Abstract
Purpose
Salinity and vegetation can significantly affect the productions and emissions of greenhouse gases (GHGs) in wetland soils. We aim to investigate the effect of salinity on the productions and emissions of GHGs in marsh soils during the decomposition of wetland plants under different scenarios.
Methods
Soils and wetland plants collected from a freshwater marsh (FM) and a saline marsh (SM) in the Louisiana Barataria Basin estuary were incubated under different levels of salinity treatment for 180 days. Gas and soil samples were periodically collected and analyzed for CO2, CH4, and N2O and for TN, TC, and DOC contents, respectively.
Results
CH4 and CO2 production rates reached maximums on day 30 as the decomposition processed. The increment of CH4 and CO2 production rates in SM soils was greater than in FM soils. The CH4 and CO2 production rates were generally more significantly correlated with DOC than with TC. Reducing salinity inhibited the productions and emissions of CO2 and N2O but significantly promoted those of CH4 in SM soils, while in FM soils, increasing salinity significantly decreased the CO2 production and emission but had no significant effect on CH4 and N2O. The CO2-equivalent cumulative emissions increased by 41% as the salinity decreased by 17.7% in saline marsh, and the percent contribution of CH4 emissions to CO2-equivalent cumulative emissions increased from 15 to 47%.
Conclusion
Our results suggested the potential that the diversion of Mississippi River water to the wetlands might promote the production and emission of CH4 in saline marsh.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Salinity is an important environmental factor to affect the biogeochemical cycle of carbon and nitrogen in the estuary wetlands (Herbert et al. 2015; Wang et al. 2017). The effects of salinity on the production and emission of the greenhouse gases (GHGs) in estuary wetlands have attracted extensive attention and research especially under the background of the global sea-level rise and salt water intrusion. In many studies, the emissions of the GHGs were measured directly in situ along the salinity gradient in marsh wetlands (DeLaune et al. 1983; Smith et al. 1983; Bartlett et al. 1987; Weston et al. 2014; Wilson et al. 2015; Welti et al. 2016), or the simulated saline intrusion experiments were carried out in situ (Neubauer et al. 2013; Herbert et al. 2018; Tong et al. 2020). These experiments have shown different changing trends of GHG discharge fluxes under the conditions of salinity variation and salt water intrusion, which could be due to the fact that the emissions of GHGs are also affected by tidal water level, temperature, vegetation community, and other key environmental factors (Olsson et al. 2015; Welti et al. 2016; Araujo et al. 2018; Chen et al. 2018; Hu et al. 2017; Sierra et al. 2020). The method of laboratory incubation has the advantage of allowing the manipulation of salinity as the main variable and avoids the influence of the complicating factors such as shifts in water level and redox status seen in the field (Marton et al. 2012). Therefore, laboratory incubations using the marsh soils have been widely used to explore the effects of salinity on the GHG production (Marton et al. 2012; Dang et al. 2017; Ardón et al. 2018; Zhang et al. 2018; Li et al. 2019; Wang et al. 2019).
Wetland plants play an important role in the nutrient cycle in the estuary wetlands. During the growing period, plants absorb nutrients from the soils, and when they die and decay, they release nutrients back to the soils (Luo et al. 2020). Moreover, the growth and decomposition of wetland plants can also change the physicochemical properties of soils, which will indirectly influence the nutrient transformation and the productions and emissions of GHGs (Chen et al. 2015; Liu et al. 2017; Luo et al. 2020). Previous research studies have shown that plant productivity/vegetation biomass (Neubauer et al. 2013; Weston et al. 2014; Xu et al. 2014; Sheng et al. 2015; Deng et al. 2021) and plant type/vegetation community/vegetation composition (Neubauer et al. 2013; Zhang et al. 2013, 2018; Xu et al. 2014; Chen et al. 2018; Herbert et al. 2018) may have strong controlling effects on the emissions of GHGs in wetlands. Furthermore, the interaction between vegetation and salinity could affect the C emission or uptake (Martin and Moseman-Valtierra 2015; Sheng et al. 2015). Nevertheless, the majority of these studies were carried out in the growing season of the wetland plants or only have focused on the impact of wetland plants in the growing season (Martin and Moseman-Valtierra 2015; Olsson et al. 2015), with less information available on how GHGs respond to plant decomposition in tidal marsh with different salinity levels (Emery and Fulweiler 2014; Luo et al. 2020). As the vegetations vary between tidal flats, the compositions of litter produced by them are different (Xu et al. 2014; Chen et al. 2018), which will also influence the productions and emissions of GHGs (Chen et al. 2015; Yakimovich et al. 2018).
Louisiana Barataria Basin estuary as part of the Louisiana’s coastal wetlands is located directly south and west of New Orleans. The Barataria Basin estuary has faced the problem of wetland loss and salt water intrusion due to the eustatic sea-level rise and anthropogenic activities such as direct discharge of brine associated with oil recovery operation (Pezeshki et al. 1989; DeLaune et al. 2013; Wang et al. 2015). As a restoration effort, the Mississippi River water has been periodically diverted into the northern region of the basin, which also reduces salt water intrusion and leads to lower basin salinities (DeLaune et al. 2008, 2013). Previous studies in marshes of Barataria Basin paid more attention to the effect of freshwater diversions on soil C and N (DeLaune et al. 2005, 2008, 2013; Yu et al. 2006; Wang et al. 2011); however, less information is available on the productions and emissions of GHGs in the basin soils at the different scenarios of salinity change, especially with respect to plant decomposition. Therefore, in this research, soils and vegetation from the freshwater marsh (FM) and saline marsh (SM) were collected, respectively, and used to prepare the soil subsamples with different salinities in laboratory. The objectives of this research were (1) to observe the change of the C and N content and the production rates and emissions of the GHGs in the soils with different salinities during the decomposition of wetland plants and (2) to explore the interaction effect of salinity and decomposition of vegetations on the productions and emissions of the GHGs and identify the main factors.
2 Materials and methods
2.1 Site description and sampling
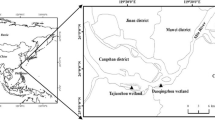
Composite samples of about 20 kg soil at the depth of 0–20 cm were taken using a stainless shovel from a freshwater marsh (S1, 29°52′N lat. and 90°14′W long.) and saline marsh (S2, 29°28′N lat. and 89°56′W long.) located in the Louisiana Barataria Basin estuary (Fig. 1). The vegetation in the freshwater marsh is dominated by a mixture of Alternanthera philoxeroides (A. philoxeroides) and Sagittaria lancifolia (S. lancifolia), while the saline marsh is dominated by Spartina alterniflora (S. alterniflora). The aboveground part of the dominant plants was also collected at the same time. The soil at the freshwater marsh site is classified as an Allemands muck (Terric Haplosaprists) and, at the saline site, as a Timbalier muck (Typic Haplosaprists) (Dodla et al. 2008; Wang et al. 2011).
