Abstract
Legume proteins were utilized in this study to electrospin an bioactive component for use in food packaging. Firstly, the protein extraction from legumes with ultrasound assistance with the use of deep eutectic solvents (UA-DES) has been studied. The optimum extraction conditions were determined. Secondly, lentil protein isolate (LPI) and chickpea protein isolate (CPI) produced by the UA-DES were used in nanofiber production by electrospinning using polyvinyl alcohol (PVA) at different ratios. Lastly, the usability of fibers obtained from protein isolates and PVA in coating bioactive components was tested using ferulic acid (FA). The encapsulation properties of nanofibers produced at a ratio of 50:50 (PVA:PI) were investigated. The characteristic and antibacterial properties and release kinetics of the produced FA-loaded nanofibers were evaluated. The distribution of FA within the fibers was confirmed by fluorescence microscopy. Furthermore, the antibacterial properties were proven and the release kinetics of the produced FA-loaded fibers in different food simulants were determined. As a result, the final encapsulated material obtained by coating FA with nanofibers created with PVA and LPI-CPI mixtures might have suitable properties which may be used in various food applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional methods have been used to obtain protein and bioactive ingredients from plants. The disadvantage of classical extraction methods is related to solvent and energy usage, the risk of heat-labile components being destroyed, and enduring extraction [1]. Moreover, these processes are complex, expensive, and difficult to improve. Therefore, liquid–liquid extraction has been proposed as a viable alternative [2]. In the present investigation, deep eutectic solvents (DES) are used for protein extraction from green lentils and chickpeas because of their low-cost, ecological acceptability. Furthermore, they are non-volatile, non-toxic, and very stable [3]. DES is made up of substituted hydrogen bond donors such as acids, alcohols, amines, and quaternary ammonium salts. One of the most often used and recommended quaternary ammonium salts is choline chloride (ChCl) for the production of DES as it is inexpensive and easy to extract from biomass. ChCl-based DES has seen an increase in interest [4]. In the past, many DES were employed in the extraction of proteins [5,6,7,8,9].
Leguminous proteins and peptides are recognized to have antihypertensive, immunostimulating, antibacterial, and antioxidant effects [10]. For this reason, in the current study, we aimed to obtain electrospun material using legume proteins. Proteins are one of the functional products that can be used to electrospin material to create fibers [11]. Electrospinning is a technology of fiber production that may generate fibers with diameters as small as sub-micrometers and nanometers [12]. In literature, under various circumstances, zein [13], soy protein isolate [14], gelatin [15], and whey proteins [16] and have been utilized as electrospun nanofiber raw materials. Compared to polysaccharide- and lipid-based films, protein-based films offer superior mechanical and gas barrier qualities because of their distinct structure, which results in a high intermolecular binding potential. Nevertheless their reduced mechanical strength and poor water vapour resistance restrict their use in food packaging. Morevere, the electrospinning of native or denatured proteins in aqueous solutions is ineffective, and these solutions have limited electro-spinnability [17]. For this reason, fiber products can be promoted by the presence of carrier polymers. In the present study, polyvinyl alcohol (PVA) served as a polymer carrier. PVA which produces fibers with extremely desirable morphologies is a biodegradable and biocompatible polymer [18] Furthermore, [19] studied that PVA was used in the production of an orally dissolving dosage form of probiotics by high-speed electrospinning. Besides using biopolymers instead of plastics uses for food packaging is getting interest due to environmental pollution concerns [20].
Produced protein-based fibers were used for ferulic acid (FA) encapsulation in present study. FA is a hydroxycinnamic acid that is high in antioxidants. FA is approved by the FDA as an antibacterial molecule in its natural state [21, 22]. On the other hand,it has also been approved globally as a dietary additive to combat lipid peroxidation, because FA effectively scavenges the superoxide anion radical [23]. However, because of its limited solubility in water and physiochemical instability in bodily fluids, FA may exhibit poor bioavailability [24]. Transport of such bioactive molecules through biodegradable and biocompatible polymeric matrices might increase the water solubility of food additives [25]. Furthermore, micro-and nanoencapsulation could be used as an effective option for the preservation of antioxidants. Antioxidants included in packaging structures may enhance their activity, allowing them to retain an optimal impact throughout food storage because of the prolonged leaving of antioxidant compounds [26]. Electrospinning has recently been utilized to create electrospun fibers containing FA [27,28,29].
In this study, the first purpose was to protein extraction from green lentils and chickpeas using ultrasound-assisted deep eutectic solvent (UA-DES) extraction method as it is a fast, effective, environmentally friendly, and economical method. Additionally, the ideal protein extraction conditions were determined. The usability of the obtained protein which was extracted by UA-DES as an electrospun nanofiber with PVA has also been investigated. It is expected that quality fiber can be obtained with as high a protein concentration as possible. This is significant because nanofibers contain LPI and CPI can be used as a matrix for nanocapsules to boost the nutritional content of food or as a packaging material for functional ingredients. Currently, there are no reports of studies about the use of LPI and CPI for the creation of nanofibers using electrospinning according to the authors' knowledge. The second purpose of this study is to evaluate fiber formation by electrospinning LPI and CPI in various amounts and to test the usability of fibers obtained in the appropriate selected protein ratio as antioxidant (FA) carriers.
