Abstract
Supercooling preservation of fruits and vegetables (FV) is critical to food freezing. Magnetic field (MF)-assisted freezing of FV promotes supercooling; but its phenomenon is yet to be uncovered. Therefore, information on the supercooling phenomenon of MF-assisted freezing and its impacts on the quality preservation of frozen FV is critical to cellular food freezing manufacturing practices. This study reported on the supercooling phenomenon of MF-assisted freezing and its impacts on the quality preservation of frozen FV. Intrinsic factors (hydrogen bonding ordering and geometry) related to product, and extrinsic factors (types of magnetic field, field intensity, and exposure time) related to process parameters, that influenced supercooling were discussed. The study revealed that the occurrence of supercooling during MF-assisted freezing depends mainly on the types of magnetic field applied, field intensity and the direction of the applied field, which affects the effective magnetic lines of force resulting to uncompensated electron spins through samples. The exhibition of electron spins increases the order of magnetic ions and water molecules contained in cellular foods. For process design, more in-depth study and accurate understanding of the supercooling phenomenon of MF-assisted freezing and its impacts on the quality preservation of frozen FV are essential. It is hoped that this study provide better insight on the supercoling phenomenon of MF-assisted freezing and its impacts on the quality preservation of frozen FV for further studies.
Practical Applications: Application of high intensity magnetic field to cellular food freezing assists supercooling phenomenon, with advantage of enhancing quality. But the development as well as market acceptance of the technology is low because the supercooling phenomenon is not well-understood. Currently, insightful studies on the supercooling phenomenon of magnetic field-assisted freezing and its impacts on quality preservation of fruits and vegetables have been unveiled. The studies revealed that the strong magnetic field assistance to freezing is possible through the exhibition of electron spins and re-ordering of magnetic ions of water molecules contained in cellular foods. However, the results outlined in this study offer comprehensive insights into the supercooling phenomenon of magnetic field-assisted freezing and its impacts on the freezing process and the quality preservation of fruits and vegetables, offering valuable guidance for future developments of strong magnetic field-assisted freezing technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
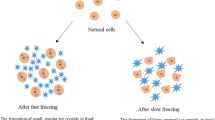
Freezing helps to preserve the nutritional content of fruits and vegetables by minimizing the loss of vitamins, minerals, and other essential nutrients [1,2,3,4,5,6,7]. Freezing is often considered as a better preservation method compared to other methods that may involve heat, which can lead to nutrient degradation. However, the undesirable loss of nutrients, color, and texture is a consequence of the physical changes that take place when freezing occurs due to the formation of ice crystals [8,9,10,11,12,13]. Therefore, the crystallization of ice plays a crucial role in determining both the efficiency of freezing and the quality of frozen products. The process of ice crystallization can be classified into two categories: (1) a slow process and (2) a fast process. A slow process results in the formation of large ice crystals that can harm cells and lead to a decline in quality. Conversely, a fast process promotes the generation of finely dispersed small ice crystals within the food matrix, which is preferable for preserving fruits and vegetables [1, 10, 14,15,16]. Conventional freezing methods like contact plate freezing and air blast freezing are slow processes that demand significant energy and incur high costs [13]. Moreover, when freezing is carried out using conventional methods, the crystallization processes are significantly impacted, and the qualities of final products are notably diminished [17,18,19,20].
In recent years, physical fields, such as magnetic fields, have been employed to facilitate rapid freezing - a technique used in the food industry to control freezing process, and preserve the quality, texture, and nutritional value of perishable products like fruits and vegetables. Unlike slow freezing, which can cause large ice crystals to form and damage cell structures, rapid freezing quickly lowers the temperature of the product to well below its freezing point. This process helps in maintaining the freshness and extending the shelf life of the product, making it a preferred method in food preservation. This approach offers cost-effective and energy-efficient solutions [21,22,23,24]. In addition, the utilization of a magnetic field in the freezing process, referred to as Magnetic Field-Assisted Freezing (MFAF), induces supercooling - a phenomenon wherein the product’s temperature is lowered below its freezing point without ice formation. In the context of MFAF preservation, particularly with fruits and vegetables, supercooling is achieved by maintaining the product in a supercooled state at temperatures where it would typically freeze. This is advantageous because it prevents ice crystal formation, which can damage the cell structure of the produce. This, in turn, improves the quality of the frozen product once freezing eventually occurs, by leveraging on magnetic wave vibrations [16]. The disruptive effect of magnetic wave vibrations on the intra-cluster hydrogen bonds of water molecules present in fresh fruits and vegetables results in an increased number of hydrogen bonds and a decreased in self-diffusion coefficient. This process lowers energy level and promotes supercooling [16, 17].
By design, a magnetic field is created by passing a current through a coil of wire wound around a magnetic core, typically made of iron or steel, with numerous turns, in accordance with Ampere’s law, as expressed in the following equation:
Where;
\(\:B\) = Magnetic flux density (tesla) or Magnetic field
\(\:{\mu\:}_{o}\) = Permeability of free space (\(\:4\pi\:\times\:{10}^{-7}\))
\(\:N\) = Number of turns of wire
\(\:L\) = Number of turns per unit length (m)
\(\:I\) = Current flowing through the wire (A)
Fleming’s right-hand rule dictates this law, proposing that when a current traverses a magnetic field, it produces an electromotive force (EMF). The EMF, often termed magnetic field strength, is contingent on both the number of wire turns (N) and the current (I) running through the wire. Exposure of EMF to foods induces advantageous alterations in their physical and chemical properties [10, 25].
