Abstract
The development of oleogels is currently at the stage of searching for optimal methods of structure formation to obtain the specified product properties. One of the approaches is to optimize the composition of the gelator. The current problem is the lack of information about the role of gelators in their combination in oleogel formation process. The study aimed to determine the effect of combining individual fractions from beeswax on the properties of oleogels. For this purpose, differential scanning calorimetry, polarized light microscopy, texture analysis, and oil-binding capacity measuring methods were used to study sunflower oil-based oleogels with the addition of a 6 wt. % gelator. Beeswax or combinations of its fractions obtained by preparative liquid chromatography were used as different gelators. A close linear correlation was shown between the onset temperature of crystallization and the content of hydrocarbons (r = -0.804, p < 0.001) and wax esters (r = 0.925, p < 0.001). It was found that the strongest gels were formed when the content of wax esters was from 64.74% to 89.36% with the addition of 7.86% to 20.64% of a mixture of free fatty acids and alcohols. The onset temperature of crystallization can be corrected by varying the content of hydrocarbons in the system up to 80.18% while maintaining firmness higher than that of beeswax oleogel. The obtained data are useful for directed regulation of oleogels properties by varying the gelator composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fats and oils are a source of essential fatty acids in the human diet and play an important role in food texture formation. At the same time, solid fats traditionally used to form the texture of a product are a source of saturated and trans-isomeric fatty acids, excessive consumption of which is associated with the risk of cardiovascular disease. In this regard, finding promising ways to replace such fats becomes relevant [1]. Oleogels can act as such an alternative, representing one of the most developing areas of research in solid fat replacement to reduce saturated and trans-isomeric fatty acids in the diet [2]. Oleogels may include various gelling agents (usually less than 10%) dissolved in oil when heated and, upon cooling, forming a thermally reversible three-dimensional network of crystals in which the liquid oil is encased [3].
Generally, low molecular weight compounds such as waxes [4] or high molecular weight compounds such as ethylcellulose [5] are used as a gelator. Among many natural waxes, beeswax is one of the most studied gelling agents due to its multi-component nature [6, 7] and low cost [8]. The main components of beeswax that can be used as structuring agents [7, 9] are hydrocarbons (HC), wax esters (WE), free fatty acids (FFA), and free fatty alcohols (FAl) [6, 10]. According to [6, 11] beeswax hydrocarbons are represented mainly by C27, C29, C31, and C33 compounds. The wax esters are a mixture of mono-, di-, and triesters. Monoesters are esters consisting of long-chain alcohols: C24, C26, C28, C30 and C32 and long-chain fatty acids: C16, C18 and C24. Di- and tri- esters also consist of diols: C24, C26, C28 and C30 and hydroxy acids: C16, C18 and C24.
A big step in the field of wax oleogels was made thanks to the study [6], where the component composition of some natural waxes was analyzed and their different effects on the gelation process in oils were shown. It was found that liquid oils structured by waxes with a large amount of WEs in their composition form hard but brittle oleogels, and the presence of HC and FFA contribute to the formation of firm oleogels. For a more detailed understanding of the role of individual components in the gelling process and the creation of the properties of oleogels, the authors [7] first developed and subsequently modified [12] the method for beeswax fractionation by preparative flash chromatography using solvents approved for use in the food industry. Individual fractions of beeswax with different component compositions were obtained. The fractionation of beeswax made it possible to analyze its components and identify differences in the content of volatile substances, chemical composition, thermal properties, and morphological features. In addition, this made it possible to evaluate their gel-forming properties in structuring oils, either individually or in combinations [7]. By using individual wax components, it was shown that the statement reported in [13] that faster cooling rates increase the firmness of wax oleogels is true for oleogels structured with beeswax, but the contrary effect was shown for samples structured with HC [14].
Oleogels have some disadvantages, i. e. they can release oil during storage due to syneresis, which at the moment does not allow us to consider them a complete substitute for solid fats [15]. Technological features of the oleogels production process include heating of the dispersion medium (oil) up to 90–100 °C, which, in turn, can lead to the development of oxidative processes. In the aspect of sensory properties of oleogels, an increase in the concentration of wax gelators is accompanied by the formation of a waxy mouthfeel [16]. In the study [17] on the example of cookies it is shown that when using oleogels structured by individual fractions of beeswax, it is possible to obtain a food product that does not differ from the solid fat-based product. Despite this, research on ways to improve oleogels and search for modifications of gelling agents, including for reducing their concentration in the oleogel, is constantly continuing.
There is evidence that the nature of vegetable oil influences the properties of oleogels structured both by multi-component gelators [18, 19] and by pure alcohols and acids [20, 21]. As is known that oleogels structured with native wax and beeswax HCs cooled at different rates [14] can have diametrically opposite properties. To better understand the effect of combining beeswax fractions on the properties of oleogels, it is also necessary to study these combinations in different oils under various processing conditions, such as different cooling rates.