Material and methods
Material
Green lentil and chickpea samples were purchased from a local market. Choline chloride (C-1879), polyvinyl alcohol (P8136,70,000, %87–90 hydrolyzed), ferulic acid (128,708), phosphate buffer (17,202), and glucose (G7021) were obtained from Sigma Aldrich (St. Louis, MO, USA). Fructose (57–48-7), acetic acid (64–19-7), citric acid (5949–29-1), glycerol (56–81-5) from Merck (Darmstadt, Germany), and urea (57–13-6) from Isolab (Istanbul, Türkiye) were purchased. The chemicals were analytical grade. All analyses were carried out in three parallels with two replicates.
Methods
Choosing of deep eutectic solvent (DES)
The DES was created in accordance with Rajha et al. (2019). Choline chloride was used as the hydrogen acceptor while glycerol, urea, acetic acid, glucose, and fructose were used as the hydrogen donor. Initially, the components of the DES were a mixture 1:1:1 M ratio. All DES contained water as 3rd component [7]. The prepared different DES have used protein extraction from green lentils and chickpeas at the lowest time (3 min) and lowest ultrasound power (%30) of optimization. The amount of protein in the extract was calculated by the Bradford assay [30] DES prepared for each sample was used as a blank solution. The outcomes were presented as mg BSA equivalent/g. The BSA solution was prepared using water.
Optimization of Ultrasound-Assisted (UA) Extraction with DES and Protein Isolate Preparation
BBD design of Design Expert (Trial Version 7.0.0, Stat-Ease Inc., USA) was used for the optimization procedure. Extraction was performed with an ultrasound device (UP400S, Hielscher, Germany) using different times (3,9, and15 min), power (30%, 45%, and 60% amplitude), the molar ratio of urea (1, 1.5, and 2 M), and the molar ratio of water (1,3, and 5 M) as independent variables. While duration and power were determined according to preliminary studies, the urea molar ratio was determined according to preliminary studies and literature [8]. The amount of water was limited to 5 M according to preliminary study and literature.When the studies were examined, it was seen that adding appropriate amounts of water into DES would increase the solubility of proteins [8], and the diffusion rate of the solvent into the cell matrix would increase as the viscosity decreased thanks to the added water [31].
Five center points were chosen in the design. Accordingly, extraction was performed at 29 different points in total. Samples were prepared in 100 mL beakers with a ratio of 30 mg/mL [7]. The ultrasound probe was immersed up to its center point and then turned on. The beaker was surrounded by ice to provide temperature control throughout the application. After sonication, the extracts were taken into a centrifuge tube. The samples were centrifuged (NF 1200R, Nüve, Turkey) at 4200xg for 10 min after extraction. The protein amounts of supernatant were determined according to the Bradford method [30].
The protein extraction was carried out for green lentils (1.1 M urea, 4.3 M water, 34% amplitude power, 14.5 min time) and chickpeas (1.1 M urea, 4.9 M water, 59% amplitude power, 10.6 min time) at the determined optimum points before the samples underwent centrifugation at 4000xg for 10 min and the supernatants were gathered. The cold EtOH (15 mL, 4 °C, 24 h) was used to precipitate the proteins in the supernatants and further centrifuged (4000 × g, 10 min). The pellet was cleaned once again using the same method. In a lyophilizer(Christ Alpha, 1 2 LD plus, Germany) the resultant pellet was freeze-dried at 30 °C, under a vacuum of 0.04 mbar, and stored at -20 °C [7].
Preparation of Polymer Solutions for Electrospinning
A preliminary study was conducted and determined the protein isolates obtained from green lentil protein isolate (LPI) and chickpea protein isolate (CPI) alone were not capable of forming fibers. Therefore, PVA was used for preparing the electrospinning solution. The protein solutions both LPI and CPI were prepared at 150 g/L according to [32]. The PVA solution was prepared by magnetic stirrer at 30% at 90 °C, for 1 h. After that, the PVA: LPI and PVA: CPI mixtures at different ratios (70:30, 60:40, 50:50, and 40:60 (w:w)) were prepared at 90 °C 1 h [18, 20, 32].
Characterization of Polymer Solutions
Rheological Properties
The rheological properties of solutions of PVA: LPI and PVA: CPI were measured using a shear control and Peltier system rheometer (Thermo HAAKE, Mars III, Karlsruhe, Germany). Measurements were made with the cone-plate configuration (cone diameter: 35 mm, angle: 1°, cone plate spacing: 0.5 mm). The sample (1 mL) was placed between the cone and the plate, and shearing was applied in the range of 0.1–100 s−1. After 10 s of waiting at each shear rate value, the apparent viscosity values, shear stress, and shear rate data of the samples were determined. The temperature is set at 25 °C ± 1. The data are generated according to the power-law model [33].