Interestingly, fresh fruits and vegetables possess cellular water that exhibits diamagnetic properties which enable the induction of a magnetic dipole moment within their mass when it comes in contact with an external magnetic field [26]. The magnetic dipole moment gives rise to robust inter-molecule hydrogen bonds, altering the physical properties (such as viscosity and surface tension), electromagnetic characteristics (including refractive index, dielectric constant, and electrical conductivity), thermodynamic attributes (such as enthalpy of vaporization and heat capacity), and dynamic features (like the self-diffusion coefficient) of cellular food materials [27,28,29,30,31]. In the year 2010, it was reported that MF in the order of 14 T affects the optical properties of food model and the effects remain for considerable time after the magnetic field is removed [31]. Some literatures on the use of magnetic fields to increase the number of hydrogen bonds and ordered stable configuration in cellular foods and aqueous solutions are available [5, 11,12,13, 25, 27, 32, 33]. The findings of Zhang et al. (2010) revealed the increase of hydrogen bonding of water molecule under magnetic field of 10 T. They noted that the presence of increased hydrogen bonding and the ordering of water molecules can result in modifications of water properties, such as an elevation in the freezing point. An increased in freezing point causes solidification of 0.5 g of distilled water under a magnetic field of 0.5 T (Aleksandrov et al., 2000). Aside from water, a rise in the freezing point under the impact of a magnetic field was also documented in certain diamagnetic food models [13].
In the food industry, there are typically three types of magnetic fields utilized: permanent or static magnetic field (SMF), resonance or oscillating magnetic field (OMF), and pulsed magnetic field (PMF). Figure 1 illustrates the various types of magnetic fields employed in the freezing of cellular foods. The static magnetic field (SMF) is present nearly ubiquitously across the planet, with its intensity ranging from 25 to 65 mT [13, 34]. SMF can be categorized into two types: (a) attractive static magnetic field (ASMF), and (b) repulsive static magnetic field (RSMF). The former extends the phase transition time, while the latter is reported to shortens the phase transition time during the freezing of food models [27]. Mok et al. (2015) reported that RSMF (of 480 and 50 mT) reduced phase transition time of 0.9% NaCl by 32.1% and 42.0%, when compared to the control (2215 ± 16 s), and ASMF (2593 ± 15 s). This phenomenon arises from the distortion of hydrogen bonds caused by the formation of weak polygonal rings under unidirectional ASMF, leading to a high degree of supercooling. Also, the cryoprotective effects of SMF on human dental pulp stem cells (DPSCs) are observed during the cryopreservation process [23]. Lin et al. (2015) showed that the survival rates of the thawed DPSCs increased 2- or 2.5-fold when the cells were exposed to SMF of 0.4 T or 0.8 T, respectively. The results also indicated that the cryoprotective impact of static the SMF was attributed to an enhancement in the biophysical stability of the cell membrane within the supercooled zone. In the same vein, certain investigations demonstrated that freezing aided by OMF encourages supercooling, preserving the quality of cellular foods through rapid freezing and the generation of finely uniform ice crystals throughout the entire food volume [10, 14, 34, 35]. Enzo-Aldoradin (2019), reported that OMF in-conjunction with cells alive system (CAS) freezer improved the supercooling process and quality of frozen mango when compared with samples frozen without OMF.
In the case PMF–assisted freezing, Iwasaka et al. (2011) reported that magnetic field of up to 325 T/s at 6.5 mT causes more uniform grains in aqueous solutions than the samples frozen without PMF. The findings hypothesized that a time-varying magnetic field is one of the factors which affect food supercooling.
However, the supercooling phenomenon of magnetic field-assisted freezing remains unclear. Although, various literatures have been reviewed and published. In 2010, [36] reviewed the effect of electric and magnetic field on freezing and possible relevance in freeze drying. In 2017, [13] published an overview on magnetic field and electric field interactions with ice crystallization; application in the case of frozen food. More so, for an in-depth knowledge on ice nucleation control during freezing using emerging technologies, [8] provided a review on the control of ice nucleation by ultrasound waves, electric and magnetic fields. In spite of the above; to the best of our knowledge, no study about the supercooling phenomenon of magnetic field-assisted freezing and its impacts on the quality of preservation of fruits and vegetables have been conducted. Therefore, in the current study, the fundamentals including the relevance and supercooling phenomenon of magnetic field-assisted freezing and its impacts on the quality of preservation of fruits and vegetables are introduced, intrinsic factors (i.e. hydrogen bonding ordering and geometry) related to product, and extrinsic factors (i.e. magnetic field types, magnetic intensity, and magnetic treatment time) related to magnetic process parameters, that influence the efficacy of magnetic field-assisted freezing were discussed. The impact of a magnetic field on the quality of frozen fruits and vegetables were emphasized. It is anticipated that the insights into the supercooling phenomenon of magnetic field-assisted freezing and its impacts on the quality of preservation of fruits and vegetables will provide valuable information for the food industry.