By combining several components, lower melting temperatures of their mixtures relative to the initial compounds can be obtained [22]. This also makes it possible to produce oleogels with higher textural properties. [23]. The authors [7] show that HCs, when interacting with other wax components, form eutectics—mixtures with a lower melting point, which, in turn, makes it possible to avoid waxy mouthfeel. There are also works associated with the combination of several oils [24, 25] however the effects of such interactions are less significant in comparison with the combination of several gelators. In [9] the role of wax esters and their combinations in gel formation were investigated. It’s shown that by mixing several WEs it is possible to achieve a modification of the thermal effects and improved structuring action. However, there is a lack of systematic studies on the effects of combining all the major classes of compounds that constitute the waxes.
The study aimed to determine the effect of combining individual fractions from beeswax on the properties of oleogels.
Materials and Methods
Materials
Beeswax (BW) was purchased from a local apiary (Dom Voska, Russia) and was fractionated using preparative flash chromatography according to the method [12]. Three fractions of gelators (A—hydrocarbons > 99%, B—wax monoesters > 95%, and C—66% wax di- and triesters, > 29% free fatty acids and < 5% free fatty alcohols) were used, the component composition of which is given in Table 1 (10:0:0, 0:10:0, and 0:0:10, respectively) and corresponds to [12], in which the composition analysis was performed by thin-layer chromatography (TLC) and high-performance liquid chromatography with evaporative light-scattering detector (HPLC-ELSD). The composition of combinations 4–21 were calculated. Analytical grade solvents were used for the separations, including hexane, acetone, and isopropyl alcohol (Component-Reactiv, Russia). Refined deodorized sunflower oil was purchased from (EFKO, Russia) for the preparation of oleogels.
Oleogel Preparation
The concentration of the gelator was 6 wt. % in the sunflower oil. The choice of concentration was based on a previous study [18] in which it was shown that a this concentration of beeswax forms an oleogel with characteristic mechanical properties (firmness, oil-binding capacity). To prepare oleogels, a structure-forming agent ( individual fractions or their combinations) was added to heated oil at 90 °C. The combinations of fractions were prepared from three individual fractions of beeswax, so that their total content was equal to 6 wt. %. The samples were mixed on a magnetic stirrer at 300 rpm for 20 min. For structuring, the samples were cooled to 20 ± 1 °C at a rate of 1 °C/min and then incubated for 24 h before testing. The temperature and cooling rate were controlled in the climatic chamber KK240 (Pol-Eko-Aparatura, Poland). A total of 21 samples of oleogels were prepared and analyzed, with different compositions of the gelators used to obtain the oleogel.
Fractions A, B, and C were added to the oil in various proportions with 20% increments. The content of each fraction in the sample was marked as a ratio (A:B:C). For example, a sample containing 20% of fraction A, 20% of fraction B, and 60% of fraction C was marked as (2:2:6). The numbering and chemical composition of the gelling agent mixture are shown in Table 1.
It is worth noting that usually the wax esters are a mixture of mono-, di-, and triesters [11], but by fractionation we were able to separate monoesters, from di- and triesters [12] into the B fraction.
Analysis of Oleogels
Characteristics of Crystal Morphology
Studies of the crystal morphology were performed using a Zeiss Axio Imager Z1 microscope in polarized light mode (Carl Zeiss Microimaging GmbH. Jena, Germany) equipped with a digital camera AxioCam (Zeiss). The procedure of the specimen preparation was described in [24], with a slight difference. Specimens were cooled to 20 C with 1 °C/min cooling rate. Crystal size in the oleogels was determined using microphotographs made in two replicates using JMicroVision software.
Oil-Binding Capacity (OBC)
Studies of oil-binding capacity were carried out by centrifugation according to the method [18]. Before the study, tubes with oleogels were thermostatted at 20 ± 1 °C for 24 h. The analysis was performed in two replicates.
Qualitative Phase Diagrams
Three mililiters of oleogels prepared according to the technology described in “Oleogel preparation” section were poured into 5 mL tubes (inner diameter 19 mm). The samples of oleogels were cooled to 20 °C at 1 °C/min and incubated for 24 h. The samples were stored at a constant temperature from 20 to 50 °C, increasing the temperature by 5 °C at a rate of 1 °C/min every 24 h to prepare qualitative phase diagram. Gel formation was confirmed visually by evaluating the inability of the samples to flow after turning the test tube. The test tube was held upside down for 1 min. Samples were characterized as "gel", "thickened liquids", or "liquid" (non-viscous solution) based on the inability of samples to flow after turning over [26].
Differential Scanning Calorimetry (DSC)
The crystallization and melting temperatures of the oleogels were studied using a Mettler-Toledo DSC 3 differential scanning calorimeter (Switzerland) equipped with a TC45 cooling module (Huber, Germany). Samples (6–8 mg) were placed in aluminum pans and sealed. The measuring cycle consisted of 5 sections: cooling, stabilization, heating, stabilization, and cooling. First, the samples were cooled to -15 °C, then stabilized at this temperature for 3 min and heated to 90 °C. The samples were then stabilized at 90 °C 4 min and cooled to 10 °C. The heating and cooling rate for all steps was 10 °C/min.