(τ shear stress (Pa), γ∙ illustrates the shear rate (s−1), k defines consistency coefficient (Pa·sn), n represents flow behavior index (dimensionless), and η apparent viscosity (Pa·s).
Electrical Conductivity
A conductivity meter (HQ40d multimeter, Hach, USA) was used to determine the electrical conductivity of the polymer solutions. The probe of the device was immersed in the prepared solution and the measurement was read on the screen.
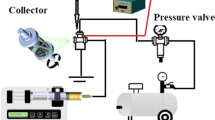
Electrospinning
Electrospinning equipment (Nanospinner, NE300, Turkey) with a variable high voltage power supply of 0–30 kV was used. The anode is attached to a 16 µm inner diameter stainless steel needle attached plastic syringe containing polymer solutions. On a syringe pump with a digital control system, the syringe needle was set horizontally and directed toward the collection. The flow rate of PVA: LPI and PVA: CPI solutions (0.3 mL/h), applied voltage (21 kV), and the separation between the needle and the collector (11 cm) were determined by preliminary experiments [32].
Characterization of Electrospun Nanofibers
Scanning electron microscopy (SEM)
A digital SEM (Leica LEO: S-440, Cambridge, USA) was used to examine the fiber morphology of electrospun nanofibers. A thin coating of gold was applied to the samples. A 25 kV excitation voltage was used for visualization. The average of diameters and diameter distributions of the fibers were determined by measuring the diameter of 100 randomly selected fibers from different images taken at 20,000 zooms using the Image J software program [18].
Ferulic Acid (FA) Loading of Selected Protein/PVA Nanofibers
Considering the particle diameters obtained with SEM images and rheological properties, the highest protein/polymer ratio for PVA: LPI and PVA: CPI was determined as 50:50 (w:w). FA weighed as 2 mg/mL (FA/ PVA: PI solution) was added to the mixtures and kept in a magnetic stirrer at room temperature for 1 h. The FA concentration in the solution was determined according to the literature [33]. For control, PVA solution was used alone and FA was added at the same rate as 2 mg/mL (FA/PVA). The flow rate of solutions (0.3 mL/h), applied voltage (21 kV), and the separation between the needle and the collector (11 cm) were determined by preliminary experiments [34].
Encapsulation Efficiency
The effectiveness of FA encapsulation in nanofibers was determined [27]. The nanofiber loaded with FA (1 mg) was placed in a test tube containing ethanol (1 mL) and kept at 300 rpm for 10 min to release the FA in the capsule. The tubes were then centrifuged at 10,000 × g for 10 min at 20 °C. The supernatant was eliminated and the precipitate was resuspended in ethanol (1 mL) and centrifuged again at 10,000 × g for 10 min at 20 °C. Absorbance measured at 324 nm (UV1700 Pharmaspec, Shimadzu, Japan). The amount of FA was determined by the standard curve. Solutions containing FA at different concentration (0.1-1 µg/mL) were prepared and a graph was created by reading the absorbance at 324 nm.
Optical Microscopy
The presence and distribution of FA encapsulated in PVA: LPI and PVA: CPI (50:50 w:w) nanofibers at a %2 ratio were observed using a digital fluorescence microscope (Nikon-Ti Eclipse 2-DS Fi2 camera) [27].
Antibacterial Activity of FA-Loaded Nanofibers
Tests were carried out by incubating 20 mg nanofibers sterilized with UV rays in 1 mL of Mueller Hinton Broth (MHB) containing 108 CFU/mL E. coli (ATCC 25922) that was activated at 37 °C and obtained from Erciyes University culture collection at 37 °C for 24 h. At the end of the incubation period, appropriate dilutions were made from the solutions and it was planted on 0.1 mL nutrient agar medium by spreading method. Colonies formed in petri dishes kept at 37 °C for 24 h were counted and calculated in CFU/mL. Finally, bacterial colonies were counted after overnight incubation at 37 °C. The outcomes were evaluated against the quantity of bacterial colonies in the control group without additional nanofibers [32, 35].
Release Kinetics of FA from Nanofibers
Two distinct food stimulants; hydrophilic (10% v/v ethanol/distilled water) and hydrophobic (50% v/v ethanol/distilled water) were used at 25 °C in the release studies [36, 37]. Furthermore, the in vitro release kinetics of nanofibers were evaluated in phosphate buffered solution with pH 7.4 at 37 °C [38]. Nanofibers were weighed at 5 mg/mL into different prepared media solutions and samples were taken at 1, 2, 4, 6, 8, 24, and 36 h. The concentration of FA released into the environment was determined by absorbance measurement at 324 nm (UV1700 Pharmaspec, Shimadzu, Japan). All conditions were kept the same for control (PVA) and PVA-PI samples. The kinetics of FA release from nanofibers were interpreted according to the Fick diffusion law (Eq-1), Power-law (Eq-2), and Weibull model (Eq-3) [36] using the Statistica (Tibco Software Inc. USA).