Fundamentals of Magnetic Fields-assisted Freezing
Relevance of Magnetic Fields to Freezing
The relevance of MF to freezing of FV includes non thermal treatment for enzyme inhibition [37], promotion of supercooling [15], and control of ice nucleation [8, 9]. The cellular water enclosed within the cell structure of fresh fruits and vegetables promotes the proliferation of pathogenic and spoilage organisms, resulting in abnormal chemical reactions [6]. The chemical reactions affect physiological properties and shelf-life of fresh FV [3]. In a ‘standard’ storage procedure, [18] observed significant growth of L. monocytogenes on fresh-cut cantaloupe (∼0.8 log) and fresh-cut mango (∼0.6 log). Subjecting fresh fruits and vegetables to a magnetic field (MF) can decrease the growth of spoilage organisms and chemical reactions. For example, an MF of 396.8 mT has been demonstrated to potentially reduce microbial loads from 8.40 × 105 to 5.86 × 105 CFU/ml, 7.03 × 105 to 5.89 × 105 CFU/ml and 6.00 × 105 to 4.0 × 105 CFU/ml (at P ≤ 0.05) for cooled jute mallow, fluted pumpkin and bitter leaf respectively, and significantly preserved their quality [2]. [37] reported that employing static magnetic field cold-water shock treatment did not necessarily influenced supercooling, but resulted in increased dismutation activities of catalase and superoxide, leading to a reduction in malondialdehyde compared to the conventional cold-water shock treatment technique. Furthermore, the application of a magnetic field induced forces of magnetic vibration in cellular water molecules within plant-based materials, delaying the initial formation and growth of ice crystals, thereby fostering a high degree of supercooling [13, 14, 38,39,40]. MF can penetrate deeply into the cells of fruits and vegetables, elongating their cell walls, thereby promoting a high nucleation rate. Increased nucleation rate reduces phase transition time and exerts control over ice nucleation [16]. The controlled crystallization phenomenon holds promise as an approach to enhance the efficiency of the freezing process and prevent damage to fresh fruits and vegetables caused by conventional freezing. Table 1 shows the effects of MF on the supercooling process and quality of supercooled FV.
Supercooling Phenomenon of Magnetic field-assisted Freezing of Fruits and Vegetables
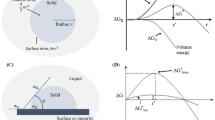
The supercooling phenomenon of magnetic field-assisted freezing can be adequately explained in the three (3) primary stages of freezing. The first stage involves pre-freezing or cooling the product from the initial temperature to the freezing temperature. The second stage is known as the freezing stage or phase change stage, and the third stage is called the sub-cooling stage or reduction to the storage temperature [4]. The phase change stage holds particular significance among the three stages, as it involves crystallization. As highlighted in the introduction, the preferred method, for promoting quality during freezing of cellular foods, is to generate small ice crystals that are evenly dispersed throughout the product [42,43,44,45,46,47, 32, 33, 48]. Achieving small ice crystals necessitates inducing significant supercooling in the product slated for freezing. Typically, the decision to apply a magnetic field during freezing is rooted in its potential to sustain the product in a supercooled state (unfrozen) at a temperature below its freezing point. A greater degree of supercooling (the difference between freezing and nucleation temperatures) results in higher nucleation rates and a shorter phase transition time [15, 16]. For example, Zhang et al. (2021) reported that PMF can significantly cause electron spins of cellular water, promotes high nucleation rates and short phase transition time, leading to creation of small ice crystals in cucumber tissue. Subsequently, the presence of smaller ice crystals minimizes cellular damage and enhances the characteristics of frozen products in contrast to the effects of conventional slow freezing techniques. Therefore, if the use of a magnetic field during the freezing of fresh fruits and vegetables leads to a higher degree of supercooling, its mechanisms can be examined based on the predominant constituents of cellular foods—specifically, magnetic elements or ions and water. Fruits and vegetables comprise a significant amount of water molecules, often ranging from 80 to 90% in content [4]. When dipolar water molecules are subjected to an external magnetic field, an electromotive force is applied to either charge of the polar molecule, inducing vibration or rotary movement of the charge center in the direction of the field [29]. According to thermodynamic principles; the faster the movements of molecules, the more rapid the heats transfer. Consequently, the vibration of magnetic elements induced by the electromotive force enhances the heat conductivity of cellular water. This increased heat conductivity elevates the degree of supercooling, thereby regulating the formation and growth of ice crystals [29]. Ice crystals containing parental water molecules exhibit numerous ordered hydrogen-bonded chains, facilitating significant permeability to magnetic ions. This phenomenon accelerates the descent of product freezing points at a much faster rate (up to ten fold per each degree Celsius) than the local temperature [40]. A heightened nucleation rate facilitates the rise of magnetic ions, reduces the self-diffusion coefficient, and consequently enhances the tendency of water molecule clustering. The clustering of water molecules results in the formation of numerous small ice crystals arranged uniformly within the sample [30]. Figure 2 shows a schematic supercooling phenomenon of MF-assisted freezing of FV: (a) supercooling process, and (b) water molecular cluster.