2.2 Soil and plant material preparation
The soil samples from freshwater marsh and saline marsh were thoroughly mixed, respectively, before analysis and preparation for subsamples with different salinities, while the plant materials were first washed with tap water and then rinsed with deionized water followed by air-drying. The air-dried plant materials were cut to the size of about 1 cm in length and stored in paper bags at room temperature prior to experimental use.
Five portions (each 2000.00 g) of saline marsh soil samples were prepared with one being kept as the original soil sample (S1), and the other four being leached with different amount of deionized water to eliminate salts (McClung and Frankenberger 1987). As such, five subsamples with a decreased salinity gradient (represented by S1, S2, S3, S4, and S5, respectively) were prepared. Likewise, five portions (each 2000.00 g) of freshwater marsh soil were prepared with one as the original soil sample (F1), and the other four being added with different concentrations of NaCl solutions to increase salts (Liu et al. 2017; Maucieri et al. 2017; Chen et al. 2018). Consequently, five subsamples with an increased salinity gradient (represented by F1, F2, F3, F4, and F5, respectively) were prepared.
2.3 Incubation experiment and analytical method
About 300.00 g of each prepared soil subsample was weighted and put in a 500 ml triangular flask, and four replicates were made for each salinity treatment. All the soils in the flasks were aerobically pre-incubated for 7 days to stabilize the microbial activity (Huang et al. 2004). Pre-incubation and the afterwards incubation of the soil samples were carried out in the dark at 25 ± 1 ℃, and the flasks were weighted daily to correct the soil moisture by adding deionized water.
To determine the initial greenhouse gas production rates (0 day, before adding the plant materials), incubating flasks were thoroughly flushed with ambient air using an air compressor for 5 min to ventilate air so as to avoid the accumulation of GHGs between the sampling intervals, and then the mouth of each flask was sealed with a gas tight rubber plug. The rubber plug was made with a 10-cm plastic tube through so that one end of which was connecting the air inside the flask and the other end outside the flask was equipped with a three-way valve to allow collection of gas samples from the headspace of the flask using a syringe. For the gas sampling, all the flasks were incubated for 24 h, and then 20 ml of the headspace air of each flask was collected in triplicate using the 20-ml syringes, which was taken as the initial gas samples in the flasks just before the incubation with plant materials. After all the gas samples were collected, the rubber plugs were removed, and about 30.00 g soil was collected and analyzed for C and N contents. Then, for the freshwater marsh soil incubation, about 10.00 g of mixed materials of A. Philoxeroides and S. lancifolia (N content 1.912 ± 0.115%, C content 38.717 ± 0.037%, n = 3) was added into each flask. For the saline marsh soil incubation, about 10.00 g of S. alterniflora (N content 1.125 ± 0.016%, C content 45.532 ± 0.236%, n = 3) was added into each flask. The plant materials and the soil in each flask were mixed thoroughly using a glass rod. The same incubation and gas sampling procedures as those on 0 day were carried out every 30 days. Overall, the soil samples were collected seven times (at days 0, 30, 60, 90, 120, 150, and 180) in total during the 180 days of aerobic incubation.
Soil water content and bulk density were determined using field moist soil samples, whereas other properties were determined using air-dried samples. Water content of soil was measured by oven drying method and expressed as a percentage of the wet soil weight, while bulk density was determined using the core cutter method and expressed as oven-dried soil mass (g) per unit volume (m3). Soil pH was measured using a pH meter in 1:2.5 soil-to-water suspension. The soil electrical conductivity (EC) and salinity were determined by an EC meter and the gravimetric method, respectively (at soil:water = 1:5). Soil was ground and analyzed for TC and TN using an Elementar Vario EL Cube elemental analyzer by dry combustion at 900 ℃. Dissolved organic carbon (DOC) was extracted by 0.5 mol L−1 K2SO4 (Maucieri et al. 2017) and measured by a TC analyzer (TOC-VCSH, Shimadzu, USA) after filtration through a 0.45-μm cellulose nylon membrane filter. Gas samples were analyzed for CO2, CH4, and N2O using gas chromatography (CP-3800G, Varian, CA), and the analysis for the gas samples of each incubation began as soon as the sampling ended and finished within 6 h.
2.4 Data analysis and statistics
The production rates of the greenhouse gases (CO2, CH4, and N2O) were calculated according to the formula as follows:
where R is the production rates of greenhouse gases (μmol kg−1 day−1); CH-m and CH-v are the mass concentrations (μmol L−1) and volume concentrations (ppmv) of greenhouse gases in the headspace of the flask after the 24 incubation; CA-m and CA-v are the initial mass concentrations (μmol L−1) and volume concentrations (ppmv) of greenhouse gases in the flasks before the incubation; VH is the volume of flask headspace (mL); α is the Bunsen solubility coefficients of greenhouse gases in water given by Weiss (1974) for CO2, by Weiss and Price (1980) for N2O, and by Wiesenburg and Guinasso (1979) for CH4; Vw is the volume of water in the flask (mL), which is calculated according to the soil water content and soil weight in the flask; and Ws is the dry weight of soil in the flask (g).
The cumulative emissions of GHGs were calculated using the following equation (Chen et al. 2015):
where R is the production rate of CO2, CH4, or N2O; i describes the ith measurement; and 30 is the days between the two adjacent measurements.
As CH4 and N2O had higher global warming potential (GWP) than CO2, which are approximately 28 and 265 times larger (mass basis) (IPCC 2013), we calculated the CO2-equivalent cumulative emissions (\({\mathrm{CE}}_{{\mathrm{CO}}_{2}-\mathrm{eq}}\)) of the three GHGs by using the following equation (Xu et al. 2014):
Repeated-measures ANOVA was used to detect the effect of salinity on the soil properties and production rates of GHGs during the decomposition of wetland plants (main effect, salinity; within-subjects term, 0 day, 30 days, 60 days, 90 days, 120 days, 150 days, and 180 days). The least significant difference (LSD) post hoc test was used to delineate the differences among salinity treatments after a significant main effect of salinity. The correlations between the soil properties and the production rates of GHGs were also analyzed using the Spearman’s rank-order correlation model as not all the data were normally distributed. All the statistical analyses were conducted using SPSS 25.0.