Statistical Analysis
Minitab 17 (Minitab Ltd., Coventry, UK) was used to conduct the statistical analysis. The ANOVA and Tukey tests were employed to establish statistical significance with a 95% confidence level. The optimization process was conducted by a Design Expert (Trial Version 7, Stat-Ease Inc., Minneapolis, MN). Lack of fit, R2 of the ANOVA-derived parameters, or coefficient of determination, and the F-test were used to assess the suitability of the model.
Result and Discussion
Choosing Appropriate DES
The highest protein yields in both green lentils and chickpeas were obtained from DES extractions produced with ChCl:urea:water (1:1:1) (Fig. 1). According to the study by [7], the maximum yield of protein extraction was obtained from ChCl: acetic acid (15 mg/g) and ChCl:urea (14 mg/g) from pomegranate peel. In a study in which polysaccharide extraction was optimized with US-DES, it was reported that higher extraction efficiency could be achieved with DES when comparing US-DES with hot water extraction under optimum extraction conditions [39]. Another study reported an increase in yield and a significant reduction in extraction time, although DES-based extraction was performed at lower temperatures compared to the traditional water extraction method. This was attributed to the higher solubility of curcuminoids in DES than in ethanol [31]. In the current study, it is seen that the extracts using DES have a higher efficiency than the extracts obtained with water. This situation can similarly be attributed to the higher protein solubility in DES.
RSM Optimization for UA-DES
Optimum protein extraction conditions were determined according to BBD in RSM using urea molar ratio (A), water molar ratio (B), ultrasonic power (% amplitude) (C), and application time (D) as independent variables. Among these independent factors, the urea molar ratio was 1–3, the water molar ratio was 1–5, the ultrasound power was 30–60%, and the time was 3–15 min (Table 1). The model's ability to fully forecast the variation is confirmed using the lack of fit test, which illustrates how well the models fit [40]. There was a value of > 0.05 for the lack of fit for the protein extraction optimization from green lentils and chickpeas. The model was valid according to the p-value used to assign the interaction of the independent variables. The R2 of the sample was determined as %94 and %94 for green lentils and chickpeas, consecutively. It was found that the optimization model created with BBD was meaningful (Table 2).
The ideal circumstances for green lentil protein extraction were obtained as 1.13 M urea, 4.34 M water, 34.07% amplitude, and 14.53 min. The theoretical maximum content of protein was 38.12 mg GAE/g. The experimental protein content of extracts was found as 38.50 mg BSA/g. The discrepancy between the predicted and experimental values was minor. Therefore, the pattern was competent and dependable for the protein extraction from green lentils for forecasting. It was presented the second-order polynomial equation for green lentils in terms of coded components;
The ideal circumstances for chickpea extraction depending on the BBD of RSM were 1.08 M urea, 4.91 M water, 49.14% amplitude, and 10.59 min. The determined predicted protein content of extracts by RSM was 25.81 mg BSA/g, while the experimental value was found as 25.83 mg BSA/g. According to the data, there were only slight discrepancies between the expected and experimental values, and the model was satisfactory. It was presented the second-order polynomial equation for chickpeas in terms of coded components;
It has been depicted in Fig. 2, the relationship between the variables as well as how the independent variables affected the outcome.
Electrospinning
Electrical Conductivity and Rheological Properties
A slight decrease was observed in the conductivity of electricity of the sample containing FA compared to the solution containing the same ratio of protein. In a study conducted to produce hybrid electrospun gliadin nanofibers containing FA/hydroxypropyl-beta-cyclodextrin inclusion complexes, it was reported that solutions containing FA had lower conductivity than pure gliadin solutions [41]. As the amount of protein increased, the electrical conductivity rose. In a study, nanofibers production was performed with black bean protein isolates and PVA reported that conductivity increased as protein concentration increased, similar to the present study. The evidence suggested that the bean protein isolate, which has 10 times higher electrical conductivity compared to the PVA solution, increased the electrical conductivity [18].[42] reported that the conductivity increased with the increasing ratio of sunflower protein isolates in PVA. In the current study, the electrical conductivity of LPI was approximately 20 times that of PVA polymer, and the conductivity of CPI was approximately 14 times. Therefore, as the amount of protein isolates increased, the conductivity increased (Table 3). All of the polymeric solutions prepared for use in electrospinning showed Newtonian flow behavior. Viscosity is an important parameter for Taylor cone formation stability in electrospinning. Beaded structure occurs when viscous forces and surface tension create an unstable jet and liquid properties cause capillary fragmentation [43]. At a higher viscosity, the jets produce smooth fibers that are mostly bead-free, as it improves the steadiness of the jet due to the cohesive properties of the polymers [44]. When the rheological properties of the prepared solutions are examined, it is observed that the PVA solution prepared at a 30% concentration has the highest viscosity. As the protein ratio increased in the PVA:PI mixture, the viscosity decreased. For PVA: PI, no fibers could be produced at 40:60 PVA:PI concentrations with a viscosity below 0.1 Pa.s in both LPI and CPI. It was seen that k and n50 values increased with increasing PVA ratio (Table 3). Shear tension and viscosity have a linear relationship for one-dimensional Newtonian fluids. In a study using pea protein isolate, the viscosity increased with an increasing PVA ratio from 50 to 80%. Also, a decrease in electrical conductivity was observed with increasing PVA content and decreasing protein content [32]. It is possible that the tendency of polymers to form hydrogen bonds is what causes blends with higher overall polymer concentrations to have a higher viscosity. Consequently, these solutions with high bonding capabilities will become viscous [45]. When 2% FA has been added to the PVA: PI mixture, the viscosity decreased slightly. However, this decrease did not have a negative effect on fiber production. [46] reported that FA reduced the hot dough viscosity, setback viscosity, and final viscosity of corn and sorghum starch pastes (Fig. 3).