In summary, the supercooling phenomenon in magnetic field-assisted freezing involves maintaining the product in a supercooled state below its freezing point to achieve higher nucleation rates and smaller, uniformly distributed ice crystals, which enhance the quality of frozen foods. This is accomplished by applying a magnetic field that induces molecular vibrations and improves heat conductivity, leading to rapid nucleation and reduced cellular damage compared to conventional freezing methods.
Factors Influencing Supercooling during Magnetic Field Assisted Freezing of Fruit and Vegetables
The extent of supercooling in Magnetic Field-Assisted Freezing (MFAF) of fruits and vegetables is contingent on various factors. These include product-related factors such as hydrogen bonding ordering and geometry, as well as process parameters like magnetic field types, magnetic intensity, and magnetic treatment time. As an efficient non-thermal method for supercooling cellular foods, the magnetic field technique is employed in freezing to enhance the polar charge of cellular water. This process disrupts bonding through spontaneous changes in internal energy and heat capacity [25]. The reorientation of magnetic elements results in the alignment of water molecules, leading to the generation of high temperature and pressure. This accelerates heat transfer through the electromotive force exerted on \(2{H^ + }\) and \(\:{O}_{2}^{-}\) ions and molecules [16]. Due to the thermal motion of water molecules, hydrogen intermolecular bonds weaken as the water temperature rises, resulting in the formation of smaller clusters with a greater number of neighbors [21]. As numbers of neighbors increase, thermal motion of water molecule becomes stronger [49]. The robust thermal motion of water molecules aligns with the thermodynamic principle of magnetized plant-based materials, wherein hydrogen bonding in cellular water supports the paramagnetic nature of food materials [15, 49]. The paramagnetic characteristics of magnetized plant-based materials result in the generation of Lorentz force, acting on the charge center and causing a rotation in its motion. This rotation of the charge center leads to a greater separation between bonded molecules, thereby causing an augmentation in thermal motions [50]. As the thermal motions of the bonded molecules increase, the strength of hydrogen bonding diminishes. Therefore, the weaker the hydrogen bonds become, the greater the supercooling, and vice versa.
Moreover, fruits and vegetables exhibit intricate compositions with diverse geometries, and these compositions play a role in influencing supercooling. The supercooling process in fruits and vegetables is notably influenced by the efficiency of heat transfer, which varies with product geometry, encompassing factors like size and shape. Research exploring the influence of product geometry on the supercooling in Magnetic Field-Assisted Freezing of commonly found fruits and vegetables is accessible [20, 30]. Supercooling is influenced by the size or shape of products due to variations in the length of mass transfer, particularly concerning common fruits and vegetables. Larger samples especially cut or minimally processed products, demonstrate slower supercooling behavior owing to their lower heat transfer efficiency. Conversely, smaller samples exhibit higher heat transfer efficiency, facilitating faster supercooling by minimizing mass transfer resistance [30]. Panayampadan (2022) investigated the effects of alternating magnetic field (AMF; 2.4 mT, 5.6 mT, and 8.8 mT) and cube size (2 cm, 3 cm, and 4 cm) on the freezing characteristics of minimally processed guava. Their findings revealed significant reductions in phase transition times of frozen guava cubes totaling 62.4%, 44.71%, and 27.36% for cube 4 cm, 3 cm, and 2 cm under AMF intensity of 2.4 mT, 5.6 mT, and 8.8 mT, respectively. The study indicated that for the freezing of different sizes of raw materials, heat transfer efficiency is the main influencing factor.
Figure 2 show the freezing curve of the frozen guava cubes of different sizes. Leng et al.(2022) investigated and compared the effects of SMF (0–45 mT) on four different kinds of cellular foods (i.e. apple, peach, cucumber, and Indian jujube) under the same experimental conditions. Their results show that SMF tended to decrease the phase transition time as well as the ice crystals size for all the four kinds of cellular foods, and the optimal SMF intensity varied with the types of foods.
Magnetic field process parameters such as magnetic types (i.e. OMF, PMF, and SMF), and intensity influence supercooling of FV [34]. Tang et al. (2020) reported that the supercooling behavior and inhibition of ice nucleation during MFAF of cherry are influenced by the types of magnetic fields and their strength. Among other magnetic types applied, SMF (at 10 mT) is considered for the MFAF of cherry because of its achievement in promotion of supercooling, reduction in drip loss and cellular damage. Figure 3 show the drip loss variation and microstructure of cherry frozen under two different magnetic fields with different intensities.