Firmness Determination
The firmness of the oleogels was measured by a texture analyzer using EZ Test SX machine (Shimadzu, Japan) with a cylindrical probe (12 mm diameter, Perspex). Oleogels for this study were prepared under standard conditions and poured by 3 ml into 5 ml cylindrical tubes with an inner diameter of 14 mm. All the samples were incubated for 24 h at 20 °C in the climatic chamber KK240 (Pol-Eko-Aparatura, Poland) to determine the firmness of the oleogel samples. The room temperature during the analysis was 20 ± 2 °C, maintained by a climate control system. Then the samples were compressed by the cylindrical probe, moving at a speed of 5 mm/min over a distance of 6 mm into the sample. Firmness was measured automatically by Trapezium X (Shimadzu, Japan) software.
Statistical Analysis
Statistical data analysis and plotting of triple diagrams were performed using OriginPro 2018 software [27]. Multiple Correspondence Analysis was performed using RStudio software package (version 1.1.463) with FactoMineR and factoextra modules [28, 29], R package version 1.0.5. The significance level was p < 0.05 with a 95% confidence level.
Results
Microscopy
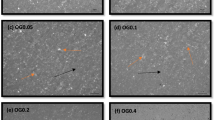
One of the most important parameters characterizing the morphology of crystals is their shape and average crystal length (Lc) [30]. Figure 1 shows microphotographs of oleogel crystals structured by combinations of beeswax fractions at a concentration of 6%. Oleogels prepared on the individual fractions A—1 (10:0:0), B—2 (0:10:0) and C—3 (0:0:10) are placed in the vertices of the triangle.
Polarized light microscopy of oleogels based on fractions A, B, C and their combinations (Plan-Neofluar 20X objective. The horizontal scale corresponds to 100 µm. On the upper right is the sample number. On the upper left are mass fractions of individual fractions in the format A:B:C). A hydrocarbons > 99%, B wax monoesters > 95%, and C 66% wax di- and triesters, > 29% free fatty acids and < 5% free fatty alcohols
Among the samples studied, the crystal length varied significantly, varying from a minimum value of 7.55 ± 1.97 μm for the oleogel crystals of sample 3 (0:0:10) to a maximum of 69.54 ± 18.44 μm for the crystals of sample 1 (10:0:0). The crystals of the oleogels of sample 2 (0:10:0) had a length equal to 46.43 ± 11.53 μm. Crystals in the form of large polygonal plates were formed in oleogels structured by fraction A (sample 1 (10:0:0)), which is typical for hydrocarbon crystals [31]. Similar platelet-like crystals were formed in oleogels in fraction B (sample 2 (0:10:0)), which is consistent with earlier studies of oleogel crystals structured by beeswax monoesters [7] and pure WEs [9]. Substantial differences in oleogel crystals were found in sample 3 (0:0:10) in fraction C characterized by small crystal size (Fig. 2) but similar in shape to oleogel crystals in the beeswax [18].
For oleogels on binary and triple combinations of these fractions, crystals also differed significantly in size depending on the used ratios. Overall, the crystal size of the binary and ternary mixtures also varied considerably from 5.23 ± 1.84 μm to 63.93 ± 22.62 μm. Oleogel crystals on mixtures of fractions A and C the smallest values of average length (Fig. 2) and, conversely, when combining fractions A and B, large crystals were formed, on average more than 30 µm. When fractions B and C were combined, both large crystals (at high concentration of fraction B) and small crystals (at high concentration of fraction C) were formed. When all three fractions were combined in oleogels, both large crystals (sample 11 (2:6:2)) and small crystals (sample 12 (2:2:6)) were formed. Crystals of sample 20 (4:4:2) were represented by aggregated plates. This is also typical of sample 21 (4:2:4), but its crystals were much smaller, probably due to higher amount of FFAs and FAls (Table 1).
A study of the morphology of oleogel crystals on the initial wax containing a large amount of hydrocarbons and oleogels structured by pure hydrocarbons (dotriacontane) was previously described in [32]. The authors showed that the crystals of oleogels structured by hydrocarbons were much larger compared to the crystals of wax-based oleogels. The authors concluded that such a tendency can be observed due to the multi-component composition of the wax, leading to the development of the mixed systems with a less developed three-dimensional structure and smaller crystals as compared to the pure substance.
Depending on the combination used and the fractions chemical composition, we observed both large and small crystals, mostly platelet-like in shape. Some samples with high concentration of fractions A and C (e.g., 3 (0:0:10), 6 (0:2:8), 9 (2:0:8), 12 (2:2:6), 16 (6:0:4), and 17 (4:0:6)) were characterized as needle-like. However, according to the publication [33], this visual effect was caused by the orientation of crystals perpendicular to the surface of the slides, but it is reported that their real shape is platelet-like [3, 14, 34].
