is restricted and scarce. Additionally, C. rickenii was included in the Current Red List of Slovak fungi, which encompasses 39 species classified as endangered according to IUCN criteria (Kautmanová 2004). It was also proposed to be included in the European Red List (Senn-Irlet 2011) and is listed in the Red Data Book as a vulnerable rare Ukrainian species with a disjunctive range (Didukh 2009). Cercopemyces ponderosus is a rarely collected species, apparently endemic to the eastern USA, and has been proposed for consideration due to its extreme rarity (Baroni et al. 2014). The results of the phylogenetic analysis indicate that sequences from different collections of C. messii form a monophyletic clade within the Cercopemyces genus, with Ripartitella and Cystodermella as a sister group.
Material examined: Cercopemyces messii. ARGENTINA, Salta, Parque Nacional Baritú, Bosque El Cedral, 1700 m asl., 03 Mar. 2023, 22°27′39.24″ S, 64°44′31.56″ W, leg. Juan M. Suárez, Agustín P. Martínez & Joaquin Aliaga (BAFC53456). Parque Nacional Baritú, Bosque El Cedral, 1700 m asl., 03 Mar. 2023, 22°27′11.2″ S, 64°44′40.26″ W, leg. Agustín P. Martínez, Juan M. Suárez & Joaquin Aliaga (BAFC53457). Parque Nacional Baritú, Bosque El Cedral, 1700 m asl., 03 Mar. 2023, 22°27′33.5″ S, 64°44′50.1″ W, leg. Joaquin Aliaga, Agustín P. Martinez & Juan M. Suárez (BAFC 53458).
Clitocybe cedrelae J.M. Suárez, A.P. Martínez, B.E. Lechner & J. Aliaga, sp. nov. (Fig. 6).
MycoBank: MB851203.
GenBank: rDNA ITS: OR886661; LSU: OR886658.
Etymology: The proposed nomenclature is derived from the genus Cedrela. One of the samples was discovered thriving on the bark of a living tree belonging to this genus, while the remainder of the samples was found growing around the base of such trees. It is worth noting that Cedrela is a genus with a scarce global distribution and limited population. Furthermore, it is essential to highlight that all collections were acquired within a forest of exceptionally ancient Cedrela trees.
Diagnosis: Clitocybe cedrelae can be distinguished by its small size and bright coloration, grayish magenta to reddish lilac when young, turning grayish red to reddish brown, featuring a translucent, striated, and slightly depressed pileus at the center, basidiospores (5.6)5.8–7.4(8.6) × (3.1)3.5–4.5(4.9) µm, hyaline, ellipsoid to pip-shaped, guttulate, with finely punctate ornamentation, basidia 21.1–33.8 × 5.9–6.8 µm, clavate, 4-sterigmate, hyaline, pileipellis forming a cutis of grayish yellow, cylindrical hyphae, 3.4–6.1 µm wide, hypodermis composed of inflated, subglobose to globose hyaline cells, 5.3–30.2 µm wide, stipitipellis grayish yellow, hyphae cylindrical 4.1–5.3 µm wide, clamp connections present.
Holotype: ARGENTINA, Salta, Parque Nacional Baritú, Bosque El Cedral, 1700 m., 03 Mar. 2023, 22°27′39.24″ S, 64°44′29.4″ W, leg. J. M. Suárez, J. Aliaga & A. P. Martínez (BAFC53464).
Description: Basidiomata (Fig. 6) small to medium sized. Pileus 25–55 mm convex; grayish magenta (13D4) to reddish lilac (14C3) when young, turning grayish red (9C4) to reddish brown (8D4) toward the center when mature, slightly depressed over the disc, translucently striate, hygrophanous and becoming opaque; margin crenate to irregularly wavy. Non-existent context. Lamellae, purplish pink (14A3), sinuate, subdistant to close, moderately thick, 10–23 mm broad, edge smooth, with lamellulae of different sizes. Stipe 55–84 × 7–9 mm, central, reddish lilac (14B4) with tones grayish magenta (13D4), brownish orange (7C4) at maturity, cartilaginous, cylindrical to slightly widened toward the base, sometimes twisted, slightly wrinkled, surface smooth. Odor and taste not recorded.