Supercooling extension during freezing of guava cubes of different sizes (a) without magnetic field, (b) at 2.4 mT, (c) at 5.6 mT, and (d) at 8.8 mT. FT represents the temperature of the freezer [30]
Depending on the field intensities applied, enhancement of supercooling, and decrease in freezing point of avocado puree [41], mango [14], and sodium chloride [27] that were supercooled by MFAF have also been reported. According to [41], the freezing point of avocado puree was decreased from − 1.2 to -6 °C, under magnetic field (at at 4 mT and 50 Hz), which indicated that the supercooling was enhanced. Among many field intensities (up to 100 mT), Kang et al. (2021) observed that OMF intensity of 50 mT increased the vibration of water molecules within mango slices and extended its supercooled state at − 5 °C. [27] liken 0.9% sodium chloride (NaCl) to a biological solution was supercooled using magnetic flux densities of range 0 to 480 mT. Their findings revealed that diamagnetic properties of water and ionic interactions, Na+ and Cl− ions cause the hydrogen bonding to be disrupted under the magnetic field, leading to molecular vibration of the ions and molecules. More so, increase of magnetic field (up to 7.2 mT) delays the phase change time, leading to a shorter phase transition duration of carrot [24].
The duration of magnetic field treatment is an additional factor that impacts supercooling in the process of Magnetic Field-Assisted Freezing MFAF of fruits and vegetables. Wang et al. (2013) indicated that increase in magnetic treatment time, the lower the friction coefficient, and the weaker the hydrogen bonding of water system.
All the above studies form the basis that magnetic fields have great potentials to influence the supercooling cellular foods system.
Impacts of Magnetic Field on the Quality Preservation of Frozen Fruits and Vegetables
External magnetic fields are reported to have potentials to promote the quality of fruits and vegetables after freeze-thawing ( [10, 30, 37]. [30] observed that alternating magnetic field significantly reduces drip loss of frozen guava when compared to the sample frozen without magnetic field. [37] developed a hurdle food preservation technique by combining SMF and low-temperature water shock (SMFT) to preserve cucumbers. Their results show that SMFT (at 70 Gs, 2oC, 40 min) lowered weight loss when compared with the ones cooled under cold-water shock treatment during storage. And the decay incidence of SMFT (42.8%) and cold-water shock treatment (52.9%), indicated 10.1% reduction by magnetic field, and confirmed that SMFT had positive influence on the preservation of quality of cucumbers. [41] investigated the effect of OMF (at 4 mT) on the freezing of avocado puree. Their findings revealed that small molecular current, exerted by the magnetic field had improvement on the quality of freeze-thawed avocado puree.
In another study by [34], application of permanent magnetic field (PMF) ranging from 0 to 20 mT and alternating magnetic field (AMF) ranging from 0 to 2 mT during freezing (at -30 oC) reduces drip loss of cherry from 10.39 to 4.64% when compared with conventional freezing. Figure 4 shows drip loss variation and microstructure of the cherry frozen under two different magnetic fields with different intensities. Contrarily, [10] reported that OMF set at 0, 30%, 50%, 75% and 100% in conjunction with cell alive freezing has no significant effect on the drip loss value when compared with conventional freezing of mango. This occurred because the frozen mangoes were thawed immediately after freezing (without storage). Thus, the liquid released from the interior to the exterior of the mango due to damaged fruits, may be strictly due to freezing in the CAS freezer whereas drip loss can only occur during frozen storage. [20] reported better frozen quality of four different kinds of fruits and vegetables after exposing them to SMF-assisted freezing (at 0–45 mT, and temperature: -5oC). Their findings revealed the positive effects of SMF on micro and macro-scale parameters including ice crystal size, drip loss and texture. Utilization of SMF in freezing maintained vitamin C content and reduced the loss of juice and total soluble solids of Korla fragrant pear [38]. All the above observations emphasize the positive effect off magnetic field on promoting freezing rate and improving the final quality of fruit and vegetables.
Drip loss variation and microstructure of cherry frozen under two different magnetic fields with different intensities (a) with PMF, and (b) with AMF [34]
Conclusion and Future Prospects
Over the last decade, an increasing number of scientific studies on the application of magnetic field to promote supercooling, control ice nucleation and preserve frozen quality of fresh fruits and vegetables have been published. Up till date, there exists no clear evidence on the supercooling phenomenon of magnetic field-assisted freezing and its corresponding effect on the quality preservation of frozen-thawed FV. To give a better insight on this subject, critical information is needed. The current study reported on the supercooling phenomenon of magnetic field-assisted freezing and its effect on the quality preservation of frozen-thawed FV. Important factors such as the hydrogen bonding ordering, product’s geometry, field intensity, field types, and field treatment time were proved to influence supercooling phenomenon during MFAF of FV. Overall, there are still some areas that need urgent attention for future studies. The studies on the effect of MF on the crystallization process of FV under MFAF suggested numbers of replicated experiment to be able to understand the stochastic nature of ice nucleation. Moreover, when freezing plant-based materials, their heterogeneous nature should be carefully considered for experimental design and analysis.
For more understanding of the underlying physic of MF promoted supercooling, intrinsic factors (i.e. hydrogen bonding system, size, shape and sample composition), and extrinsic factors (i.e. field strength, frequency and system temperature) that can influence supercooling process should be clarified by experiments. To be able to do this, experiments should be first conducted in simplex matrix, i.e. in pure water. This will assist in clear understanding of the mechanisms involved and interpret any observed results. While discerning the individual effect of SMF, OMF, and PMF in freezing, both critical field strength and frequency values, and any desirable interrelation between them should be studied. The information on the effect of field type, field strength, and frequency on the supercooling of foods with different sizes would assist in the development of appropriate MFAF technology.