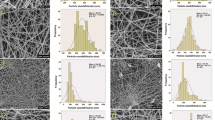
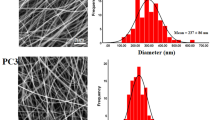
Morphology and Size Distribution of Nanofibers
Aqueous protein solutions in their natural state or denatured state can't form fibers in electrospinning without the use of a polymer to act as a carrier because protein molecules with a spherical structure do not offer enough chain structure to form fibers [17]. Therefore, the LPI and CPI were mixed with PVA at different ratios. The SEM images of nanofibers produced from PVA:LPI and PVA:CPI at different ratios were presented in Fig. 4. The fibers' diameters were 0.140 ± 0.00, 0.133 ± 0.00 and 0.114 ± 0.00 µm for 70:30, 60:40, and 50:50 for PVA:LPI (w:w), while they were 0.159 ± 0.01, 0.128 ± 0.01 and 0.11 ± 0.1 µm for 70:30, 60:40, and 50:50 for PVA:CPI (w:w), respectively. The diameter of the control fibers produced only with PVA was found as 0.165 µm. As the protein concentration in the medium increased, the diameters of the obtained fibers decreased. The viscosity of the polymer solution is one of the factors with a significant impact on the diameter of the fibers. In the electrospinning process, it is known that the increased viscosity creates resistance to elongation, and accordingly, the diameter of the fiber increases [47]. Furthermore, proteins are dissolved in water, they form ions, which are responsible for a solution's electrical conductivity. By applying a strong electric field, these charges are intended to enable the jet to extend more, resulting in a reduction in the diameter of the electrospinning fibers [48]. As the protein ratio increases, the decrease in the diameter of the fibers can also be explained by this changing electrical conductivity. Depending on the type of protein, carbohydrate, and carrier polymer used, fiber diameters can be affected differently [49].
Encapsulation of Ferulic Acid (FA) and Encapsulation Efficiency
The encapsulation efficiency for 50:50 PVA:LPI and PVA:CPI was found as 78.69% and 78.69%, respectively. It was observed that the use of LPI and CPI did not differ in terms of encapsulation efficiency. The encapsulation efficiency was reported as 83.7% in a study, in which FA was loaded into the amaranth protein isolate: pullulan nanofibers prepared at a ratio of 80:20 w:w [27]. β-carotene was encapsulated into nanofibers prepared using soy protein isolate and PVA at a 50:50 w:w ratio and the encapsulation efficiency was reported as 65% [20]. The findings of the current investigation demonstrate that legume protein isolates (LPI and CPI) are suitable sources that can be used in nanofibers production and encapsulation of antioxidants such as FA.
Antibacterial Activity
The effect of FA-loaded nanofibers produced with PVA:LPI and PVA:CPI on E. coli was given as log10CFU/mL (Fig. 5). All fibers had an antibacterial effect compared to the control sample. The nanofibers created with PVA:FA were used as a control for nanofibers of PVA:LPI:FA and PVA:CPI:FA. The number of colonies was reduced as a result of treatment with PVA:FA nanofibers compared to the control. It is known that FA has antimicrobial properties against both gram-positive and gram-negative bacteria [50]. However, the number of colonies treated with nanofibers containing LPI and CPI was also very low compared to PVA:FA. In addition to the antimicrobial effect of FA, it was said that LPI and CPI might have serious bioactive properties. Antihypertensive, immunostimulating, antimicrobial, and antioxidant properties of leguminous proteins/peptides are known [10]. Besides, it was reported that peptides and proteins derived from legumes showed high bacterial growth restriction (91–97%) [51]. Considering the reductions in the number of bacterial colonies according to control, there was a 71.3% decrease in PVA:FA while this value increased to 82.1% in PVA:LPI:FA and 78.9% in PVA:CPI:FA. Antibacterial activity of LPI nanofibers, which are rich in polyphenols, against E. coli [52, 53] and concentration dependence on antimicrobial activity for chickpea proteins [54] have been reported. Extracted proteins and peptides from legumes have indicated antibacterial activity against the most common foodborne pathogens [55]. In the current study, the antibacterial activities of the nanofibers produced with LPI and CPI were quite high compared to the control and control nanofibers of PVA:FA Fluorescent images for protein nanofibers prepared with both PVA:LPI and PVA:CPI showed that the FA was well dispersed throughout the nanofibers (Fig. 6).