Basidiospores (Fig. 7a) (5.6)5.8–7.4(8.6) × (3.1)3.5–4.5(4.9) µm; Me = 6.5 × 3.9 µm; Q = (1.3)1.5–1.8(2.1); Qe = 1.7; N = 28; hyaline, inamyloid, ellipsoid to pip-shaped, guttulate, with finely punctate ornamentation under the optical microscope, and with prominent crater-like warts as imaged under SEM (Fig. 5c, d). Basidia (Fig. 7b) 21.1–33.8 × 5.9–6.8 µm, clavate, hyaline, with granular content, 4-sterigmate, sterigmata long, 3.5 µm. Cystidia absent. Hymenophoral trama composed of subparallel, hyaline, cylindrical hyphae, 7.9–20.1 µm wide. Pileipellis (Fig. 7c) forming a cutis of grayish yellow (2B3), not incrusted, parallel, cylindrical hyphae, 3.4–6.1 µm wide. Hypodermis composed of inflated, subglobose to globose hyaline cells, 5.3–30.2 µm wide. Stipitipellis grayish yellow (2B3), hyphae cylindrical, not incrusted, 4.1–5.3 µm wide. Clamp connections present.
Habit and habitat: Solitary to scattered, growing on the ground, among leaf litter, and on the bark of standing living Cedrela spp. (Meliaceae). This species has been collected only in the Baritú National Park, Salta, within a forest primarily composed of mature Cedrela spp. specimens.
Commentary: Clitocybe cedrelae can be distinguished by its small size and bright coloration, featuring a translucent, striated, and slightly depressed pileus at the center. Due to its distinctive coloration and phylogenetic placement, C. cedrelae is classified within the group of Lepista species with violet pigmentations. Its closest phylogenetic relative is L. sordida (Schumach.) Singer is characterized by generally darker coloring, an unstriated cap, and narrow spores, 5.7–7.9 × 2.8–4.0 µm (Singer 1950a). L. argentina (Speg.) Singer was reported as a species similar to L. sordida by Wright and Albertó (2002), and it is distinguished from C. cedrelae by the presence of white lamellae and its occurrence in the winter within the Province of Buenos Aires (Singer 1950a). L. personata (Fr.) Cooke exhibits a purplish coloration primarily on the stipe, a non-violet pileus, and spores measuring 4.5–7 × 3–4 µm (Bigelow and Smith 1969). Lepista nuda (Bull.) Cooke features larger basidiomes, an unstriated cap, and a more intense purplish coloration (Bigelow and Smith 1969). L. tarda (Peck) Murril has a larger pileus, pale-violet to avellaneous and spores 7–8 × 4–5 µm (Murril 1917). L. irina (Fr.) H.E. Bigelow, L. densifolia, and L. panaeola are primarily distinguished by their non-violet coloration and larger basidiomes.
Material examined: Clitocybe cedrelae. ARGENTINA, Salta, Parque Nacional Baritú, Bosque El Cedral, 1700 m asl., 3 Mar. 2023, 22°27′39.24″ S, 64°44′29.4″ W, leg. J. M. Suárez, J. Aliaga & A. P. Martínez (BAFC53464). Parque Nacional Baritú, Bosque El Cedral, 1700 m asl., 3 Mar. 2023, 22°27′38.16″ S, 64°44′29.4″ W, leg. J. Aliaga, A. P. Martínez & J. M. Suárez (BAFC53465). Parque Nacional Baritú, Bosque El Cedral, 1700 m asl., 3 Mar. 2023, 22°27′38.16″ S, 64°44′30.84″ W, leg. A. P. Martínez, J. M. Suárez & J. Aliaga (BAFC53466).
Discussion
The Agaricales comprise a diverse group of fungi, with around 13,000 described species, though the total number is believed to be significantly higher. They play a vital role in ecosystems as decomposers of organic matter and in symbiotic relationships with certain plants. The preservation of their natural environments is of utmost importance. It ensures the continuity of research and discovery of these diverse species, maintains biodiversity, and safeguards their potential contributions in medicine, biotechnology, and ecology.