Oil-Binding Capacity
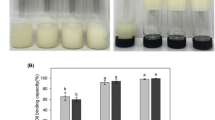
The structure's ability to holding oil after centrifugation indicates the stability of the oleogel and the efficiency of oil structuring [33]. Structuring ability is one of the critical factors in the practical application of oleogels and depends on their microstructure [35]. Data were obtained on the OBC of oleogels structured by individual beeswax fractions and their double and triple combinations (Fig. 3).
A result of the analysis (Fig. 3) is shown that using separate fractions of beeswax (samples 1 (10:0:0), 2 (0:10:0), and 3 (0:0:10) and their combination in various ratios (samples 4–21), the OBC of oleogels based on them ranges from 49.2 to 100%. The lowest OBC value is characteristic of oleogels structured with hydrocarbons (sample 1 (10:0:0)). Thus, for example, when combining fractions A (hydrocarbons) and B (monoesters), samples (4 (8:0:2), 15 (6:0:4), 13 (4:0:6), 5 (2:8:0)) were characterized by an OBC index from 68.6% to 94.5%, while increasing the hydrocarbon content in combination with monoesters leads to an increase in OBC. A similar tendency to increase OBC with an increase in hydrocarbon content was observed in samples 8 (8:0:2), 9 (2:0:8), 16 (6:0:4), and 17 (4:0:6) when combining fraction A with fraction C (wax esters, fatty alcohols, free fatty acids). At the same time, binary combinations of fractions B and C (samples 7 (0:8:2), 14 (0:6:4), 18 (0:4:6)) had 100% OBC. The exception is sample 6 (0:2:8) (93.6%), in which a minor separation of the oil phase is observed. Based on the obtained data, we shown that the use of binary compositions of beeswax fractions gelators in oleogels generally leads to an increase in OBC as compared to pure fractions. Studies of the effect of triple combinations of fractions A, B, and C (samples 10 (6:2:2), 11 (2:6:2), 12 (2:2:6), 19 (2:4:4), 20 (4:4:2), 21 (4:2:4)) showed that their use allows obtaining oleogels with high OBC from 96.3% to 100%.
Qualitative Phase Diagrams of Oleogels
The ability to structure liquid oils is due to the component composition of beeswax. Fractionation of beeswax makes it possible to evaluate the contribution of each of them to the gelling process. Since we conducted studies at the same concentration of gelators, it is possible to assess the effect of individual fractions and their combinations on gelation at different temperatures (Fig. 4).
Figure 4 shows that sample 1 (10:0:0) is the most low-melting oleogel, and samples 2 (0:10:0), 6 (0:2:8), 7 (0:8:2), 11 (2:6:2), 12 (2:2:6), 14 (0:6:4), 18 (0:4:6), and 19 (2:4:4) were the most high-melting due to their component composition. Hydrocarbons were the main component of sample 1 (10:0:0) (Table 1). Oleogels structured with beeswax and its individual fractions belong to physical (thermally reversible) gels, in connection with which the melting temperature of oleogels will be lower than the melting temperature of pure gelator during structuring. In this case, the tendency "the most high-melting gelator forms the most high-melting oleogel and contrary" can remain.
The results of the investigation of binary combinations of beeswax fractions (samples 4–9, 13–18) for gel-forming ability at different temperatures established that oleogels containing fraction A in different ratios with B fractions (samples 4 (8:2:0), 13 (4:6:0), 15 (6:4:0) and C (samples 8 (8:0:2), 9 (2:0:8), 16 (6:0:4), 17 (4:0:6)) retain gel-forming ability at temperature increase to 35 °C. The exception is sample 5 (2:8:0), which retains the gelatinous state at 40 °C. At the same time, binary combinations based on fractions B and C (samples 6 (0:2:8), 7 (0:8:2), 14 (0:6:4), 18 (0:4:6)) allow obtaining more high-melting oleogels preserving their gel state at 45 °C. Also, in the investigated compositions no transition state characterized by a fluid liquid was revealed. Thus, the content of the most low-melting fraction A (mixture of hydrocarbons) in the composition leads to obtaining low-melting oleogels. At the same time, in the case of the combination of hydrocarbons and monoesters (samples 4 (8:2:0), 5 (2:8:0), 13 (4:6:0), 15 (6:4:0)), an increase in the content of monoesters to 60 and 80% (samples 13 (4:6:0) and 5 (2:8:0), respectively) increases the temperature of the gel state.
The study of the influence of triple combinations including A, B, and C fractions on the gel-formation ability at different temperatures revealed that a decrease in the content of the most low-melting hydrocarbon fraction leads to an increase in the phase transition temperature. The most high-melting point (45 °C) was characterized by samples of oleogels (11 (2:6:2), 12 (2:2:6), and 19 (2:4:4)) with high monoesters or WEs content.