3 Results
3.1 The physicochemical properties of soils for the incubation experiment
The physicochemical properties of the initial soils were presented in Table 1. There were significantly positive correlations between the EC and salinity both in SM soils (R2 = 0.987, P < 0.001) and FM soils (R2 = 0.9995, P < 0.001). The C and N content in FM soils was significantly higher than those in SM soils (P < 0.001), which was consistent with the results found by Wang et al. (2011, 2015) in Mississippi River deltaic plain wetland soils. There was no significant difference in the soil C and N content between the salinity treatments either for the SM soils or for FM soils, indicating that the salt leaching or adding treatment had no significant impact on the soil C and N content. Compared with the original SM soil (S1), the DOC content decreased by 12.7%, 14.5%, 27.1%, and 34.4% from S2 to S5, indicating that DOC was lost during the salt leaching process. There was significant positive correlation between the DOC and salinity in SM soils (R2 = 0.966, P < 0.01). The pH of the SM soils increased with the decrease of salinity (R2 = 0.970, P < 0.01); however, the pH values increased gradually from 7.25 to 7.48 as the salinity decreased from 28.76 to 9.36 g·kg−1, indicating that the alkalization didn’t occur during the desalinization. Therefore, we supposed that the increase of pH of SM soils during salt leaching might be more related to the loss of Ca2+ and increase of HCO3− in soils than to the hydrolysis of exchangeable Na+ on soil colloids (Chen et al. 2000; Xu and Li 2019). Additional research is needed to identify the real reason of the increasing pH values during the desalinization of SM soils.
3.2 The change of C, N, and DOC content in the soils
As shown in Table 2, the soil C and N dynamics changed significantly with the vegetation decomposition time (P < 0.0001). As illustrated in Fig. 2, the C, N, and DOC contents in both SM and FM soils with different salinities increased significantly on the 30th day due to the decomposition of the added plant debris (P < 0.05). The C, N, and DOC contents generally reached maximums on day 30 for FM soils, while the occurrence of the C and N peak values in SM soils lagged behind those of FM soils.
The change of C, N, DOC content, and C:N ratios in soils during the experiment (SM means the saline marsh; FM means the freshwater marsh. The error bar represents the standard deviation of the samples (n = 4). From S1 to S5, the soil salinities decreased, while from F1 to F5, the soil salinities increased)
The soil C and C:N ratios of the saline marsh were also affected by the main effect of salinity, while salinity had no significant impact on C and N in soils of the freshwater marsh (Table 2). Specifically, the C content and C:N ratios increased most in S3 at the middle salinity (17.04 g/kg) but least in S1 (28.76 g/kg) (P < 0.05). The change of N content in soils varied greatly between salinity treatments (Fig. 2), and lower N content than the initial values was found in SM soils sometimes from day 60 to day 150 and was in F4 and F5 soils on day 90 (Fig. 2), suggesting consumption of nitrogen in the soils as the incubation progressed. The DOC levels presented a consistent significant change trend both in SM and FM, increasing to the peak values on day 60 and then decreasing; however, no significant differences were found between salinity treatments. Furthermore, the interaction effects of the decomposition time and salinity on C and N dynamics were generally observed except for DOC in SM soils and N in FM soils (Table 2).
3.3 The change of greenhouse gas production rates and cumulative emissions
As shown in Table 3, the production rates and cumulative emissions of GHGs both in SM and FM soils changed significantly with the vegetation decomposition time (P < 0.0001). The rates and emissions of all three GHGs in SM soils were significantly affected by the main effect of salinity (P < 0.05), while salinity only had a significant impact on CO2 in FM soils (P < 0.05). Moreover, the interaction effects of the decomposition time and salinity was observed in the N2O rates and emissions both in SM and FM soils as well as in the CO2 cumulative emissions (P < 0.05).
As illustrated in Fig. 3, the production rates of CH4 increased dramatically both in the SM and FM soils and then decreased significantly on the 90th day and remained low rates until the end of the experiment. The increase of the production rates of CH4 in SM soils was larger than that in FM soils. The maximums of the production rates occurred on the 30th day in SM and FM soils except those in the S3 and F1 soils on the 60th day. Moreover, the extent of increase differed between the soils with different salinities; however, the significant differences (P < 0.05) were only found in SM soils. The production rates of CO2 in soils of SM and FM showed similar change trend, which increased dramatically on the 30th day and then decreased gradually. Like the rates of CH4, SM soils also presented more increase in the production rates of CO2 than FM soils. Furthermore, the production rates of CO2 in S1 and F1 increased significantly more than those in other soils of SM and FM (P < 0.05), respectively. Unlike CH4 and CO2, the production rates of N2O didn’t show significant increase in soils of SM and FM after the addition of plant debris. The production rates of N2O in the SM soils even decreased on the 30th day and increased gradually and then decreased. The production rates of N2O in S1 increased more than in the other soils of SM (P < 0.05).
The change of the production rates of GHGs in the soils during the experiment (SM means the saline marsh; FM means the freshwater marsh. The error bar represents the standard deviation of the samples (n = 4). From S1 to S5, the soil salinities decreased, while from F1 to F5, the soil salinities increased)
The cumulative emissions of the GHGs were illustrated in Fig. 4. In general, the cumulative emissions of CH4 both in SM and FM soils increased within the first 90 days and then remained relatively stable, while the emissions of CO2 and N2O kept an increasing trend till the end of the experiment. SM soils had higher cumulative emissions of CH4 and CO2 but lower emissions of N2O than FM soils. The significant differences of the cumulative emissions of the GHGs between the salinity treatments in SM and FM soils were as same as those of the production rates of the GHGs. Moreover, differed from the production rates of CO2, there were significant interaction effects of salinity and decomposition time on the CO2 cumulative emissions both in SM and FM soils (Table 3).