References
Alvarado P, Moreno G, Vizzini A, Consiglio G, Manjón JL, Setti L (2015) Atractosporocybe, Leucocybe and Rhizocybe: three new clitocyboid genera in the Tricholomatoid clade (Agaricales) with notes on Clitocybe and Lepista. Mycologia 107(1):123–136
Baroni TJ, Albertó E, Niveiro N, Lechner B (2012) New species and records of Pouzarella (Agaricomyetes, Entolomataceae) from northern Argentina. Kurtziana 37:41–63
Baroni TJ, Kropp BR, Evenson VS, Wilhelm M (2014) Cercopemyces crocodilinus, a new genus and species related to Ripartitella, is described from North America. Mycologia 106(4):785–796
Bigelow HE, Smith AH (1969) The status of Lepista—a new section of Clitocybe. Brittonia 21:144–177
Brown AD, Malizia LR (2004) Las selvas pedemontanas de las Yungas. Ciencia Hoy 14(83):52–63
Brown AD, Pacheco S, Lomáscolo T, Malizia L (2005) Ecorregión Yungas: Situación ambiental en los Bosques andinos Yungueños. Fundación Vida Silvestre Argentina, Argentina, pp 53–72
CABI Bioscience and Landcare Research. 2020. Index fungorum. Available in https://www.indexfungorum.org/. Accessed July 2023
Cabrera AL (1994) Regiones Fitogeograficas Argentinas. Enciclopedia Argentina de Agricultura y Jardinería. Tomo II. Editorial ACME S.A.C.I., Buenos Aires
Carbone M, Boccardo F, Calledda F (2020) Tricholoma ligusticum, una specie nuova dei querceti liguri. Neotipificazione e studio genetico di Tricholoma quercetorum. RdM 63(3):197–214
Didukh YP (2009) Red data book of Ukraine. Plant kingdom, Kyyiv, Ukraine
Dios MM, Moreno G, Zamora JC, Altés A (2017) Algunos hongos gasteroides epigeos interesantes de Catamarca (Argentina). Lilloa 54(2):1–10
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32(5):1792–1797. https://doi.org/10.1093/nar/gkh340
Franco-Molano AE (1993) Studies on Cystoderma: a new species and a new combination. Mycologia 85:672–676. https://doi.org/10.2307/3760512
Garnica S, Weiss M, Walther G, Oberwinkler F (2007) Reconstructing the evolution of agarics from nuclear gene sequences and basidiospore ultrastructure. Mycol Res 111(9):1019–1029
Grau A, Brown A (2000) Development threats to biodiversity and opportunities for conservation in the Mountain Ranges of the Upper Bermejo River Basin, NW Argentina and SW Bolivia. Ambio 29:445–450
Hakizimana JCR, Amalfi M, Degreef J, Desjardin D, Decock C (2023) Ripartitella degreefii (Tricholomataceae), a new species from tropical Africa. Phytotaxa 597(3):195–207
Hall TA (1999) BioEdit: a user–friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series 41(41):95–98
Hartley AJ, de Mattos-Shipley K, Collins CM, Kilaru S, Foster GD, Bailey AM (2009) Investigating pleuromutilin–producing Clitopilus species and related basidiomycetes. FEMS Microbiol Lett 297(1):24–30
He ZM, Yang ZL (2022) The genera Bonomyces, Harmajaea and Notholepista from Northwestern China: two new species and a new record. Mycological Progress 21(2):26
He ZM, Chen ZH, Bau T, Wang GS, Yang ZL (2023) Systematic arrangement within the family Clitocybaceae (Tricholomatineae, Agaricales): phylogenetic and phylogenomic evidence, morphological data and muscarine–producing innovation. Fungal Diversity 123(1):1–47
He ZM, Yang ZL (2024) 3014 Proposal to conserve the name Clitocybe (Basidiomycota) with a conserved type. Taxon. https://doi.org/10.1002/tax.13149
Heluta V, Kulsha Y, Akata I, Sapsay V (2019) A new locality record of a rare fungus Cercopemyces rickenii (Agaricales: Tricholomataceae) in Ukraine. Eurasian J For Sci 7(3):237–242
Kautmanová I (2004) Red list species of fungi held in the collections of Slovak national museum–natural history museum (BRA). I. Extinct and critically endangered species. Acta Rer Natur Mus Nat Slovakia
Kornerup A, Wanscher JH (1987) Methuen handbook of colour. ISBN: 0413334007
Largent DL (1986) How to identify mushrooms to genus I – macroscopic features. Mad River. Eureka, California. ISBN: 978–0916422004
Lechner BE (2021) Hongos de la Argentina – Comestibles vs. Tóxicos. Volúmen 1: Los hongos con laminillas y boletáceas del Partido de Pinamar. MiBo Ediciones
Malizia L, Pacheco S, Blundo C, Brown AD (2012) Caracterización altitudinal, uso y conservación de las Yungas subtropicales de Argentina. Ecosistemas 21:53–73
