Abstract
This study aimed to develop novel gelatin nanofibers loaded with Lactarius deliciosus extract for food packaging via the electro-assisted solution-blown spinning technique. Gelatin nanofibers loaded with four different concentrations of extract were fabricated with centrifugal spinning and subsequently cross-linked. Nanofibers were characterized by morphological, chemical, thermal, and bioactivity aspects. Nanofibers exhibited antioxidant activity determined by DDPH and ABTS methods with increasing extract ratio. In addition, the nanofibers had antimicrobial activity against E. coli, S. aureus and B. cereus in relation to the mushroom extract they contained. The developed nanofibers have the potential to be used in the active edible packaging of fresh foodstuffs to extend the shelf life, as well as provide a robust solution to reduce synthetic plastic pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The packaging industry has become an important part of the global industry and constitutes a high proportion of 2% of the Gross National Product for developed countries (Robertson 2005). At this rate, the contribution of the packaging used for food is undeniable.
Food packaging is a system that protects food products at a reasonable cost while meeting the needs and expectations of consumers and the industry. Food packaging materials play an active role in possible microbiological, physical, and biochemical changes in foods. Plastic, glass, cardboard, paper, and tin packaging are widely used materials in the food industry. However, petroleum-based plastics are among the most preferred materials in the packaging industry due to their flexibility, durability, recyclability, and cheapness. However, as packages of plastic origin take a long time to break down naturally, they cause great environmental pollution problems. The amount of plastic waste is projected to double by 2030, and plastics have become a serious cross-border threat to natural ecosystems (Patrício Silva et al. 2021). In addition, when plastics are used directly as food packaging, they can cause serious problems to health due to the possible risk of migration (Wang et al. 2021). From another point of view, food packaging can also prevent food waste. According to the 2011 report of the FDA (Food and Drug Administration), approximately 1.3 billion tons of food is thrown away every year, and 20–30% of this waste consists of purchased packaged products (Dobrucka 2013).
Considering the increasing demand for food in the face of the increasing population, the need for long-distance transportation, and high transportation costs, it is important to preserve packaged foods without showing any signs of deterioration and without sacrificing quality. It is important to increase the shelf life of foods without changing their sensory properties and to reduce the associated food waste.
The active packaging concept, developed by integrating bioactive ingredients into food packaging materials, has been successfully applied to prevent microbial and oxidative food spoilage. To solve these problems, the demand for edible, sustainable, environmentally friendly, and low-cost packaging that extends the shelf life of foods has increased especially in the last ten years. In the last few years, nanofiber food packaging systems have been developed by incorporating active ingredients into natural polymers to meet sectoral and consumer needs. Nanofiber packages allow higher loading of active agents, provide higher surface area, high mechanical properties and allow controlled release. In addition, the smaller gaps formed between the fibers form a barrier against the entry of microorganisms (Doğan et al. 2022).
Nanofibers used in food packaging are generally produced by the electro-spinning technique. However, the nanofiber productivity using the solution blowing technique is 30 times higher than that using electrospinning (Sett et al. 2016). In this context, solution blowing technique is an important potential that should be evaluated in the rapid production of nanofiber mats for food packaging. However, studies on the use of solution blowing techniques to develop nanofibers for food packaging are limited.
Gelatin, as an edible material, has a good ability to form films thanks to its high flexibility. It is also biodegradable, biocompatible, inexpensive, and non-toxic. However, gelatin has hydrophilic properties, which can cause handicap in its use as a food packaging material. Although chemicals that carry the risk of toxic effects are generally preferred to provide water resistance in nanofiber mats, physical methods that provide high-temperature cross-linking can be preferred instead as they are not suitable for food packaging (Doğan et al. 2022).