Release Kinetic of FA
In the present study, diffusion was described by Fick's second law, considering the cylindrical structure of electro-nanofibers. However, many controlled release technologies are not based on pure diffusion, different approaches have been explored to consider other mechanisms [36]. In addition, the diffusion coefficient (D) obtained according to Fick's law, kinetic constant (k), and diffusion exponent (n) of the release system mechanism determined according to a power law, a and b constants of release according to the equation of Weibull were determined (Table 4). It was seen that FA was released from the fibers much faster in hydrophobic food simulant than hydrophilic. Therefore, it can be used as active packaging and additive for hydrophobic foods that require a high amount of antimicrobial and antioxidant release in a short time.
In the power-law model, the value of n characterizes the intracapsular release mechanism of the compound. In the case of cylindrical capsules, it is called 0.45 ≤ n Fickian diffusion, 0.45 < n < 0.89 non-Fickian diffusions, n = 0.89 state II diffusion, and n > 0.89 superstate II diffusions [56]. It was determined that the n value was 0.45 < except in the hydrophobic environments where the release from PVA:LPI and PVA:CPI fibers was very rapid. In the Weibull equation, the release mechanism is indicated by the b parameter: values less than 0.75 indicate that release happens according to Fick's diffusion law, and b > 1 values indicate that complex release mechanisms occur [36]. Similar to the results obtained from the power law, when the b values obtained for the fibers were examined, except for the hydrophobic environments where the release from the nanofiber produced with PVA:LPI and PVA:CPI were very rapid, the value of b was less than 0.75. In general, the hydrophilic application of the nanofibers of PVA:LPI and PVA:CPI is more convenient as the release takes longer and occurs more slowly.
The release of FA was slower in PVA control nanofibers, while the nanofibers obtained from PVA:LPI and PVA:CPI showed a similar release profile. The k values for nanofiber produced with PVA, PVA:LPI, and PVA:CPI were found as 0.14, 0.17, and 0.15, respectively. The n value was found as 0.26, 0.36, and 0.40, respectively. Similarly, b values were the lowest in PVA, while it was the highest for nanofiber of PVA:LPI. The values of k, n, and b revealed that the dominant release of FA from nanofibers in vitro environment was suitable for Fick's law (Table 3).
Conclusion
In this study, optimization of UA protein extraction from green lentils and chickpeas with DES was performed, and using ChCl:urea resulted in the highest protein content. The optimum points were determined as 1.13 M urea, 4.34 M water, 34.07% amplitude, and 14.53 min for green lentils, 1.08 M urea, 4.91 M water, 59.14% amplitude, and 10.59 min for chickpeas. The production of nanofibers by the electrospinning from protein isolates produced by UA-DES at the optimum point showed that nanofibers could not be produced at < 40:60 PVA:PI (w:w), however, rheological data and SEM images suggested that fiber could be produced at 50:50 PVA:PI (w:w). Encapsulation efficiencies were determined as 78.69% and 78.69% for 50:50 PVA:LPI and PVA:CPI, respectively. Antibacterial activity was investigated and release properties of FA-loaded nanofibers in simulated food and in vitro environments were evaluated. Our findings would seem to demonstrate that UA-DES was an effective and efficient technique for the extraction of LPI and CPI. It was also observed that nanofibers produced with LPI and CPI had a high antibacterial effect. In conclusion, The findings of the current investigation showed that legume protein-containing FA-loaded nanofibers with high antioxidant and antibacterial properties could be produced and these edible nanofibers might be used for food preservation purposes.
Data Availability
The datasets generated during the current study are available from the corresponding author upon reasonable request.