The cumulative emissions of the GHGs in the soils during the experiment (SM means the saline marsh; FM means the freshwater marsh. The error bar represents the standard deviation of the samples (n = 4). From S1 to S5, the soil salinities decreased, while from F1 to F5, the soil salinities increased)
3.4 The correlations between GHG production rates and soil C and N indexes
As showed in Fig. 5, the CH4 and CO2 production rates in the soils of SM and FM generally had significant positive correlations with the soil DOC content, while the N2O production rates were generally negatively correlated with the soil DOC content, and the significant correlations were found in S2, S4, and F2. In saline marsh, the CO2 production rates had significant positive correlations with the C content and C:N, while no significant correlations were found for the CH4 production rates except the significant negative correlation with C:N in S1. Furthermore, none of the production rates of GHGs was significantly correlated with the N content in SM soils. In freshwater marsh, the CH4 production rates were significantly positively correlated with the C content except in F5, while the significant positive correlations between the CO2 production rates and C content were only found in F1 and F2. The N2O production rates had significant negative correlations with the C content in S2 and F2; in addition, in F1, F2, and F3, the N2O production rates were significantly correlated with the N content and C:N although the correlations were not consistent. In general, the production rates of N2O were not as strongly correlated with the C and N indexes as those of CH4 and CO2.
4 Discussion
4.1 Effects on soil physicochemical properties
The decomposition of wetland plants significantly affected the C and N dynamics both in SM and FM soils (Fig. 2, Table 2). The mineralization of plant materials increased the soil C and N content, and the maximums of C, N, and DOC contents mostly occurred on day 30 for FM soils and on day 60 for SM soils, respectively. As summarized in the study of Bridgham and Richardson (2003), higher initial N content, a lower C:N ratio, and/or a lower lignin:N ratio of litter enhance short-term (months to a few years) decay rates. Specifically, Heal et al. (1997) explained that plant materials with C:N ratios < 20 decompose rapidly, and substrates with intermediate C:N ratios of 25–75 can also decompose quickly, but N mineralization activity is often reduced by increased microbial immobilization as well as protein complexation by polyphenols when the cells lyse. The C:N ratios of S. lancifolia were intermediate (40.5 ± 0.64, n = 3), while the C:N ratios of plant materials (the mixture of A. philoxeroides and S. lancifolia) were low (20.3 ± 1.22, n = 3). Moreover, the mixed plant materials also had higher N content (1.912 ± 0.115%) than S. lancifolia (1.125 ± 0.016%). Therefore, the plant materials in FM soils decomposed faster compared with that in SM soils, which resulted in the faster and greater increase of C, N, and DOC content in FM soils than in SM soils as well as general N consumption in SM soils.
Salinity has been suggested as a regulator of soil organic carbon and plant detritus decomposition due to its impact on microbial community structure and extracellular enzyme activity (Morrissey et al. 2014; Li et al. 2019). Elevated salinity has been reported to decrease decomposition rates as it inhibits microbial activities (Roache et al. 2006). In the present study, S3 with the middle salinity (17.04 g/kg) presented significant increase of C content and highest C:N (P < 0.05), while S1, the original soils with the highest salinity (28.76 g/kg), was on the contrary (P < 0.05). Considering the high water content in SM soils (Table 1), our results were somewhat consistent with Zhai et al. (2021) who found that the moderately higher salinity would increase anaerobic decomposition owing to the combined effect of salinity and inundation. In contrast to the negative effect of salinity in SM soils, we speculated that the non-significant effect of salinity on the C and N dynamics in FM soils might be related to the soil fertility (higher soil N content than SM soils as shown in Table 1) (Mendelssohn et al. 1999), the litter quality (liable to decompose) (Stagg et al. 2018), and the salt type (Berger et al. 2019) as well as the adaption of the microbial community to the increase of salinity (Roache et al. 2006; Vander Vorste et al. 2019).
4.2 Effects on the production rates and cumulative emissions of the GHGs
The incorporation of plant materials significantly enhanced carbon gas production and emission (Figs. 3, 4, and Table 3). The maximums of CO2 and CH4 production rates occurred on day 30 or day 60, which was consistent with those of soil C and DOC contents. Soil carbon especially labile C was previously shown as the main source of the CO2 and CH4 for its easily degradation and availability to microorganisms (Liu et al. 2017; Zhang et al. 2018; Li et al. 2019; Luo et al. 2020). DOC is and has been considered as a major indicator of labile C (Huang et al. 2004). In this study, the CO2 and CH4 production rates were clearly more significantly correlated with DOC than with the total C content (Fig. 5), and the exhaustion of DOC likely resulted in the slower rates of C productions after day 60 (Fig. 3). In contrast to CO2 and CH4, the N2O production rates varied greatly and differently in SM and FM soils (Fig. 3). The decrease of the N2O production rates in SM soils on day 30 might be due to the significant increase of C:N ratios (Fig. 2), which led to lower available N to soil microorganisms (Zhang et al. 2018). The negative correlations between the N2O production rates and DOC might also suggest the consumption of DOC by the nitrifiers and denitrifiers (Huang et al. 2004). Soil mineral N, including NH4+-N and NO3−-N are important factors influencing N2O productions (Huang et al. 2004). Although the correlations between the N2O production rates and total N content were not significant (Fig. 5), there were significant interaction effects of salinity and decomposition of wetland plants on the N2O production rates (Table 3), which could complicate the situation of N2O production resulting in its non-consistent correlations with N and C:N ratios in F1, F2, and F3 (Zhang et al. 2018).
Salinity had significant main effect on the production rates and cumulative emissions of all three GHGs in SM soils but only on those of CO2 in FM soils during the experiment (Fig. 3, Table 3). However, we did not find a consistent pattern of significant increase or decrease in GHG production rates and cumulative emissions with salinity gradient. Specifically, significant increase in GHG production rates and emissions was found in S4 and S2 for CH4, S1 and F1 for CO2, and S1 for N2O. Salinity has been repeatedly reported to inhibit methane emissions in tidal wetlands (Poffenbarger et al. 2011; Marton et al. 2012; Olsson et al. 2015; Sheng et al. 2015), and the reason might be the sulfate-reducing bacteria (SRB) outcompeting methanogens for substrate (Bartlett et al. 1987; Hu et al. 2017; Araujo et al. 2018). Besides, salinity was also found to have an impact on the functional mcrA gene of methanogens and pmoA gene of methanotrophs, thus affecting the production and emission of CH4 (Li et al. 2019; Tong et al. 2017). SM soils with lower salinity (S4 and S2) than S1 had shown more increase in CH4 production rates and emissions, indicating that high salinity also inhibited the production and emission of CH4 during the decomposition of wetland plants. The effect of salinity on the production and emission of CH4 in FM soils was not significant (Table 3), which might be related with the choice of salt for the salinity increasing treatment. Although NaCl is the predominant salt in sea water, it also contains other salts which may have different impact on carbon mineralization (Liu et al. 2017; Zhang et al. 2018). Besides, soil pH is an important variable that could govern the activity and function of soil microorganism (Min et al. 2014). The methanogen activity is favored by alkalescence (Tong et al. 2017), and previously, the optimum pH range for methanogens was reported to be 6.9 to 7.2 (Wang et al. 1993). In our study, the SM soil pH was 7.25 ~ 7.48, while the FM soil pH was 5.97 ~ 6.78 (Table 1), which might have contributed to the lower CH4 production rates and emissions in FM soils than in SM soils (Fig. 3).