Differential Scanning Calorimetry
Phase transition temperatures of the oleogels structured with individual beeswax fractions or their combinations were studied using DSC. The experiment revealed that oleogels based on individual fractions A and B were characterized by a single crystallization peak at 34.53 °C and 51.38 °C, respectively. The crystallization isotherm of oleogel based on fraction C is characterized by two peaks at 33.01 °C and 45.18 °C. However, in binary combinations 4 (8:2:0), 8 (8:0:2), and 16 (6:0:4) simultaneous crystallization of both components (presence of only one crystallization peak) was observed (Figure S1 in the supplementary materials), which indicate the formation of eutectic mixtures [7]. Information about the crystallization onset temperature (Tconset) of all studied samples shown in Fig. 5.
Figure 5 shows the distribution map of the crystallization initiation temperatures, which represents the liquidus surface for the three-component system. The lowest temperatures of the onset crystallization were characteristic of the combinations with high content of fraction A. The temperatures of the beginning of crystallization decrease smoothly for all combinations with increasing concentration of fraction A, except for binary combinations containing 60% of fraction C and 80% of fraction B.
Thermograms of sample melting were studied in the same way (Figure S2 in the supplementary materials). The lowest melting point has the oleogel based on fraction A (38.17 °C, sample 1 (10:0:0)), and the highest—is based on fraction B (55.47 °C, sample 2 (0:10:0)). Melting temperature of oleogel based on fraction C is 50.31 °C (sample 3 (0:0:10)). The melting thermograms were characterized by the presence of a greater number of melting peaks compared with the crystallization thermograms. It can be associated with presence of polymorphic transformations in the studied samples during melting [36].
Texture Analysis
The mechanical strength of oleogels is an important factor in their practical application in food products [35]. The mechanical strength was determined as the firmness of the oleogels. Samples were characterized at 20 °C in a “gel” state, according to Fig. 4. The firmness of the investigated oleogels ranged from 0.18 ± 0.01 N to 3.98 ± 0.49 N. The obtained results are shown in Fig. 6, where the dark and light areas correspond to the lower and higher firmness of the oleogel, respectively.
According to Fig. 6, the oleogel samples structured by fractions A, B, and C show the lowest firmness. The decrease of this parameter was observed in the range: sample 2 (0:10:0) > sample 3 (0:0:10) > sample 1 (10:0:0). Most of the investigated oleogels on combinations of fractions had higher firmness than oleogels on individual fractions. The exceptions were samples 5 (2:8:0) and 9 (2:0:8).
A significant increase in firmness of oleogels on binary combinations of fractions A and C was found in samples 8 (8:0:2), 16 (6:0:4) and 17 (4:0:6). Also, the increase of firmness parameter is typical when combining fractions A and B in samples 4 (8:2:0), 13 (4:6:0) and 15 (6:4:0), in which the firmness increases by more than 2 times, relative to samples 1 (10:0:0) and 2 (0:10:0). Analysis of binary combinations of fractions B and C showed that samples 7 (0:8:2), 14 (0:6:4), 18 (0:4:6) and 6 (0:2:8) had higher firmness compared to samples 2 (0:10:0) and 3 (0:0:10). The effect of combining all three fractions on the texture of the oleogels was further evaluated, showing a positive influence of their combination. All the triple combination samples (10 (6:2:2), 11 (2:6:2), 12 (2:2:6), 19 (2:4:4), 20 (4:4:2), 21 (4:2:4)) show increased firmness relative to oleogels structured with fractions A, B, and C.
In our study, oleogels with different crystal morphologies had the greatest strength (OBC and firmness). For example, sample 19 (2:4:4), which had large crystals (Fig. 2), had the highest firmness (Fig. 6). In contrast, some oleogels with large crystals had lower firmness and OBC (e.g., sample 1 (10:0:0)).
We found that most triple combinations of fractions had better gelling properties relative to the initial fractions (A—hydrocarbons; B—monoesters; C—wax esters, fatty alcohols, and free fatty acids). It agrees to the study [7], which shows high gelling properties of hydrocarbons in combination with WEs and FFAs. At the same time, the present study found that it is also possible to obtain solid gels with a high Tonset crystallization without the use of hydrocarbons. The analysis of the OBC of the samples shows that by combining the gelators (6 wt. %), we can achieve high values of this parameter.
Statistical analysis of the data was performed to analyze the relationship between the studied parameters.
Statistical Analysis
Using the data obtained in this work the analysis of correlations between the studied indicators was carried out (Table 2).