Edible mushrooms have high nutritional values as well as superior bioactive and medicinal properties. Thanks to the bioactive compounds they contain, mushrooms provide antioxidant, antimicrobial, and various benefits to health, one example being Lactarius deliciosus (L. deliciosus) (Kosanić et al. 2016). These bioactive compounds are already used in the direct consumption of mushrooms or, more recently, in the development of food and medicines. In recent years, studies have increasingly found that the shelf life of bioactive compounds can be extended when used in edible food packaging (Mudenur et al. 2021). In the present study, bovine gelatin-based edible nanofiber was produced by using L. deliciosus extract, which is widely found in the flora of Turkey, as an active ingredient. It was assumed that the use of mushroom extract (ME) in the production of nanofibers would increase the antimicrobial and antioxidative properties of the mats. Although studies on nanofiber food packaging produced by using electrically assisted solution blowing are insufficient, no studies have been found on the production, characterization, antioxidant, and antimicrobial activity of L. deliciosus loaded mats.
Therefore, in this study, gelatin nanofibers loaded with different ME ratios (0, 1, 3, 5 and 10% w/w) were produced by the electrically assisted solution blowing technique and thermally crosslinked. The nanofibers were then characterized conformationally. Afterward, the changes in the antimicrobial and antioxidant properties of the nanofiber food packaging were evaluated.
Materials and methods
Mushroom samples, pathogen culture and chemicals
Mushroom (L. deliciosus) was supplied by the local greengrocer in Karabük, Turkey in November 2022. Type A bovine gelatin in powder form (Bloom 250–270) was purchased in Halavet Gida LLC (Istanbul, Turkey). The pathogen microorganisms, Escherichia coli (E. coli) ATCC 35,218, Staphylococcus aureus (S. aureus) ATCC 29,213, Bacillus cereus (B. cereus) NCIMB 7464, and Candida albicans (C. albicans) ATCC 90,028 were obtained from the culture collection (Yozgat Bozok University, Bogazliyan Vocational School, Turkey). The chemicals and microbial media used in the study were obtained from Merck/Sigma-Aldrich (Darmstadt, Germany) unless otherwise stated.
Treatments and extraction of mushroom
The mushrooms were cleaned, cut into 2-cm slices then, sliced mushrooms were dried in an oven for 12 h at 60 °C until they reached 10% humidity. Dried mushrooms were powdered using a grinder (Bosch MKM6000, Germany) (Doğan and Doğan 2022). 10 g mushroom powder was added to 100 ml of ethanol at 90% concentration and extracted in a shaking incubator at 50 °C at 125 rpm for 60 min. The extract was centrifuged for 20 min/2000 g, it was filtered with a syringe filter (0.45 μm pore size filter, Whatman) and the supernatant was obtained. After that, the ethanol in the supernatant was evaporated in a rotary evaporator at 40 oC and the ME was obtained.
Preparation of solutions
Gelatin solution (15% w/v) was prepared by dissolving gelatin powder in a 9:1 co-solvent mixture of acetic acid and distilled water. After that, a particular percentage (1%, 3%, 5%, and 10% v/v) of the ME was added to the mixture and stirred for 8 h at 40 °C using a magnetic stirrer to obtain homogeneous solutions.
Fabrication of cross-linked bovine gelatin-fungal nanofibers
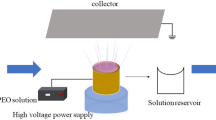
The acetic acid/water co-solvent combination with a volumetric ratio of 9:1 was chosen for the synthesis of ME blended gelatin nanofibers by the electrical-assisted solution-blowing (ESB) technique (Fig. 1). The ESB device is typically comprised of a syringe pump, a nozzle, a high-velocity air supply, and a collector (Benavides et al. 2012; Cui et al. 2020). The nozzle is concentric, with an outer and inner nozzle to pump high-velocity airflow and polymer solution. Unlike electrospinning, which uses electric force to create nanofibers, EBS employs gas to stretch a polymer solution into filaments, which are then formed into nanofiber membranes on the collector after the solvent evaporates (Dadol et al. 2020). The resulting solution was placed into the ESB apparatus, and the production process was run for 1 h at a 10 ml/h feed rate, 10 kV voltage, and 35 cm nozzle-to-collector distance. The produced samples were designated as pure gelatin (M0) as control, gelatin with 1% ME (M1), gelatin with 3% ME (M3), gelatin with 5% ME (M5), and gelatin with 10% ME (M10) throughout the entire work. Thermal crosslinking was applied to the nanofibers by applying heat treatment at 140 °C for 8 h with the help of an oven to gain water resistance.