The effect of salinity on the CO2 production rates and emissions was different between SM and FM soils. For SM soils, S1 with the highest salinity (28.76 g·kg−1) had more increase in the CO2 production rates and emissions compared with the other soils with reduced salinities. It seemed that the decrease of salinity in SM soils had not promoted the production of CO2, which might be related to the leaching loss of DOC (Table 1). Wang et al. (2019) also found that decreasing the salinity from 26 to 19 ppt in salt marsh soils had no effect on CO2 fluxes. For FM soils, F1 with the lowest salinity (1.95 g·kg−1) showed significantly higher increase in the CO2 production rates and emissions than those with increased salinities. This indicated that the increase in the salinity of freshwater marsh soils might reduce CO2 productions and emissions during the decomposition of wetland plants. Saviozzi et al. (2011) has reported that the size of microbial biomass, the enzyme activity number, and soil respiration in the artificially salinized soil samples were decreased by increasing salinity and protease and dehydrogenase which were inhibited by NaCl during laboratory incubation. The adverse effects of salinity could inhibit the growth and activity of heterotrophic soil microorganisms (Hu et al. 2016; Wang et al. 2020) and thus reduce the production and emission of CO2 (Sheng et al. 2015; Ardón et al. 2018; Zhang et al. 2018). The negative effect of salinity on CO2 productions found in our study are consistent with the findings of several previous studies in laboratory incubation experiments (Maucieri et al. 2017; Wang et al. 2017; Ardón et al. 2018; Zhang et al. 2018). Nevertheless, no significant difference was observed in CO2 production rates between the higher salinity treatments (F2 ~ F5, salinity, 9.96 ~ 33.94 g·kg−1 presented in Table 1). This might be related to the salinity thresholds (~ 10–15‰) above which potential CO2 productions were inhibited (Wang et al. 2017). Besides, the increase in carbon content due to the decomposition of plant materials could assist soil microbes to adapt to adverse environmental conditions and multiply rapidly, resulting in no significant difference in soil CO2 production rates among the salinity treatments (Dang et al. 2017).
Several studies have reported that the N2O production increased with increasing salinity in laboratory-incubated tidal soils (Marton et al. 2012; Li et al. 2019). However, in the present study, only S1 showed significantly more increase in N2O production rates and emissions compared with the other soils with the reduced salinities in SM soils, and no significant difference was found in FM soils with different salinities (Table 3). Marton et al. (2012) also reported that denitrification was the greatest in the highest salinity treatment. N2O is produced in soils through the microbial processes of nitrification and denitrification (Welti et al. 2016), and the N2O production is sensitive to soil moisture or water level (Zhang et al. 2013, 2018; Liu et al. 2017; Welti et al. 2016). Under wet (anaerobic) soil conditions, heterotrophic denitrification is the predominant N2O-producing process (Huang et al. 2004; Hu et al. 2015; Welti et al. 2016). The nirS, nirK, and nosZ of denitrifier communities control the production and emission of N2O as nirS and nirK are the microbial producers of N2O, while nosZ reduces N2O to N2 (Henry et al. 2006; Zhao et al. 2018). Li et al. (2019) have reported that the copy number of nirS and nirK genes increased, while those of nosZ gene decreased along with the salinity gradients in an incubation experiment. Therefore, the higher water content in the soils (80.5%, shown in Table 1) and the denitrifier community might favor the denitrification leading to the higher production rates and more emissions of N2O in S1. Moreover, the lower C:N ratios in S1 also created favorable conditions for preventing complete N2O reduction (Welti et al. 2016). However, some other studies also reported that an increase in salinity could decrease the N2O production and emission because high levels of salt can suppress both nitrification and denitrification (Dang et al. 2017; Liu et al. 2017; Ardón et al. 2018), while Wang et al. (2017) found no relationship between potential N2O production and salinity. Therefore, the complex effects of salinity on N cycling and the interaction of salinity and plant decomposition (Table 3) might be the reasons for the insignificant salinity effect on the N2O production rates in FM soils. Besides, it might also be related to the type of salt used for developing salinity because different salts may have different impact on nitrogen mineralization (McClung and Frankenberger 1987; Ardón et al. 2018).
4.3 Study implications and limitations
Our study indicated that in saline marsh, the decrease of salinity promoted the production and emission of CH4 but inhibited those of CO2 and N2O, while in freshwater marsh, the increase of salinity decreased the production and emission of CO2, with the cumulative emissions of CO2 in F2 at the middle salinity of 9.96 g·kg−1 being reduced by 14.3% compared with the original soil (F1, 1.95 g·kg−1). While the production and emissions of CO2, CH4, and N2O could be influenced by various intrinsic nutrient and carbon sources in these wetlands as reported in our previous observations (Dodla et al. 2008, 2009; Wei et al. 2020), the current study provides the information regarding to GHG production and emission under controlled salinity plant decomposition conditions, which have not been investigated. Moreover, we calculated the CO2-equivalent cumulative emissions of the three GHGs in S1 (the original soil, 28.76 g·kg−1) and S2 with the reduced salinity of 23.67 g·kg−1 according to formula (3). The results showed that the CO2-equivalent cumulative emissions increased by 41% as the salinity decreased by 17.7% (i.e., from 28.76 g·kg−1 of S1 to 23.67 g·kg−1of S2) in saline marsh. Correspondingly, the contribution of CH4 emissions to \({\mathrm{CE}}_{{\mathrm{CO}}_{2}-\mathrm{eq}}\) increased from 15 to 47%, while the contribution of CO2 emissions decreased from 83 to 52%. This suggested a potential that the diversion of the Mississippi River water to the Louisiana coastal wetlands might induce the increase of CH4 production and emission in the saline marsh and accelerate the global warming effect.