Data Availability
No datasets were generated or analysed during the current study.
References
K.P. Alabi, P. Ayoola, M. Olalusi, Adesoji, Olaniyan, and and, L.O.G. Fadeyibi, Adeshina, Effects of osmotic dehydration pretreatment on freezing characteristics and quality of frozen fruits and vegetables. J. Food Process Eng. 1–13 (2022). https://doi.org/10.1111/jfpe.14037
K. Alabi, A. Peter, Fadeyibi, O. Faith Tinuade, Magnetic field Hydrocooling System: Effect of Field intensities on the cooling characteristics of three different Leavy vegetables. Innovative Food Sci. Emerg. Technol. 86, 1–8 (2023). https://doi.org/10.1016/j.ifset.2023.103347
K. Alabi, R.A. Peter, Oladipupo, T. Obateru, Faith, and Adedamola Musbaudeen, Novel pre-cooling techniques and their effect on the quality of cooled fruits and vegetables. J. Agricultural Eng. Technol. (JAET). 26(1), 88–103 (2021)
K. Alabi, Z. Peter, Zhu, S. Da Wen, Transport Phenomena and their effect on microstructure of frozen fruits and vegetables. Trends Food Sci. Technol. 101, 63–72 (2020). https://doi.org/10.1016/j.tifs.2020.04.016
V.D. Aleksandrov, A.A. Barannikov, N.V. Dobritsa, Effect of Magnetic Field on the Supercooling of Water drops. Inorg. Mater. 36(9), 895–898 (2000)
I. Arah, G.K. Kojo, E.K. Ahorbo, E.K. Anku, Kumah, and Harrison Amaglo, Postharvest Handling Practices and Treatment Methods for Tomato Handlers in developing countries: a Mini Review. Adv. Agric. 1–8 (2016). https://doi.org/10.1155/2016/6436945
H. Arteaga, A.C. Silva, De Sousa, Tambelli, Caio Eduardo De Campos, Souto, Sergio, and Coster, Ernane Jose Xavier. 2022. Using pulsed magnetic fields to improve the quality of frozen blueberry: a Bio-impedance Approach. LWT - Food Sci. Technol. 169:1–11
N. Dalvi-isfahan, Mohsen, E. Hamdami, Xanthakis, A. Le-bail, Review on the control of ice nucleation by Ultrasound waves, Electric and magnetic fields. J. Food Eng. 195, 222–234 (2017)
M. Dalvi-Isfahan, P.K. Jha, J. Tavakoli, A. Daraei-Garmakhany, E. Xanthakis, Alain Le-Bail, Review on identification, underlying mechanisms and evaluation of freezing damage. J. Food Eng. 255, 50–60 (2019)
P. Enzo-Aldoradin, F. Esteban, C. Mayo, José Miguel Alemán Polo, Angel Perea De La Matta, Javier Sánchez Espinoza, Javier Castillo Alva. 2019. Effect of freezing with oscillating magnetic fields on the physical and Sensorial Characteristics of Mango. Braz. J. Food Technol. 22:1–11
M. Iwasaka, M. Onishi, S. Kurita, N. Owada, Effects of pulsed magnetic fields on the light scattering property of the freezing process of Aqueous solutions. J. Appl. Phys. 109, 1–4 (2011)
P. Jha, E. Kumar, S. Xanthakis, V. Chevallier, Jury, and Alain Le-Bail, Assessment of Freeze damage in fruits and vegetables. Food Res. Int. 121, 479–496 (2018). https://doi.org/10.1016/j.foodres.2018.12.002
P. Jha, E. Xanthakis, V. Jury, and Alain Le-Bail, An overview on Magnetic Field and Electric Field interactions with ice crystallisation; application in the case of frozen food. Crystals. 7(10), 299–310 (2017)
T. Kang, Y. You, R. Hoptowit, M.M. Wall, and Soojin Jun, Effect of an oscillating magnetic field on the inhibition of ice nucleation and its application for supercooling preservation of fresh-cut mango slices. J. Food Eng. 300, 1–12 (2021). https://doi.org/10.1016/j.jfoodeng.2021.110541
T. Kang, Y. Youngsang, and and Soojin Jun, Supercooling Preservation Technology in Food and Biological Samples: a review focused on Electric and magnetic field applications. Food Sci. Biotechnol. 29(3), 303–321 (2020). https://doi.org/10.1007/s10068-020-00750-6
M. Kaur, and Mahesh Kumar, An Innovation in magnetic field assisted freezing of perishable fruits and vegetables: a review. Food Reviews Int. 36(8), 761–780 (2020). https://doi.org/10.1080/87559129.2019.1683746
R. Kobayashi, and Toru Suzuki, Effect of supercooling accompanying the freezing process on ice crystals and the Quality of Frozen Strawberry tissue. Int. J. Refrig. 99, 94–100 (2019). https://doi.org/10.1016/j.ijrefrig.2018.11.045
B. Kroft, S.B. Gu, Y.L. Ganyu, A. Shirley, P.M. Micallef, X. Nou, Effects of temperature abuse on the growth and survival of Listeria Monocytogenes on a wide Variety of whole and fresh-cut fruits and vegetables during storage. Food Control. 137, 1–32 (2022)