Characterizations of nanofibers
Fiber morphology by scanning electron microscopy (SEM)
The morphological characteristics of the nanofibers were investigated using a Carl Zeiss Ultra Plus Field Emission Scanning Electron Microscope (FE-SEM). Diameter measurements were taken from 100 different randomly selected images of nanofibers. The average diameters and distributions of nanofibers were measured from SEM images using ImageJ program 24.
Fourier transform infrared spectroscopy analysis (FT-IR)
FT-IR spectra of nanofibers were recorded using Bruker ALPHA FTIR Spectrometers (total of 24 scans) in the 400–4000 cm− 1 wavelength range.
Thermogravimetric analysis (TGA)
The thermal behavior of the nanofilms was determined with the aid of a TGA/SDTA 851 Mettler Toledo instrument. The nanofibers were heated up to 500 °C at a heating rate of 10 °C min− 1 and a gas flow rate of 2 mL min− 1 under an N2 atmosphere, and their thermal behavior was investigated.
In-vitro antimicrobial activity
Antimicrobial activities of nanofibers were determined based on the agar disk diffusion method and spectrophotometric technique. Antimicrobial effects were tested against Gram-negative bacteria E. coli, Gram-positive bacteria S. aureus, Bacillus cereus, and Candida albicans, which are foodborne pathogens. First, each microorganism was transferred into Mueller Hinton Broth (MHB) loop and incubated at 35 °C for 18 h. After incubation, the OD600 was adjusted until the absorbance reached 108 CFU/mL. 100 µL of bacterial cell suspension was injected into Mueller Hinton Agar, spread on a sterile swab, and left for 5 min for absorption. The nanofibers were portioned into discs (6 mm diameter) and exposed to UV light for 30 min. Discs were placed on pathogen-inoculated media and incubated at 35 °C. The zone diameters formed around the discs were measured with a caliper and recorded (Doğan et al. 2022).
Analysis of antioxidant activity
In vitro antioxidant activities (AA) of the films were determined by monitoring the discoloration of DPPH (2,2’-diphenyl-1-picrylhydrazil) and ABTS (2,2’-azino-bis-3ethylbenzothiazoline-6-sulfonic acid) radicals. The DPPH and ABTS assays were done as described by Başyiğit et al. (2020) with some modifications. Firstly, solutions were prepared for analysis. For the DPPH test, 3.9 mL of 25 mg/L methanolic DPPH solution was prepared. For the ABTS test, ABTS radical solution containing 2 mL of 7 mM ABTS with 2.45 mM potassium peroxodisulphate (K2S2O8) was diluted with phosphate-buffered saline (PBS, pH 7.6) until the absorbance reached 0.700 at 734 nm. Then, 5 mg of film was added to the solutions in a rectangle cuvette. For DPPH, it was incubated for 30 min in a dark place, then filtered and the absorbance measured at 515 nm. For ABTS, it was incubated for 6 min, then filtered and the absorbance measured at 734 nm. DPPH radical inhibition (RIDPPH) and ABTS radical inhibition (RIABTS) of nanofilms were calculated according to Eq. (1) and Eq. (2) respectively.
Statistical analysis
The data were subjected to analysis of variance and results were presented as mean ± standard deviation. Significant differences between means (p < 0.05) were detected and lettered by Duncan post-hoc test (SPSS 22.0 software, IBM). Experiments were carried out in triplicate.