Nevertheless, our experiments were conducted using laboratory incubations, and the production rates may differ from those of flux chambers or other measurements of in situ greenhouse gas emissions; hence, it has to be considered that results from a simplified laboratory incubation cannot directly be transferred to conditions in the field which are much more complex. We are also unable to predict from these results the long-term consequences of salinity change on the productions and emissions of GHGs. Therefore, to better elucidate the role of salinity associated with plant decomposition in the productions and emissions of GHGs, further studies under natural conditions are needed, especially to characterize those mechanisms from a microbial perspective.
5 Conclusion
Our findings indicated that reducing the salinity of the soils in saline marsh significantly promoted the production and emission of CH4 but inhibited those of CO2 and N2O, while in freshwater marsh, increasing the salinity significantly decreased the production and emission of CO2 but had no significant impact on those of CH4 and CO2. Furthermore, the CO2-equivalent cumulative emissions in soils with reduced salinities from saline marsh increased significantly compared with the original soil. This implied that the diversion of the Mississippi River water to the wetlands might promote the production and emission of CH4 and increase the global warming potential. However, due to the limitations of the laboratory incubation experiment, the response of the productions and emissions of the GHGs to the salinity during the decomposition of wetland plants warrants additional study under field conditions, especially from a microbial perspective.
References
Araujo J, Pratihary A, Naik R, Naik H, Naqvi SWA (2018) Benthic fluxes of methane along the salinity gradient of a tropical monsoonal estuary: implications for CH4 supersaturation and emission. Mar Chem 202:73–85. https://doi.org/10.1016/j.marchem.2018.03.008
Ardón M, Helton AM, Bernhardt ES (2018) Salinity effects on greenhouse gas emissions from wetland soils are contingent upon hydrologic setting: a microcosm experiment. Biogeochemistry 140(2):217–232. https://doi.org/10.1007/s10533-018-0486-2
Bartlett KB, Bartlett DS, Harriss RC, Sebacher DL (1987) Methane emissions along a salt marsh salinity gradient. Biogeochemistry 4:183–202. https://doi.org/10.1007/BF02187365
Berger E, Frör O, Schäfer RB (2019) Salinity impacts on river ecosystem processes: a critical mini-review. Philos Trans R Soc B 374:20180010. https://doi.org/10.1098/rstb.2018.0010
Bridgham SD, Richardson CJ (2003) Endogenous versus exogenous nutrient control over decomposition and mineralization in North Carolina peatlands. Biogeochemistry 65:151–178. https://doi.org/10.2307/1469850
Chen Q, Guo B, Zhao C, Xing B (2018) Characteristics of CH4 and CO2 emissions and influence of water and salinity in the Yellow River delta wetland, China. Environ Pollut 239:289–299. https://doi.org/10.1016/j.envpol.2018.04.043
Chen W, Chen BB, Shen QR (2000) Studies of the changes of pH value and alkalization of heavily saline soil in seabeach during its desalting process. Acta Pedologica Sinica 37(4):521–528. (in Chinese). https://doi.org/10.11766/trxb199911300412
Chen YC, Sun J, Xie FT, Yan Y, Wang XD, Cheng GW, Lu XY (2015) Non-Additive Effects of Litter Diversity on Greenhouse Gas Emissions from Alpine Steppe Soil in Northern Tibet. Sci Rep-UK 5:17664. https://doi.org/10.1038/srep17664
Dang DM, Macdonald B, Warneke S, White I (2017) Available carbon and nitrate increase greenhouse gas emissions from soils affected by salinity. Soil Res 55(1):47–57. https://doi.org/10.1071/SR16010
DeLaune RD, Johnson CB, Gambrell RP, Jugsujinda A (2008) Export of dissolved organic carbon from a ponded freshwater marsh receiving diverted Mississippi River water. Sci World J 8:1210–1218. https://doi.org/10.1100/tsw.2008.156
Delaune RD, Jugsujinda A, West JL, Johnson CB, Kongchum M (2005) A screening of the capacity of Louisiana freshwater wetlands to process nitrate in diverted Mississippi River water. Ecol Eng 25:315–321. https://doi.org/10.1016/j.ecoleng.2005.06.001
DeLaune RD, Kongchum M, White JR, Jugsujinda A (2013) Freshwater diversions as an ecosystem management tool for maintaining soil organic matter accretion in coastal marshes. Catena 107:139–144. https://doi.org/10.1016/j.catena.2013.02.012
DeLaune RD, Smith CJ, Patrick WH Jr (1983) Methane release from Gulf coast wetlands. Tellus B 35(1):8–15. https://doi.org/10.1111/j.1600-0889.1983.tb00002.x
Deng HG, Zhang J, Wu JJ, Yang LW, Zhang YH, Yao X (2021) Physicochemical properties and greenhouse gas emissions of water body during the decomposition of Potamogeton crispus with different values of initial debris biomass. Environ Sci Pollut R 29:5505–5516. https://doi.org/10.1007/s11356-021-15823-0
Dodla SK, Wang JJ, Delaune RD, Breitenbeck G (2009) Carbon gas production under different electron acceptors in a freshwater marsh soil. Chemosphere 76:517–522. https://doi.org/10.1016/j.chemosphere.2009.03.013
Dodla SK, Wang JJ, Delaune RD, Cook RL (2008) Denitrification potential and its relation to organic carbon quality in three coastal wetland soils. Sci Total Environ 407:471–480. https://doi.org/10.1016/j.scitotenv.2008.08.022
Emery HE, Fulweiler RW (2014) Spartina alterniflora and invasive Phragmites australis stands have similar greenhouse gas emissions in a New England marsh. Aquat Bot 116:83–92. https://doi.org/10.1016/j.aquabot.2014.01.010
Heal OW, Anderson JM, Swift MJ (1997) Plant litter quality and decomposition: an historical overview. In: Cadisch G, Giller KE (eds) Driven by nature: plant litter quality and decomposition. CAB International, Wallingford, Oxfordshire, pp 3–30
Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol 72:5181–5189. https://doi.org/10.1128/AEM.00231-06