References
K. Kumar et al., Food waste: a potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresources and Bioprocessing 4(1), 1–14 (2017)
K. Shrivas, H.-F. Wu, Rapid and highly sensitive protein extraction via cobalt oxide nanoparticle-based liquid–liquid microextraction coupled with MALDI mass spectrometry. Analyst 137(4), 890–895 (2012)
L. Benvenutti, A.A.F. Zielinski, S.R.S. Ferreira, Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 90, 133–146 (2019)
A.P. Abbott et al., Selective extraction of metals from mixed oxide matrixes using choline-based ionic liquids. Inorg. Chem. 44(19), 6497–6499 (2005)
C. Bai, Q. Wei, X. Ren, Selective extraction of collagen peptides with high purity from cod skins by deep eutectic solvents. ACS Sustain. Chem. Eng. 5(8), 7220–7227 (2017)
A. Grudniewska et al., Enhanced protein extraction from oilseed cakes using glycerol–choline chloride deep eutectic solvents: a biorefinery approach. ACS Sustain. Chem. Eng. 6(11), 15791–15800 (2018)
E. Hernández-Corroto et al., Sustainable extraction of proteins and bioactive substances from pomegranate peel (Punica granatum L.) using pressurized liquids and deep eutectic solvents. Innov. Food Sci. Emerg. Technol.. Food Sci. Emerg. Technol. 60, 102314 (2020)
R. Wahlström et al., High yield protein extraction from Brewer’s spent grain with novel carboxylate salt-urea aqueous deep eutectic solvents. ChemistrySelect 2(29), 9355–9363 (2017)
K. Xu et al., A green deep eutectic solvent-based aqueous two-phase system for protein extracting. Anal. Chim. Acta 864, 9–20 (2015)
F. Kamran, N. Reddy, Bioactive peptides from legumes: Functional and nutraceutical potential. Recent Adv. Food Sci. 1(3), 134–149 (2018)
S. Tansaz et al., Soy protein meets bioactive glass: Electrospun composite fibers for tissue engineering applications. Mater. Lett. 199, 143–146 (2017)
A. Formhals, United States: Patent Application Publication. US patent 1(975), 504 (1934)
H. Azizi, A. Koocheki, B. Ghorani, Structural elucidation of Gluten/Zein nanofibers prepared by electrospinning process: Focus on the effect of zein on properties of nanofibers. Polym. Testing 128, 108231 (2023)
Y. Dong et al., Development of composite electrospun films utilizing soy protein amyloid fibrils and pullulan for food packaging applications. Food Chem.: X 20, 100995 (2023)
J. Dai et al., The improvement of sodium dodecyl sulfate on the electrospinning of gelatin O/W emulsions for production of core-shell nanofibers. Food Hydrocolloids 145, 109092 (2023)
M. AmanMohammadi et al., Fabrication of food-grade nanofibers of whey protein Isolate-Guar gum using the electrospinning method. Food Hydrocolloids 90, 99–104 (2019)
A. López-Rubio, J.M. Lagaron, Whey protein capsules obtained through electrospraying for the encapsulation of bioactives. Innov. Food Sci. Emerg. Technol. 13, 200–206 (2012)
S.L.M. El Halal et al., Electrospun ultrafine fibers from black bean protein concentrates and polyvinyl alcohol. Food Biophys. 14(4), 446–455 (2019)
E. Hirsch et al., Probiotic bacteria stabilized in orally dissolving nanofibers prepared by high-speed electrospinning. Food Bioprod. Process. 128, 84–94 (2021)
G.P. Bruni et al., Electrospun β-carotene–loaded SPI: PVA fiber mats produced by emulsion-electrospinning as bioactive coatings for food packaging. Food Packag. Shelf Life 23, 100426 (2020)
J.-Y. Cho, J.-H. Moon, K.-H. Park, Isolation and identification of 3-methoxy-4-hydroxybenzoic acid and 3-methoxy-4-hydroxycinnamic acid from hot water extracts of Hovenia dulcis Thunb and confirmation of their antioxidative and antimicrobial activity. Korean J. Food Sci. Technol. 32(6), 1403–1408 (2000)
E. Graf, Antioxidant potential of ferulic acid. Free Radical Biol. Med. 13(4), 435–448 (1992)
N. Kumar, V. Pruthi, Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 4, 86–93 (2014)
M.A. Ouimet et al., Biodegradable ferulic acid-containing poly (anhydride-ester): degradation products with controlled release and sustained antioxidant activity. Biomacromol 14(3), 854–861 (2013)
Y. Huang, W.-G. Dai, Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharmaceutica Sinica B 4(1), 18–25 (2014)
E. Atay et al., Development and characterization of chitosan/gelatin electrosprayed microparticles as food grade delivery vehicles for anthocyanin extracts. Food Hydrocolloids 77, 699–710 (2018)
M. Aceituno-Medina et al., Improved antioxidant capacity of quercetin and ferulic acid during in-vitro digestion through encapsulation within food-grade electrospun fibers. J. Funct. Foods 12, 332–341 (2015)
J. Yan et al., Sustained-release multiple-component cellulose acetate nanofibers fabricated using a modified coaxial electrospinning process. J. Mater. Sci. 49(2), 538–547 (2014)
D.-G. Yu et al., Third generation solid dispersions of ferulic acid in electrospun composite nanofibers. Int. J. Pharm. 400(1–2), 158–164 (2010)
M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1–2), 248–254 (1976)