As shown in Table 2, Firmness and OBC values had statistically significant correlation only between each other (r = 0.631, p < 0.01). Thus, in firmer samples, the oil-binding capacity was predominantly higher, which is consistent with the work [37]. However, there were samples that did not follow this pattern. According to [38] the oil-binding capacity of wax oleogels is affected by many factors, including the crystal structure in the solid state, crystal size and shape, as well as their spatial distribution and order. Interactions responsive to gel formation are generally considered to have a significant influence on the oil-binding capacity. According to [39] such interactions include hydrogen bonding, π-π-stacking, electrostatic and van der Waals interactions. The authors [40] also state that the crystal network structure responsible for the mechanical properties of the gel is strongly influenced by the type of molecular interactions and the orientation of the gel-forming molecules.
Firmness also depends on different factors, such as the influence of the dispersion medium [18, 41] and the dispersed phase [6, 42]. According to [43] firmness also depends on the polymorphic transformations of the structure-forming crystals during storage. The firmness depends on the technological methods used, such as sintering [43], cooling rate [13, 14, 44, 45], shear rate during preparation [45] or the application of ultrasound [46]. The presence of different mechanisms affecting the firmness and oil-binding capacity of oleogels explains the low degree of linear relationship between these parameters with statistical significance of their dependence.
The closest correlations (p < 0.001) between composition and Tonset of crystallization were observed for HC fractions (r = -0.804) and WEs (r = 0.925). This is connected to the fact that during the mixing of beeswax fractions the systems without congruent melting point were formed, that is, the crystallization Tonset of the mixture never exceeds the crystallization Tonset of its most high-melting component (Figure S1 in supplementary materials). This corresponds to previously obtained data on crystallization of various binary combinations of beeswax fractions [7]. In the studied systems the highest Tonset of crystallization had sample 2 (0:10:0), containing mainly WEs, and the lowest—sample 1(10:0:0), containing only HCs. In this regard, increasing the fraction of WEs leads to an increase in Tonset of crystallization, and increasing the fraction of HCs leads to its decrease. The slightly lower values of the correlation coefficient between Tonset of crystallization and HCs content were due to combinations 4 (8:2:0), 8 (8:0:2), and 16 (6:0:4), containing large amounts of HCs, were eutectics and their Tonset of crystallization decreases non-linearly.
Crystal length in oleogel shows a close negative correlation with the content of FFAs and FAls (r = -0.626, p < 0.01), therefore, an increase in their content leads to a decrease in crystal length. This relationship is due to the fact that FFAs with FAl form mixed crystals as a result of co-crystallization, which leads to the formation of a small crystals [35]. Therefore, more FFA and FAl in the oleogel leads to more small-sized crystals, which reduces the average size of crystals in the oleogel.
In this work, the concentration of the gelling agent in all the investigated oleogels were equal; therefore, a change in the amount of one of the fractions resulted in a proportional change in the amount of another fractions. This is the reason of the observed negative correlations between HC content and WE content (r = -0.945, p < 0.001) as well as with FFA + FAL content (r = -0.555, p < 0.01).
No correlation (r = 0.152, p > 0.05) was found between firmness and average crystal length. It indicates that the size of crystals, is not the key factor influencing on firmness of oleogels based on different gelators.
The values of OBC, firmness and Tonset crystallization of oleogels structured with combinations of wax fractions were then compared with the values measured for oleogel based on beeswax (OBC = 100%, Firmness = 1.7 N, Tonset of crystallization = 48.14 °C). Two groups parameter (higher or lower than that of beeswax oleogels) were distinguished and examined by Multiple Correspondence Analysis (Fig. 7).
Figure 7 shows that the first two dimensions describe 84.6% of the variation between the samples studied, indicating the representativeness of the data presented. The first dimension (Dim1) mainly describes the variation in Firmness and OBC. To the right are the oleogel samples with higher values than the beeswax-based oleogel. On the left are those with lower values. The second dimension (Dim2) mainly describes the difference in the Tonset crystallization index. At the top are samples of oleogels with higher Tonset crystallization than that of beeswax oleogels, and at the bottom, on the contrary, lower. Onset temperature of crystallization is positively correlated with concentration of WE and negatively with HC. Concentration of FFA + FAL is positively correlated with Firmness and OBC.
We can distinguish four main groups of samples depending on the intensity of their parameters. The first group includes oleogels with high Tonset crystallization, Firmness and OBC (samples 7 (0:8:2), 14 (0:6:4), 18 (0:4:6), 19 (2:4:4)). These samples contain predominantly wax esters (64.74–89.36% WE) and free fatty acids and alcohols (7.86–20.64% FFA + FAl).
The second group consists of samples with low Tonset crystallization but high Firmness and OBC (samples 8 (8:0:2), 10 (6:2:2), 12 (2:2:6)). This group mainly contains samples containing high amounts of hydrocarbons. Among them, sample № 8 (8:0:2) is a eutectic mixture of predominantly hydrocarbons with WEs, FFAs, and FAls. Among the samples obtained from the combination of the three fractions in this group, sample 12 (2:2:6) stands out with the highest OBC and firmness.
The third group includes oleogels with low Tonset crystallization, Firmness and OBC (samples 1 (10:0:0), 3 (0:0:10), 4 (8:2:0), 6 (0:2:8), 9 (2:0:8), and 15 (6:4:0). This group includes samples with a low content of refractory B fraction (monoesters).