Matheny PB, Curtis JM, Hofstetter V, Aime MC, Moncalvo JM, Ge ZW, Slot JC, Ammirati JF, Baroni TJ, Bougher NL, Hughes KW, Lodge DJ, Kerrigan RW, Seidl MT, Aanen DK, DeNitis M, Daniele GM, Desjardin DE, Kropp BR, Norvell LL, Parker A, Vellinga EC, Vilgalys R, Hibbett DS (2006) Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98:982–995. https://doi.org/10.3852/mycologia.98.6.982
Moncalvo JM, Lutzoni FM, Rehner SA, Johnson J, Vilgalys R (2000) Phylogenetic relationships of agaric fungi based on nuclear large subunit ribosomal DNA sequences. Syst Biol 49(2):278–305
Murril WA (1917) North American Flora, vol 10, Part 2, (Agaricales). The New York Botanical Garden
Niveiro N (2012) Agaricales sensu lato (Agaricomycetes) de las Selvas del Dominio Amazónico de la Argentina. Tesis Doctoral. Universidad Nacional de Córdoba, Diversidad, Distribución y Abundancia
Niveiro N, Popof O, Desjardin D, Albertó E (2012) Mycena moconensis, a new species of section Polyadelphia from Argentina. Mycotaxon 119:167–173. https://doi.org/10.5248/119.167
Niveiro N, Popof O, Lechner B, Albertó E (2014a) Pholiota oblita, new species in sect. Adiposae stirps (Subfammans Strophariaceae), Agaricomycetes from the Argentinean Yungas. Phytotaxa 167:276–282
Niveiro N, Popof O, Albertó E (2014b) Hemimycena longipleurocystidiata (Mycenaceae, Agaricomycetes), a new species from the Argentinean Atlantic Forest. Phytotaxa 177:49–55. https://doi.org/10.11646/phytotaxa.177.1.4
Niveiro N, Zuliani P, Ramírez NA, Popoff OF, Albertó EO (2014c) Hongos agaricoides de las Yungas argentinas. Clave De Géneros Lilloa 51(1):74–86
Nouhra E, Soteras F, Pastor N, Geml J (2018) Richness, species composition and functional groups in Agaricomycetes communities along a vegetation and elevational gradient in the Andean Yungas of Argentina. Biodivers Conserv 27:1849–1871
Putra IP, Hermawan R, Sibero MT, Puspita Sari AA, Nurhayat OD (2022) Morphological and molecular study of Lepista sordida in Indonesia. Philipp J Sci 151(4):1333–1336
Rambaut A (2009) FigTree v1. 3.1. http://tree.bio.ed.ac.uk/software/fgtree/. Accessed 1 Sep 2023
Saar I, Põldmaa K, Kõljalg U (2009) The phylogeny and taxonomy of genera Cystoderma and Cystodermella (Agaricales) based on nuclear ITS and LSU sequences. Mycol Prog 8:59–73
Senn–Irlet B (2011) European Council for the Conservation of Fungi. European Red List of endangered macrofungi. Red List candidates. https://www.wsl.ch/eccf/candlist-subtotals.xls
Singer R (1950a) Die höheren Pilze Argentiniens. Bulletin Suisse De Mycologia 28(1):181–196
Singer R (1953) Four years of mycological work in southern South America. Mycologia 45:865–891
Singer R (1955) New and interesting species of Basidiomycetes IV. Mycologia 47(5):763–777. https://doi.org/10.2307/3755585
Singer R (1959) New and interesting species of Basidiomycetes VI. Mycologia 51(3):375–400. https://doi.org/10.2307/3756058
Singer R (1960) Monographs of South American Basidiomycetes, especially these of the east slope of the Andes and Brazil. 3. Reduced Marasmioid genera of South America. 1. The genus Gloiocephala, Manuripia, Epicnaphus and Hymenogloea and their taxonomic position. Sydowia 14:258–280
Singer R (1965a) Monographs of South America Basidyomycetes, especially those of the east slope of the Andes and Brazil X. Xeromphalina Bol Soc Argent Bot 10(4):302–310
Singer R (1965b) Monographic studies on South American Basidiomycetes, especially those of the east slope of the Andes and Brazil. 2. The Genus Marasmius in South America. Sydowia 18(1–6):106–358
Singer R (1969) Mycoflora australis. Beih. Nova Hedwigia 29:1–405
Singer R (1970) Omphalinae (Clitocybeae, Tricholomataceae, Basidiomycetes). Fl Neotropica Monogr 3:1–84
Singer R (1973a) The genera Marasmiellus, Crepidotus, and Simocybe in the Neotropics. Beih Nova Hedwigia 44:1–517
Singer R (1973b) Diagnoses fungorum novorum Agaricalium III. Beih Sydowia 7:1–106
Singer R (1975) The neotropical species of Campanella and Aphyllotus with notes of some species of Marasmiellus. Nova Hedwigia 26:847–896
Singer R (1976) Marasmieae (Basidiomycetes, Tricholomataceae). Fl Neotropica Monogr 17:1–347
Singer R (1986) The Agaricales in Modern Taxonomy. Koeltz vol. 4. ISBN: 9783874292542
Singer R, Digilio APL (1951) Pródromo de la flora agaricina Argentina. Lilloa 25:6–461
Singer R, Morello JH (1960) Ectotrophic forest tree mycorrhizae and forest communities. Ecology 41:549–551