Result and discussion
SEM analysis
SEM and Image J were used to study morphology and mean diameter of the produced nanofiber mats. Figure 2 illustrates the SEM images and nanofiber diameter distribution of the samples. The produced nanofibers had a smooth surface, and there were no notable changes in the morphological properties of the nanofibers produced. Increasing the concentration of ME resulted in no significant change in fiber morphology other than a decrease in nanofiber diameter. The average diameter of the produced samples (M0, M1, M3, M5, and M10) were 471.089 ± 9.249, 397.478 ± 8.458, 357.143 ± 21.924, 235.140 ± 2.163, and 332.825 ± 2.997 nm respectively. As can be seen from the results increasing the ME content reduced the mean diameter of the produced nanofibers up to 5% ME. This may be a result of the decrease in the polymer solution’s viscosity brought on by the addition of ME because it is well known that when polymer solution viscosity is decreased average nanofiber diameter is reduced (Gundogdu et al. 2018; Tepekiran et al. 2019). Contrarily, when 10% ME was added, the fiber diameter increased, which may be a result of the solution’s pseudo-plastic nature. This is entirely consistent with results reported by Teilaghi et al. (2020). According to their research, the pseudo-plastic behavior (degree of pseudo-plasticity) rises as the solution’s Nigella sativa extract concentration increases.
FTIR analysis
Figure 3. shows FTIR spectra of gelatin and gelatin containing various quantities of ME (M1, M3, M5, and M10). These spectra were compared to examine the bond alterations in the chemical structures of the nanofiber mats. The strong peak with a range of 3100 to 3625 cm− 1 and a maximum of 3265 cm− 1 in all samples, as depicted in Fig. 3, was the typical peak for N-H and O-H stretching vibration. Absorption bands were seen at 1523 cm− 1 (CO stretching vibration), 1444 cm− 1 (NH bending vibration), 1437 cm− 1, and 1236 cm− 1 (CH bending and CN stretching vibration, respectively) in the FTIR analysis of the gelatin nanofibers. The amide I was demonstrated for the gelatin at 1643 cm− 1, whereas the amide II band was indicated at 1566 cm− 1 (Habibi and Hajinasrollah 2018). The presence of protein is shown by two signals at 1645 cm-1 (Amide I) and 1535 cm-1 (Amide II). They are the result of C = O stretching vibrations (Amide I) and bending vibrations of N-H groups (Amide II), and they have previously been seen in L. edodes -glucans (Surenjav et al. 2006). The polysaccharide peaks at 868 cm − 1 were present because of’ the structure’s β-glucan. Due to molecular interactions between gelatin and the ME, the amide I and amide III peak intensities decreased, whilst the amide II peak intensity rose as the percentage concentration increased (Gonzaga et al. 2013). Previous research by Liu et al. (2018) had also found signal peaks at a nearly identical range of wavelengths; as a result, their study findings concurred with the results of this spectra analysis.
TGA analysis
Nanofibers’ thermal characteristic represents their ability to withstand breakdown at high temperatures (Zhang et al. 2019). Figure 4. depicts the results of the TGA analysis of gelatin blended with ME nanofibers. TGA curves showed similar two major weight loss stages. the first step of thermal deterioration occurred up to temperatures of 118 °C and was attributable to the evaporation of physically weak and chemically strong bound water (Liu et al. 2019). The second stage, which occurred between 180 °C and 505 °C, was linked to the thermal degradation of nanofibers (Roy and Rhim 2020). The weight loss in this stage was attributed to the elimination of chemisorbed water and the breakdown of polymer chains. The thermal resistance was improved with the addition of ME to the polymer matrix. Adding ME could have enhanced the fiber’s thermal stability by rearranging polymeric chains, forming intermolecular hydrogen bonds, and crystallizing during fiber production (Rojas et al. 2021).
In-vitro antimicrobial activity of nanofibers
The antimicrobial activity results of gelatin-based nanofibers loaded with ME against E.coli, S. aureus, B.cereus and C.albicans are given in Table 1. Even the nanofiber sample loaded with the highest percentage of ME did not show antifungal effect against C. albicans. Candida albicans can form a biofilm to increase its resistance to antimicrobial agents. Biofilm is the functioning of a matrix in which microorganisms are bound together. This matrix can increase the resistance of microorganisms to antimicrobial agents by different mechanisms. For example, microorganisms on the biofilm can inhibit the penetration of antifungal products. In addition, cells on the biofilm are exposed to less oxygen, which can reduce the effectiveness of antimicrobial agents (Tobudic et al. 2012).