M. Kurokawa, T. Kasai, A. Sugino, Y. Okada, R. Kobayashi, Investigation of the key factors that affect drip loss in Japanese Strawberry cultivars as a result of freezing and thawing. Int. J. Refrig. 134, 189–196 (2022). https://doi.org/10.1016/j.ijrefrig.2021.11.004
D. Leng, C. Zhang, Hainan, Tian, P. Li, F. Kong, B. Zhan, Static magnetic field assisted freezing of four kinds of fruits and vegetables: Micro and Macro effects. Int. J. Refrig. 146(1), 1–11 (2022). https://doi.org/10.1016/j.ijrefrig.2022.10.018
C. Li, Chen Long, and, Z. Ren, Surface tensions of non-polar liquids in high magnetic fields. J. Mol. Liq. 181, 51–54 (2013). https://doi.org/10.1016/j.molliq.2013.02.010
D. Li, Z. Zhu, S. Da-wen, Effects of freezing on cell structure of Fresh Cellular Food materials: a review. Trends Food Sci. Technol. 75, 46–55 (2018)
S.-L. Lin, W.-J. Chang, C.-Y. Lin, S.-C. Hsieh, S.-Y. Lee, K.-H. Fan, C.T. Lin and, and, H.M. Huang, Static magnetic field increases Survival Rate of Dental Pulp Stem cells during DMSO-Free cryopreservation. Electromagn. Biol. Med. 34(4), 302–308 (2015)
B. Liu, J. Song, Z. Yao, R. Bennacer, Effects of magnetic field on the Phase Change cells and the formation of ice crystals in Biomaterials: Carrot Case. J. Therm. Sci. Eng. Appl. 9(3), 1–6 (2017). https://doi.org/10.1115/1.4035936
E. Xanthakis, Alain Le-Bail, and Michel Havet. 2014. Freezing Combined with Electrical and Magnetic Disturbances. In Emerging Technology for Food Processing 2nd Edition, Author: Da_Wen Sun, Elsevier Ltd.pg 563–579
Y. Wang, T. Xu, G. Tan, H. Chen, T. Li, and Dongxing Du, Effects of low-intensity DC magnetic field on the freezing process of aqueous solution and beef. Food Sci. Technol. 2061, 1–11 (2022)
J.H. Mok, W. Choi, S.H. Park, S.H. Lee, and Soojin Jun, Emerging Pulsed Electric Field (PEF) and static magnetic field (SMF) Combination Technology for Food freezing. Int. J. Refrig. 50, 137–145 (2015). https://doi.org/10.1016/j.ijrefrig.2014.10.025
L. Otero, A.C. Rodríguez, I. Morales, R. Costo, P. De, P.D. Sanz, Effect of oscillating magnetic fields on freezing of a Colloidal Dispersion of Superparamagnetic nanoparticles. J. Food Eng. 347, 111440 (2023). https://doi.org/10.1016/j.jfoodeng.2023.111440
L. Otero, A.C. Rodríguez, Miriam Pérez-Mateos, and, P.D. Sanz, 2016. Effects of Magnetic Fields on Freezing: Application to Biological Products. Comprehensive Reviews in Food Science and Food Safety 15(3):646–67. https://doi.org/10.1111/1541-4337.12202
A. Panayampadan, M.S. Saeed, R. Alam, S.K. Aslam, Gupta, S. Gagandeep Kaur, Effects of alternating magnetic field on freezing of minimally processed Guava. LWT-Food Sci. Technol. 163, 1–10 (2022). https://doi.org/10.1016/j.lwt.2022.113544
X. Pang, B. Deng, Infrared absorption Spectra of pure and magnetized water at elevated temperatures. Frontier Phys. 92(6), 1–7 (2010)
G. Zhang, W. Zhang, H. Dong, Magnetic freezing of Confined Water. J. Chem. Phys. 133, 1–6 (2010)
L. Zhang, Z. Yang, Q. Deng, Effects of pulsed magnetic field on Freezing Kinetics and Physical Properties of Water and Cucumber tissue fluid. J. Food Eng. 288, 1–8 (2021)
J. Tang, H. Zhang, C. Tian, S. Shao, Effects of different magnetic fields on the freezing parameters of Cherry. J. Food Eng. 278, 1–7 (2020). https://doi.org/10.1016/j.jfoodeng.2020.109949
G. Purnell, James Christian, and, J. James Stephen, The effects of applying oscillating magnetic fields during the freezing of Apple and Potato. Food Bioprocess. Technol. 10(12), 2113–2122 (2017). https://doi.org/10.1007/s11947-017-1983-3
M.W. Woo, A.S. Mujumdar, Effects of Electric and magnetic field on freezing and possible relevance in Freeze Drying. Drying Technol. 28(4), 433–443 (2010). https://doi.org/10.1080/07373930903202077
S. Zhao, Z. Yang, L. Zhang, N. Luo, X. Li, Effect of combined static magnetic field and Cold Water Shock Treatment on the Physicochemical properties of Cucumbers. J. Food Eng. 217, 24–33 (2018). https://doi.org/10.1016/j.jfoodeng.2017.08.011