Singer R (1950b) Type Studies on Basidiomycetes IV. Lilloa 23:147–246
Sjökvist E, Larsson E, Eberhardt U, Ryvarden L, Larsson KH (2012) Stipitate stereoid basidiocarps have evolved multiple times. Mycologia 104(5):1046–1055
Spegazzini C (1912) Mycetes argentinenses (series VI). Anales Del Museo Nacional De Historia Natural De Buenos Aires 23:167–244
Spegazzini C (1919) Los Hongos del Tucumán. Primera Reunión Nacional de la Sociedad Argentina de Ciencias Naturales, Tucumán, pp. 254–274
Steiner JJ, Poklemba CJ, Fjellstrom RG, Elliott LF (1995) A rapid one–tube genomic DNA extraction process for PCR and RAPD analyses. Nucleic Acids Res 23:2569
Thiers B (2012) Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih. Accessed July 2023
Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li DZ, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Glashütten: Koeltz Botanical Books. https://doi.org/10.12705/Code.2018
Varga T, Krizsán K, Földi C, Dima B, Sánchez-García M, Sánchez-Ramírez S, Nagy LG et al (2019) Megaphylogeny resolves global patterns of mushroom evolution. Nature Ecology & Evolution 3(4):668–678
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
Vizzini A, Contu M, Ercole E (2011) Musumecia gen. nov. in the Tricholomatoid clade (Basidiomycota, Agaricales) related to Pseudoclitocybe. Nordic Journal of Botany 29(6):734–740
Wang S, Guo H, Li J, Li W, Wang Q, Yu X (2019) Evaluation of five regions as DNA barcodes for identification of Lepista species (Tricholomataceae, Basidiomycota) from China. PeerJ 7:e7307. https://doi.org/10.7717/peerj.7307
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Academic Press, San Diego, pp. 315–322. https://doi.org/10.1016/B978-0-12-372180-850042-1
Wicaksono CY, Aguirre-Guitierrez J, Nouhra E, Pastor N, Raes N, Pacheco S, Geml J (2017) Contracting montane cloud forests: a case study of the Andean alder (Alnus acuminata) and associated fungi in the Yungas. Biotropica 49:141–152. https://doi.org/10.1111/btp.12394
Wilson AW, Hosaka K, Mueller GM (2017) Evolution of ectomycorrhizas as a driver of diversification and biogeographic patterns in the model mycorrhizal mushroom genus Laccaria. New Phytol 213(4):1862–1873
Wright JE, Albertó E (2002) Guía de hongos de la región pampeana. I. Hongos con laminillas. L.O.L.A., Buenos Aires. ISBN: 978–9–509725–46–1
Zapater MA, Del Castillo EM, Pennington TD (2004) El género Cedrela (Meliaceae) en la Argentina. Darwiniana, Nueva Serie 42(1–4):347–356
Zhang M, Li TH, Wei TZ, Liang XS, Liu ZX (2019) Ripartitella brunnea, a new species from subtropical China. Phytotaxa 387(3):255–261
Zhang M, Gao XL, Mu LQ, Deng WQ (2023) Morphology and molecular phylogeny reveal five new species of Laccaria (Hydnangiaceae, Agaricales) from Southern China. J Fungus 9(12):1179
Zuloaga FO, Morrone O (1999) Catálogo de las plantas vasculares de la República Argentina II. Missouri Botanical Garden Press
Acknowledgements
Additionally, we extend our heartfelt appreciation to the park rangers of Baritú National Park and El Nogalar De Los Toldos National Reserve for their invaluable collaboration during the sampling conducted within the reserve. Their support, provision, and the use of facilities in Lipeo greatly contributed to the discovery of a significant number of collections. Our appreciation is also extended to M. Collazo for his computing assistance.
Funding
CONICET and UBA provided funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the studies described in the paper and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All authors confirm that no research involving humans or animals was involved in the current study, that there are no issues relating to animal welfare relating to the current study, and that they have approval to participate in the current study.
Consent for publication
All authors have given explicit consent to the submitted paper and to the inclusion of their data in it.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Zhu-Liang Yang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martínez, A.P., Suárez, J.M., Aliaga, J. et al. New species from Argentinean Yungas, Cercopemyces messii and Clitocybe cedrelae (Agaricomycetes, Agaricales). Mycol Progress 23, 33 (2024). https://doi.org/10.1007/s11557-024-01971-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-024-01971-3