Gram (+) S.aureus and B.cereus were more sensitive to mushroom-loaded nanofibers than Gram (-) E.coli. Gram (-) bacteria have a thicker and layered cell membrane, a Lipopolysaccharide layer inside the layer, and a less tight peptidoglycan membrane. These structures can prevent antimicrobial agents from acting on Gram (-) bacteria and increase the resistance of bacteria to antimicrobial agents (Silhavy et al. 2010).
Fungi within the genus Lactarius exhibit an intriguing chemical transformation during the early stages of fruit body development. At this initial phase, they contain fatty acid esters that are inherently biologically inactive. However, as the fruit body continues to mature, a fascinating enzymatic process takes place, converting these inert compounds into potent dialdehydes and sesquiterpenes (Xu et al. 2019). These newly formed substances exhibit remarkable antimicrobial activity, serving as a chemical defense mechanism for the fungus against external invaders. Interestingly, as the fruit body reaches its final stages of maturation, there is a noticeable decline in antimicrobial activity. This reduction can be attributed to the gradual degradation of these once-active compounds, marking a fascinating lifecycle progression within the Lactarius genus (Barros et al. 2007). In our study, no antimicrobial activity was detected in the control samples that did not contain fungal extracts. As the extract ratio increased, the antibacterial effect due to the above-mentioned components increased. The findings indicated that the obtained edible active nanofibers could be used in antimicrobial packaging.
In-vitro antioxidant activity of nanofibers
Packaging materials endowed with antioxidant properties represent a resilient approach for safeguarding food products against oxidative degradation. Beyond their protective role, edible packaging serves as coatings that can be ingested along with the food, facilitating the uptake of antioxidant components into cells (Sahraee et al. 2019). Consequently, these intracellular antioxidants play a pivotal role in promoting the proper functioning of cellular processes and serve as a defense mechanism against DNA damage during cell division ( Petkoska et al. 2021).
Antioxidant activity of gelatin-based edible nanofibers loaded with ME was determined by DDPH and ABTS methods and presented in Fig. 5. Unsurprisingly, the incorporation of ME into the nanofibers resulted in a discernible enhancement of their antioxidant properties. Nonetheless, it is noteworthy that augmenting the ME concentration in the samples didn’t yield a corresponding linear improvement in antioxidant activity. In fact, the disparity in antioxidant performance between samples containing 5% and those with 10% ME was remarkably subtle, indicating a plateau effect in terms of enhancement. Presumably, the phenolic compounds in the extract obtained from L. deliciosus played a role in the antioxidant activities (Palacios et al. 2011) of the nanofibers. However, in our study, gelatin was used as a matrix polymer in the production of nanofibers. The peptides of gelatin are rich in hydrophobic amino acids, which may provide partial antioxidant activity (Gómez-Guillén et al. 2011). This situation can explain the antioxidant activity of control samples that did not contain ME in our study. Proteins such as gelatin and phenolic compounds interact with various types of bond such as hydrogen, Van der Waals and covalent. Although these interactions generally improve the technological properties of the conjugate, they cause a decrease in the antioxidant activity of the phenolic compounds (Ozdal et al. 2013). The results in our study were consistent with the above phenomenon and literature.
Conclusion
In this study, edible active packaging containing different levels of L. deliciosus extract and gelatin was developed with a new technique, electro assisted solution blow spinning. ME added up to 5% to the polymer solution reduced the diameter of the nanofibers. Although the increase in ME increased the thermal stability of the nanofibers, no significant increase in antioxidant activity was detected after the addition of 5% ME. Moreover, nanofibers with high concentration showed antimicrobial activity against E. coli, B. cereus and S. aureus, except for C. albicans. To conclude, this study revealed that L. deliciosus extract-loaded nanofibers rapidly fabricated with electro-assisted solution-blown spinning technology is promising for use in novel edible food packaging applications.