J. Qiao, M. Zhang, Z. Fang, Jian Fu, Effect of static magnetic field assisted freezing on the productquality of Korla fragrant pear. J. Food Process Eng. 144, 1–10 (2023). https://doi.org/10.1111/jfpe.14404
L. Qiu, M. Zhang, B. Chitrakar, B. Bhandari, Application of Power Ultrasound in freezing and thawing processes: effect on process efficiency and product quality. Ultrason. Sonochem. 06, 1–13 (2020). https://doi.org/10.1016/j.ultsonch.2020.105230
A.C. Rodríguez, L. Otero, J.A. Cobos, P.D. Sanz, Electromagnetic freezing in a widespread frequency range of alternating magnetic fields. Food Eng. Rev. 11(2), 93–103 (2019). https://doi.org/10.1007/s12393-019-09190-3
Y. Tan, Y. Jin, N. Yang, Z. Wang, Z. Xie, X. Xu, Z. Jin, X. Liao, and Han Sun, Influence of uniform magnetic field on Physicochemical properties of Freeze-Thawed Avocado Puree. Royal Soc. Chem. 9(68), 39595–39603 (2019). https://doi.org/10.1039/c9ra05280a
Y. Tian, P. Zhang, Z. Zhu, S. Da Wen, Development of a Single/Dual-Frequency Orthogonal Ultrasound-assisted Rapid freezing technique and its effects on Quality attributes of Frozen Potatoes. J. Food Eng. 286, 1–11 (2020). https://doi.org/10.1016/j.jfoodeng.2020.110112
Y. Tian, Z. Zhang, Z. Zhu, S. Da Wen, Effects of Nano-bubbles and Constant/Variable-Frequency ultrasound-assisted freezing on freezing Behaviour of Viscous Food Model systems. J. Food Eng. 292, 1–10 (2021). https://doi.org/10.1016/j.jfoodeng.2020.110284
V. Vicent, F.T. Ndoye, P. Verboven, B.M. Nicolaï, G. Alvarez, Quality Changes kinetics of Apple Tissue during frozen storage with temperature fluctuations. Int. J. Refrig. 92, 165–175 (2018)
V. Vicent, P. Verboven, F.T. Ndoye, G. Alvarez, Bart, Nicolaï, A New Method developed to characterize the 3D microstructure of frozen Apple using X-Ray Micro-CT. J. Food Eng. 212, 154–164 (2017)
A. Voda, N. Homan, M. Witek, A. Duijster, G. van Dalen, Ruud, van der J. Sman, L. Nijsse, van H. Vliet, Van As, and John van Duynhoven,. 2012. The Impact of Freeze-Drying on MicrostructureRehydration Properties of Carrot. Food Research International 49(2):687–93. https://doi.org/10.1016/j.foodres.2012.08.019
J. Wu, X. Jia, and Kai Fan, Recent advances in the Improvement of Freezing Time and Physicochemical Quality of Frozen fruits and vegetables by Ultrasound Application. Int. J. Food Sci. Technol. 57(6), 3352–3360 (2022). https://doi.org/10.1111/ijfs.15744
Y. Zhang, J.H. Zhao, Y. Ding, H.W. Xiao, S.S. Sablani, Y. Nie, S.J. Wu, Xuan Ming Tang, Changes in the Vitamin C Content of Mango with Water State and Ice crystals under State/Phase transitions during Frozen Storage. J. Food Eng. 222, 49–53 (2018)
E.J.L. Toledo, C. Ramalho Teodorico, M. Zuy, Magriotis, Influence of magnetic field on Physical-Chemical Properties of the Liquid Water: insights from experimental and theoretical models. J. Mol. Struct. 888(3), 409–415 (2008). https://doi.org/10.1016/j.molstruc.2008.01.010
Y. Wang, B. Zhang, Z. Gong, K. Gao, Y. Ou, J. Zhang, The Effect of a static magnetic field on the Hydrogen Bonding in Water using Frictional experiments. J. Mol. Struct. 1052, 102–104 (2013). https://doi.org/10.1016/j.molstruc.2013.08.021
Acknowledgements
The authors are grateful to the technical staff of the Crop Processing and Storage Laboratory, Department of Food and Agricultural Engineering, Kwara State University, Malete, Nigeria. And the staff of the Department of Agricultural and Environmental Engineering, Federal University of Technology Akure, Ondo State, Nigeria.
Funding
There was no funding for the study.
Author information
Authors and Affiliations
Contributions
Kehinde Peter Alabi compiled information from the literature, analyzed the data, and wrote the manuscript. Ayoola Patrick Olalusi contributed to the editing and supervision, whereas John Isa contributed to the data gathering and co-supervision. Kehinde Folake Jaiyeoba did the editing and co-supervision.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alabi, K.P., Olalusi, A.P., Isa, J. et al. Supercooling Phenomenon of Magnetic Field-Assisted Freezing and its Impacts on Quality Preservation of Frozen Fruits and Vegetables. Food Biophysics (2024). https://doi.org/10.1007/s11483-024-09873-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11483-024-09873-3