S.S. Patil, A. Pathak, V.K. Rathod, Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: A greener route for extraction of curcuminoids from Curcuma longa. Ultrason. Sonochem. 70, 105267 (2021)
N. Maftoonazad et al., Development and evaluation of antibacterial electrospun pea protein isolate-polyvinyl alcohol nanocomposite mats incorporated with cinnamaldehyde. Mater. Sci. Eng. C 94, 393–402 (2019)
M. Hayta, M. Dogan, D. Aslan Türker, Rheology and microstructure of galactomannan–xanthan gum systems at different pH values. J. Food Process Eng 43(12), e13573 (2020)
P. Vashisth et al., Biomedical applications of ferulic acid encapsulated electrospun nanofibers. Biotechnol. Rep. 8, 36–44 (2015)
C. Remoroza et al., Combined HILIC-ELSD/ESI-MSn enables the separation, identification and quantification of sugar beet pectin derived oligomers. Carbohyd. Polym. 90(1), 41–48 (2012)
S. Estevez-Areco et al., Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag. Shelf Life 18, 42–50 (2018)
E. Commission, Commission regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union. 12(1), 1–89 (2011)
M.M. Mahmud et al., Controlled release of curcumin from electrospun fiber mats with antibacterial activity. J. Drug Del. Sci. Technol. 55, 101386 (2020)
L. Zhang, M. Wang, Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 95, 675–681 (2017)
L. Quanhong, F. Caili, Application of response surface methodology for extraction optimization of germinant pumpkin seeds protein. Food Chem. 92(4), 701–706 (2005)
N. Sharif et al., Active food packaging coatings based on hybrid electrospun gliadin nanofibers containing ferulic acid/hydroxypropyl-beta-cyclodextrin inclusion complexes. Nanomaterials 8(11), 919 (2018)
E. Shanesazzadeh, M. Kadivar, M. Fathi, Production and characterization of hydrophilic and hydrophobic sunflower protein isolate nanofibers by electrospinning method. Int. J. Biol. Macromol. 119, 1–7 (2018)
A.L. Yarin, S. Koombhongse, D.H. Reneker, Bending instability in electrospinning of nanofibers. J. Appl. Phys. 89(5), 3018–3026 (2001)
L. Shahreen, G.G. Chase, Effects of electrospinning solution properties on formation of beads in TiO2 fibers with PdO particles. J. Eng. Fibers Fabr. 10(3), 155892501501000320 (2015)
C. Kriegel et al., Fabrication, functionalization, and application of electrospun biopolymer nanofibers. Crit. Rev. Food Sci. Nutr. 48(8), 775–797 (2008)
T. Beta, H. Corke, Effect of ferulic acid and catechin on sorghum and maize starch pasting properties. Cereal Chem. 81(3), 418–422 (2004)
Z.-M. Huang et al., A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 63(15), 2223–2253 (2003)
C. Drosou, M. Krokida, C.G. Biliaderis, Composite pullulan-whey protein nanofibers made by electrospinning: Impact of process parameters on fiber morphology and physical properties. Food Hydrocolloids 77, 726–735 (2018)
N. Tam et al., Influence of solution properties and pH on the fabrication of electrospun lentil flour/HPMC blend nanofibers. Food Res. Int. 102, 616–624 (2017)
A. Khatkar et al., Synthesis and antimicrobial evaluation of ferulic acid derivatives. Res. Chem. Intermed. 41(1), 299–309 (2015)
M.C. Pina-Pérez, M.A. Ferrús Pérez, Antimicrobial potential of legume extracts against foodborne pathogens: A review. Trends Food Sci. Technol. 72, 114–124 (2018)
D.A. Khan et al., Antibacterial activity of Phyllantus emblica, Coriandrum sativum, Culinaris medic, Lawsonia alba and Cucumis sativus. Acta Pol. Pharm. Drug Res. 70(5), 855–860 (2013)
S.S. Nair et al., Comparative analysis of the antibacterial activity of some phytolectins. Int. Curr. Pharmaceut. J. 2(2), 18–22 (2013)
A. Kan et al., In vitro antimicrobial activities of Cicer arietinum L (Chickpea). Trop. J. Pharmaceut. Res. 9(5), 75–481 (2010)
M. González-Montoya, E. Cano-Sampedro, R. Mora-Escobedo, Bioactive Peptides from Le-gumes as Anticancer Therapeutic Agents. Int J Cancer Clin Res 4, 081 (2017)
J. Siepmann, N. Peppas, Mathematical modeling of controlled drug delivery. Adv. Drug Del. Rev. 48(2–3), 229 (2001)
Acknowledgements
The present study is funded by the Erciyes University Scientific Research Unit (ERU-BAP) as a part of a research project (FDK-2020-10341). ERU-BAP provided financial support, while ERNAM, the ERU's Nanotechnology Research Center, offered technical help.
Author information
Authors and Affiliations
Contributions
Elif Meltem İŞÇİMEN: Conceptualization, Data curation, Software, Writing-original draft, Writing-review&editing.
Mehmet HAYTA: Conceptualization, Data curation, Software, Writing-original draft, Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
We know of no conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome. As the corresponding author, I confirm that the manuscript has been read and approved for submission by all the named authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Işçimen, E.M., Hayta, M. Fabrication of Legume Protein Isolate-Polyvinyl Alcohol Nanofibers Incorporated with Ferulic Acid. Food Biophysics 19, 347–359 (2024). https://doi.org/10.1007/s11483-024-09827-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-024-09827-9