Data availability
Data and material are available if necessary.
Code availability
Not applicable.
References
Barros L, Baptista P, Estevinho LM, Ferreira ICFR (2007) Effect of fruiting body maturity stage on Chemical composition and antimicrobial activity of Lactarius sp. Mushrooms. J Agric Food Chem 55:8766–8771. https://doi.org/10.1021/jf071435+
Başyiğit B, Alaşalvar H, Doğan N et al (2020) Wild mustard (Sinapis arvensis) parts: compositional analysis, antioxidant capacity and determination of individual phenolic fractions by LC–ESI–MS/MS. Food Measure 14:1671–1681. https://doi.org/10.1007/s11694-020-00415-2
Benavides RE, Jana SC, Reneker DH (2012) Nanofibers from scalable gas jet process. ACS Macro Lett 1:1032–1036. https://doi.org/10.1021/mz300297g
Cui T, Yu J, Li Q et al (2020) Large-scale fabrication of Robust Artificial skins from a biodegradable sealant‐loaded Nanofiber Scaffold to skin tissue via Microfluidic blow‐spinning. Adv Mater 32:2000982. https://doi.org/10.1002/adma.202000982
Dadol GC, Lim KJA, Cabatingan LK, Tan NPB (2020) Solution blow spinning–polyacrylonitrile–assisted cellulose acetate nanofiber membrane. Nanotechnology 31:345602
Dobrucka R (2013) The future of active and intelligent packaging industry. LogForum 9:103–110
Doğan N, Doğan C (2022) Egzotik Bazı Mantarların (Pleurotus Ostreatus, Pleurotus Eryngii, Hericium erinaceus) Fizikokimyasal, Biyoaktif Ve Duyusal Özelliklerinin Belirlenmesi. J Fungus Nisan 13:30–36.https://doi.org/10.30708/mantar
Doğan N, Doğan C, Eticha AK et al (2022) Centrifugally spun micro-nanofibers based on lemon peel oil/gelatin as novel edible active food packaging: fabrication, characterization, and application to prevent foodborne pathogens E. coli and S. Aureus in cheese. Food Control 139:109081. https://doi.org/10.1016/j.foodcont.2022.109081
Gómez-Guillén MC, Giménez B, López-Caballero ME, Montero MP (2011) Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocolloids 25:1813–1827. https://doi.org/10.1016/j.foodhyd.2011.02.007
Gonzaga MLC, Menezes TM, de Souza JRR et al (2013) Structural characterization of β glucans isolated from Agaricus Blazei Murill using NMR and FTIR spectroscopy. Bioactive Carbohydr Diet Fibre 2:152–156
Gundogdu NS, Akgul Y, Kilic A (2018) Optimization of centrifugally spun thermoplastic polyurethane nanofibers for air filtration applications. Aerosol Sci Technol 52:515–523
Habibi S, Hajinasrollah K (2018) Electrospinning of nanofibers based on chitosan/gelatin blend for antibacterial uses. Russ J Appl Chem 91:877–881
Kosanić M, Ranković B, Rančić A, Stanojković T (2016) Evaluation of metal concentration and antioxidant, antimicrobial, and anticancer potentials of two edible mushrooms Lactarius deliciosus and macrolepiota procera. J Food Drug Anal 24:477–484. https://doi.org/10.1016/J.JFDA.2016.01.008
Liu Y, Li Y, Deng L et al (2018) Hydrophobic Ethylcellulose/Gelatin nanofibers containing zinc oxide nanoparticles for antimicrobial packaging. J Agric Food Chem 66:9498–9506. https://doi.org/10.1021/acs.jafc.8b03267
Liu Y, Qin Y, Bai R et al (2019) Preparation of pH-sensitive and antioxidant packaging films based on κ-carrageenan and mulberry polyphenolic extract. Int J Biol Macromol 134:993–1001
Mudenur C, Ghosh T, Katiyar V (2021) Nanodelivery System of Bioactive compounds in Edible Food Packaging. 273–298. https://doi.org/10.1007/978-981-33-6169-0_11
Ozdal T, Capanoglu E, Altay F (2013) A review on protein–phenolic interactions and associated changes. Food Res Int 51:954–970. https://doi.org/10.1016/j.foodres.2013.02.009