Herbert ER, Boon P, Burgin AJ, Neubauer SC, Franklin RB, Ardón M, Hopfensperger KN, Lamers LPM, Gell P (2015) A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6(10):206. https://doi.org/10.1890/ES14-00534.1
Herbert ER, Schubauer-Berigan J, Craft CB (2018) Differential effects of chronic and acute simulated seawater intrusion on tidal freshwater marsh carbon cycling. Biogeochemistry 138(2):137–154. https://doi.org/10.1007/s10533-018-0436-z
Hu HW, Chen DL, He JZ (2015) Microbial regulation of terrestrial nitrous oxide formation: understanding the biological pathways for prediction of emission rates. FEMS Microbiol Rev 39:729–749. https://doi.org/10.1093/femsre/fuv021
Hu M, Ren H, Ren P, Li J, Wilson BJ, Tong C (2017) Response of gaseous carbon emissions to low-level salinity increase in tidal marsh ecosystem of the Min River estuary, southeastern China. J Environ Sci 52:210–222. https://doi.org/10.1016/j.jes.2016.05.009
Hu Y, Wang L, Xi XF, Hu JJ, Hou YH, Le YQ, Fu XH (2016) Effects of salinity on soil bacterial and archaeal community in estuarine wetlands and its implications for carbon sequestration: verification in the Yellow River Delta. Chem Ecol 32(7):669–683. https://doi.org/10.1080/02757540.2016.1177519
Huang Y, Zou J, Zheng X, Wang Y, Xu X (2004) Nitrous oxide emissions as influenced by amendment of plant residues with different C: N ratios. Soil Biol Biochem 36:973–981. https://doi.org/10.1016/j.soilbio.2004.02.009
IPCC (2013) Climate change 2013: the physical science basis. Contribution of Working Group 1 to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom, New York, NY, USA
Li XF, Gao DZ, Hou LJ, Liu M (2019) Salinity stress changed the biogeochemical controls on CH4 and N2O emissions of estuarine and intertidal sediments. Sci Total Environ 652:593–601. https://doi.org/10.1016/j.scitotenv.2018.10.294
Liu X, Ruecker A, Song B, Xing J, Conner WH, Chow AT (2017) Effects of salinity and wet-dry treatments on C and N dynamics in coastal-forested wetland soils: implications of sea level rise. Soil Biol Biochem 112:56–67. https://doi.org/10.1016/j.soilbio.2017.04.002
Luo P, Tong X, Liu F, Huang M, Xu J, Xiao RL, Wu JS (2020) Nutrients release and greenhouse gas emission during decomposition of Myriophyllum aquaticum in a sediment-water system. Environ Pollut 260:114015. https://doi.org/10.1016/j.envpol.2020.114015
Martin RM, Moseman-Valtierra S (2015) Greenhouse gas fluxes vary between Phragmites Australis and native vegetation zones in coastal wetlands along a salinity gradient. Wetlands 35(6):1021–1031. https://doi.org/10.1007/s13157-015-0690-y
Marton JM, Herbert ER, Craft CB (2012) Effects of salinity on denitrification and greenhouse gas production from laboratory-incubated tidal forest soils. Wetlands 32(2):347–357. https://doi.org/10.1007/s13157-012-0270-3
Maucieri C, Zhang Y, McDaniel MD, Borin M, Adams MA (2017) Short-term effects of biochar and salinity on soil greenhouse gas emissions from a semi-arid Australian soil after re-wetting. Geoderma 307:267–276. https://doi.org/10.1016/j.geoderma.2017.07.028
McClung G, Frankenberger WT (1987) Nitrogen mineralization rates in saline vs. salt-amended soils. Plant Soil 104 (1):13–21. https://doi.org/10.1007/BF02370619
Mendelssohn IA, Sorrell BK, Brix H, Schierup HH, Lorenzen B, Maltby E (1999) Controls on soil cellulose decomposition along a salinity gradient in a Phragmites australis wetland in Denmark. Aquat Bot 64:381–398. https://doi.org/10.1016/S0304-3770(99)00065-0
Min K, Lehmeier CA, Ballantyne F, Tatarko A, Billings SA (2014) Differential effects of pH on temperature sensitivity of organic carbon and nitrogen decay. Soil Biol Biochem 76:193–200. https://doi.org/10.1016/j.soilbio.2014.05.021
Morrissey EM, Gillespie JL, Morina JC, Franklin RB (2014) Salinity affects microbial activity and soil organic matter content in tidal wetlands. Global Change Biol 20:1351–1362. https://doi.org/10.1111/gcb.12431
Neubauer SC, Franklin RB, Berrier DJ (2013) Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeoscience 10:8171–8183. https://doi.org/10.5194/bg-10-8171-2013
Olsson L, Ye S, Yu X, Wei M, Krauss KW, Brix H (2015) Factors influencing CO2 and CH4 emissions from coastal wetlands in the Liaohe Delta. Northeast China Biogeosci 12(16):4965–4977. https://doi.org/10.5194/bg-12-4965-2015
Pezeshki SR, Delaune RD, Patrick WH Jr (1989) Assessment of saltwater intrusion impact on gas exchange behavior of Louisiana Gulf Coast wetland species. Wetl Ecol Manag 1(1):21–30. https://doi.org/10.1007/BF00177887
Poffenbarger HJ, Needelman BA, Megonigal JP (2011) Salinity influence on methane emissions from tidal marshes. Wetlands 31:831–842. https://doi.org/10.1007/s13157-011-0197-0
Roache MC, Bailey PC, Boon PI (2006) Effects of salinity on the decay of the freshwater macrophyte, Triglochin procerum. Aquat Bot 84:45–52. https://doi.org/10.1016/j.aquabot.2005.07.014
Saviozzi A, Cardelli R, Di Puccio R (2011) Impact of salinity on soil biological activities: a laboratory experiment. Commun Soil Sci Plan 42(3):358–367. https://doi.org/10.1080/00103624.2011.542226
Sheng Q, Wang L, Wu JH (2015) Vegetation alters the effects of salinity on greenhouse gas emissions and carbon sequestration in a newly created wetland. Ecol Eng 84:542–550. https://doi.org/10.1016/j.ecoleng.2015.09.047
Sierra A, Jimenez-Lopez D, Ortega T, Gomez-Parra A, Forja J (2020) Factors controlling the variability and emissions of greenhouse gases (CO2, CH4 and N2O) in three estuaries of the Southern Iberian Atlantic Basin during July 2017. Mar Chem 226:103867. https://doi.org/10.1016/j.marchem.2020.103867
Smith CJ, DeLaune RD, Patrick WH Jr (1983) Carbon dioxide emission and carbon accumulation in coastal wetlands. Estuar Coast Shelf S 17(1):21–29. https://doi.org/10.1016/0272-7714(83)90042-2