The fourth group includes oleogels with high Tonset crystallization but low Firmness and OBC (samples 2 (0:10:0), 5 (2:8:0), 13 (4:6:0)), which contain mainly wax esters (57.34–95.10% WE) and hydrocarbons (22.82–41.94% HC).
The sample 11 (2:6:2) with high Firmness, Tonset crystallization, and low OBC, as well as oleogels with low Tconset, having low OBC with high Firmness (samples 17 (4:0:6), 21(4:2:4)), and high OBC with a low Firmness (samples 16 (6:0:4), and 20 (4:4:2)) stand out separately.
Reducing the Tonset crystallization may be preferable from an organoleptic point of view as a way to reduce the waxy mouthfeel described in [16, 47]. The most preferable in this context were samples from the second group with low Tonset crystallization and high Firmness and OBC.
All samples of oleogels obtained using individual beeswax fractions fell into the groups with reduced firmness and OBC. In this regard, their individual use for structuring sunflower oil is inappropriate.
Conclusion
We evaluated the possibility of structuring sunflower oil by binary and triple combinations of beeswax fractions (A, B, and C) in different ratios. Studies of crystal morphology, phase diagrams, oil-binding capacity, the temperature of phase transitions, and textural properties of oleogels were carried out. As a result of the study, the influence of beeswax fractions and their combinations on the gelling of sunflower oil was established. It was shown that the ratio between the studied fractions predominantly affects the properties of oleogel in a non-linear way. The exception is the Tonset crystallization, which is in a close positive linear correlation with the WE content and in a negative correlation with the HC content.
Conditions for obtaining stronger gels with either high or low Tonset crystallization was identified. It was shown that WEs (64.74–89.36%) with the addition of FFAs and FAls (7.86–20.64% FFA + FAl) form oleogels with high Tonset crystallization, Firmness and OBC. To reduce Tonset crystallization it is necessary to increase hydrocarbon content (up to 80.18%) at the expense of WEs content. These results can be used for directed correction of wax gelators composition to regulate the oleogel properties. In this case, the correction can be carried out both by fractionation and by combining individual types of waxes with different chemical compositions.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Y.V. Frolova, A.A. Kochetkova, R.V. Sobolev, V.M. Vorobyeva, V.M. Kodentsova, Vopr. Pitan. 90, 64 (2021)
A. Singh, F.-I. Auzanneau, M.A. Rogers, Food Res. Int. 97, 307 (2017)
A.I. Blake, A.G. Marangoni, Food Biophys. 10, 403 (2015)
A.I. Blake, J.F. Toro-Vazquez, and H.-S. Hwang, in Edible Oleogels (Elsevier, 2018), pp. 133–171
M. Davidovich-Pinhas, A.J. Gravelle, S. Barbut, A.G. Marangoni, Food Hydrocoll. 46, 76 (2015)
C.D. Doan, C.M. To, M. De Vrieze, F. Lynen, S. Danthine, A. Brown, K. Dewettinck, A.R. Patel, Food Chem. 214, 717 (2017)
V. Sarkisyan, R. Sobolev, Y. Frolova, A. Malinkin, M. Makarenko, A. Kochetkova, J. Am. Oil Chem. Soc. 98, 281 (2021)
A. Shirvani, S.A.H. Goli, J. Varshosaz, L. Salvia-Trujillo, O. Martín-Belloso, Food Chem. 387, 132934 (2022)
H. Brykczynski, T. Wettlaufer, E. Flöter, J. Am. Oil Chem. Soc. (2022). https://doi.org/10.1002/aocs.12589
A.P. Tulloch, Bee World 61, 47 (1980)
A.P. Tulloch, Lipids 5, 247 (1970)
R. Sobolev, Y. Frolova, V. Sarkisyan, M. Makarenko, A. Kochetkova, Food Biosci. 48, 101744 (2022)
Y. Yao, H. Zhou, W. Liu, C. Li, S. Wang, J. Oleo Sci. 70, 135 (2021)
V. Sarkisyan, R. Sobolev, Y. Frolova, I. Vorobiova, A. Kochetkova, Gels 8, 39 (2022)
M.A. Rogers, A.J. Wright, A.G. Marangoni, Soft Matter 5, 1594 (2009)
Y. Shi, C. Liu, Z. Zheng, X. Chai, W. Han, Y. Liu, J. Food Sci. 86, 3987 (2021)
Y.V. Frolova, R.V. Sobolev, A.A. Kochetkova, I.O.P. Conf, Ser. Earth Environ. Sci. 941, 012033 (2021)
Y. Frolova, V. Sarkisyan, R. Sobolev, M. Makarenko, M. Semin, A. Kochetkova, Gels 8, 48 (2022)
A. Borriello, N. Antonella Miele, P. Masi, A. Aiello, and S. Cavella, Food Chem. 375, 131805 (2022)
F.G. Gandolfo, A. Bot, E. Flöter, J. Am. Oil Chem. Soc. 81, 1 (2004)
A.J. Gravelle, A.G. Marangoni, Adv. Food Nutr. Res. 84, 1 (2018)
S. Jana, S. Martini, J. Am. Oil Chem. Soc. 93, 543 (2016)
J.K. Winkler-Moser, J. Anderson, F.C. Felker, H.-S. Hwang, J. Am. Oil Chem. Soc. 96, 1125 (2019)
Y. Frolova, R. Sobolev, and A. Kochetkova, E3S Web Conf. 285, 05009 (2021)
J. Gómez-Estaca, A.M. Herrero, B. Herranz, M.D. Álvarez, F. Jiménez-Colmenero, S. Cofrades, Food Hydrocoll. 87, 960 (2019)
K. Wijarnprecha, K. Aryusuk, P. Santiwattana, S. Sonwai, D. Rousseau, Food Res. Int. 112, 199 (2018)
J.G. Moberly, M.T. Bernards, K.V. Waynant, J. Cheminform. 10(1), 1–2 (2018)
S. Lê, J. Josse, F. Husson, J. Stat. Softw. 25(1), 1–18 (2008)
A. Kassambara and F. Mundt, Package ‘Factoextra’. Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.3 (2017). https://CRAN.R-project.org/package=factoextra Assessed 7 October 2022.