Palacios I, Lozano M, Moro C et al (2011) Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem 128:674–678. https://doi.org/10.1016/j.foodchem.2011.03.085
Patrício Silva AL, Prata JC, Walker TR et al (2021) Increased plastic pollution due to COVID-19 pandemic: challenges and recommendations. Chem Eng J 405:126683. https://doi.org/10.1016/J.CEJ.2020.126683
Robertson GL (2005) Food Packaging: Principles and Practice, Second Edition. https://doi.org/10.1201/9781420056150
Rojas A, Velasquez E, Pina C et al (2021) Designing active mats based on cellulose acetate/polycaprolactone core/shell structures with different release kinetics. Carbohydr Polym 261:117849
Roy S, Rhim J-W (2020) Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocolloids 98:105302
Sahraee S, Milani JM, Regenstein JM, Kafil HS (2019) Protection of foods against oxidative deterioration using edible films and coatings: a review. Food Bioscience 32:100451. https://doi.org/10.1016/j.fbio.2019.100451
Sett S, Stephansen K, Yarin AL (2016) Solution-blown nanofiber mats from fish sarcoplasmic protein. Polymer 93:78–87. https://doi.org/10.1016/J.POLYMER.2016.04.019
Silhavy TJ, Kahne D, Walker S (2010) The bacterial cell envelope. Cold Spring Harb Perspect Biol 2:a000414. https://doi.org/10.1101/cshperspect.a000414
Surenjav U, Zhang L, Xu X et al (2006) Effects of molecular structure on antitumor activities of (1→ 3)-β-D-glucans from different Lentinus edodes. Carbohydr Polym 63:97–104
Teilaghi S, Movaffagh J, Bayat Z (2020) Preparation as Well as evaluation of the Nanofiber membrane loaded with Nigella sativa Extract using the Electrospinning Method. J Polym Environ 28:1614–1625. https://doi.org/10.1007/s10924-020-01700-3
Tepekiran BN, Calisir MD, Polat Y et al (2019) Centrifugally spun silica (SiO2) nanofibers for high-temperature air filtration. Aerosol Sci Technol 53:921–932
Tobudic S, Kratzer C, Lassnigg A, Presterl E (2012) Antifungal susceptibility of Candida albicans in biofilms. Mycoses 55:199–204. https://doi.org/10.1111/j.1439-0507.2011.02076.x
Wang C, Huang P, Qiu C et al (2021) Occurrence, migration and health risk of phthalates in tap water, barreled water and bottled water in Tianjin, China. J Hazard Mater 408:124891. https://doi.org/10.1016/J.JHAZMAT.2020.124891
Xu Z, Fu L, Feng S et al (2019) Chemical composition, antioxidant and antihyperglycemic activities of the wild Lactarius deliciosus from China. Molecules 24:1357. https://doi.org/10.3390/molecules24071357
Zhang X, Liu Y, Yong H et al (2019) Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocolloids 94:80–92
Funding
This study was funded by Karabuk University Scientific Research Coordination Unit with grant number KBUBAP-22-ABP-07.
Author information
Authors and Affiliations
Contributions
CD, ND, YA involved in conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing—original draft and writing—review and editing; SBA involved in formal analysis, investigation, methodology and validation; SBA, IB involved in formal analysis, methodology and supervision; IB involved in funding acquisition, project administration.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Doğan, N., Doğan, C., Akgul, Y. et al. Gelatin/mushroom (Lactarius deliciosus) extract nanofibers fabricated by electro-assisted solution blow spinning as a potential edible active food packaging. J Food Sci Technol (2024). https://doi.org/10.1007/s13197-024-06080-3
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13197-024-06080-3