A. Marangoni, in Food Science and Technology (CRC Press, 2004), pp. 21–82
P. Bennema, X.Y. Liu, K. Lewtas, R.D. Tack, J.J.M. Rijpkema, K.J. Roberts, J. Cryst. Growth 121, 679 (1992)
J.A. Morales-Rueda, E. Dibildox-Alvarado, M.A. Charó-Alonso, J.F. Toro-Vazquez, J. Am. Oil Chem. Soc. 86, 765 (2009)
C. Blach, A.J. Gravelle, F. Peyronel, J. Weiss, S. Barbut, A.G. Marangoni, RSC Adv. 6, 81151 (2016)
A.J. Martins, A.A. Vicente, R.L. Cunha, M.A. Cerqueira, Food Funct. 9, 758 (2018)
M. Callau, K. Sow-Kébé, L. Nicolas-Morgantini, A.-L. Fameau, J. Colloid Interface Sci. 560, 874 (2020)
L. Bayés-García, T. Calvet, M. Àngel Cuevas-Diarte, S. Ueno, and K. Sato, CrystEngComm 15, 302 (2013)
K.-O. Choi, H.-S. Hwang, S. Jeong, S. Kim, S. Lee, J. Food Sci. 85, 3432 (2020)
S.M. Ghazani, S. Dobson, A.G. Marangoni, Curr Res Food Sci 5, 998 (2022)
S. Manzoor, F.A. Masoodi, F. Naqash, R. Rashid, Food Hydrocolloids for Health 2, 100058 (2022)
J. Rosen-Kligvasser, M. Davidovich-Pinhas, Food Chem. 334, 127585 (2021)
M. Scharfe, J. Niksch, E. Flöter, Eur. J. Lipid Sci. Technol. 124(7), 2100068 (2022)
E. Yilmaz, E. Keskin Uslu, and C. Öz, J. Am. Oil Chem. Soc. 98, 643 (2021)
I. Tavernier, C.D. Doan, D. Van de Walle, S. Danthine, T. Rimaux, K. Dewettinck, RSC Adv. 7, 12113 (2017)
A.I. Blake, A.G. Marangoni, Food Biophys. 10, 456 (2015)
C.D. Doan, I. Tavernier, P.K. Okuro, K. Dewettinck, Innov. Food Sci. Emerg. Technol. 45, 42 (2018)
T.L.T. da Silva, S. Danthine, J. Food Sci. 86, 343 (2021)
T. Wettlaufer, B. Hetzer, E. Flöter, Eur. J. Lipid Sci. Technol. 123, 2000345 (2021)
Acknowledgements
This work is supported by the Russian Science Foundation under grant (Project No. 19-16-00113).
Author information
Authors and Affiliations
Contributions
Conceptualization: [Varuzhan Sarkisyan], Methodology: [Yuliya Frolova, Varuzhan Sarkisyan], Formal analysis and investigation: [Varuzhan Sarkisyan, Yuliya Frolova, Roman Sobolev], Writing—original draft preparation: [Roman Sobolev], Writing—review and editing: [Varuzhan Sarkisyan, Yuliya Frolova, Alla Kochetkova], Supervision: [Alla Kochetkova].
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarkisyan, V., Frolova, Y., Sobolev, R. et al. On the Role of Beeswax Components in the Regulation of Sunflower Oil Oleogel Properties. Food Biophysics 18, 262–272 (2023). https://doi.org/10.1007/s11483-022-09769-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-022-09769-0