Stagg CL, Baustian MM, Perry CL, Carruthers TJB, Hall CT (2018) Direct and indirect controls on organic matter decomposition in four coastal wetland communities along a landscape salinity gradient. J Ecol 106(2):655–670. https://doi.org/10.1111/1365-2745.12901
Tong C, Luo M, Huang JF, She CX, Li YL, Ren P (2020) Greenhouse gas fluxes and porewater geochemistry following short-term pulses of saltwater and Fe(III) in a subtropical tidal freshwater estuarine marsh. Geoderma 369:114340. https://doi.org/10.1016/j.geoderma.2020.114340
Tong C, Cadillo-Quiroz H, Zeng ZH, She CX, Yang P, Huang JF (2017) Changes of community structure and abundance of methanogens in soils along a freshwater-brackish water gradient in subtropical estuarine marshes. Geoderma 299:101–110. https://doi.org/10.1016/j.geoderma.2017.03.026
Vander Vorste R, Timpano AJ, Cappellin C, Badgley BD, Zipper CE, Schoenholtz SH (2019) Microbial and macroinvertebrate communities, but not leaf decomposition, change along a mining-induced salinity gradient. Freshwater Biol 64(4):671–684. https://doi.org/10.1111/fwb.13253
Wang C, Tong C, Chambers LG, Liu X (2017) Identifying the salinity thresholds that impact greenhouse gas production in subtropical tidal freshwater marsh soils. Wetlands 37(3):559–571. https://doi.org/10.1007/s13157-017-0890-8
Wang FM, Kroeger KD, Gonneea ME, Pohlman JW, Tang JW (2019) Water salinity and inundation control soil carbon decomposition during salt marsh restoration: an incubation experiment. Ecol Evol 9(4):1911–1921. https://doi.org/10.1002/ece3.4884
Wang JJ, Dodla SK, DeLaune RD (2015) Characteristics and functions of labile organic carbon in coastal wetland soils of the Mississippi River Deltaic Plain, in: He, Z., Wu, F. (Eds.), Labile Organic Matter-Chemical Compositions, Function, and Significance in Soil and the Environment. SSSA Special Publication 62, pp 315–336. https://doi.org/10.2136/sssaspecpub62.2014.0046
Wang JJ, Dodla SK, DeLaune RD, Hudnall WH, Cook RL (2011) Soil carbon characteristics in two Mississippi River deltaic marshland profiles. Wetlands 31:157–166. https://doi.org/10.1007/s13157-010-0130-y
Wang KC, Xu JZ, Li YW, Wang HY, Wei Q, Liao LX, Liu XY (2020) Response of soil respiration and microbial biomass to soil salinity under different water content in the coastal areas of eastern China. Eurasian Soil Sci 53(1):82–89. https://doi.org/10.1134/S1064229320010159
Wang ZP, Lindau CW, Delaune RD, Patrick WH Jr (1993) Methane emission and entrapment in flooded rice soils as affected by soil properties. Biol Fert Soils 16(3):163–168. https://doi.org/10.1007/BF00361401
Wei Z, Wang JJ, Dodla SK, Fultz LM, Gaston LA, Park J-H, DeLaune RD, Meng Y (2020) Exploring anaerobic CO2 production response to elevated nitrate levels in Gulf of Mexico coastal wetlands: phenomena and relationships. Sci Total Environ 709:136158. https://doi.org/10.1016/j.scitotenv.2019.136158
Weiss RF (1974) Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Mar Chem 2:203–215. https://doi.org/10.1016/0304-4203(74)90015-2
Weiss RF, Price BA (1980) Nitrous oxide solubility in water and seawater. Mar Chem 8:347–359. https://doi.org/10.1016/0304-4203(80)90024-9
Welti N, Hayes M, Lockington D (2016) Seasonal nitrous oxide and methane emissions across a subtropical estuarine salinity gradient. Biogeochemistry 132(1–2):55–69. https://doi.org/10.1007/s10533-016-0287-4
Weston NB, Neubauer SC, Velinsky DJ, Vile MA (2014) Net ecosystem carbon exchange and the greenhouse gas balance of tidal marshes along an estuarine salinity gradient. Biogeochemistry 120(1–3):163–189. https://doi.org/10.1007/s10533-014-9989-7
Wiesenburg DA, Guinasso NL (1979) Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea water. J Chem Eng Data 24:356–360. https://doi.org/10.1021/je60083a006
Wilson BJ, Mortazavi B, Kiene RP (2015) Spatial and temporal variability in carbon dioxide and methane exchange at three coastal marshes along a salinity gradient in a northern Gulf of Mexico estuary. Biogeochemistry 123(3):329–347. https://doi.org/10.1007/s10533-015-0085-4
Xu JC, Li XB (2019) Reclamation of coastal saline wasteland using drip irrigation and embedded subsurface pipes. Agron J 111(6):2881–2887. https://doi.org/10.2134/agronj2019.03.0179
Xu X, Zou X, Cao L, Zhamangulova N, Zhao Y, Tang D, Liu D (2014) Seasonal and spatial dynamics of greenhouse gas emissions under various vegetation covers in a coastal saline wetland in southeast China. Ecol Eng 73:469–477. https://doi.org/10.1016/j.ecoleng.2014.09.087
Yakimovich KM, Emilson EJS, Carson MA, Tanentzap AJ, Basiliko N, Mykytczuk NCS (2018) Plant litter type dictates microbial communities responsible for greenhouse gas production in amended lake sediments. Front Microbiol 9:2662. https://doi.org/10.3389/fmicb.2018.02662
Yu K, Delaune RD, Boeckx P (2006) Direct measurement of denitrification activity in a Gulf coast freshwater marsh receiving diverted Mississippi River water. Chemosphere 65:2449–2455. https://doi.org/10.1016/j.chemosphere.2006.04.046
Zhai JX, Anderson JT, Yan GX, Cong L, Wu YN, Dai LY, Liu JK, Zhang ZM (2021) Decomposition and nutrient dynamics responses of plant litter to interactive effects of flooding and salinity in Yellow River Delta wetland in northeastern China. Ecol Indic 120:106943. https://doi.org/10.1016/j.ecolind.2020.106943
Zhang L, Song L, Wang B, Shao H, Zhang L, Qin X (2018) Co-effects of salinity and moisture on CO2 and N2O emissions of laboratory-incubated salt-affected soils from different vegetation types. Geoderma 332:109–120. https://doi.org/10.1016/j.geoderma.2018.06.025
Zhang L, Song L, Zhang L, Shao H, Chen X, Yan K (2013) Seasonal dynamics in nitrous oxide emissions under different types of vegetation in saline-alkaline soils of the Yellow River Delta, China and implications for eco-restoring coastal wetland. Ecol Eng 61:82–89. https://doi.org/10.1016/j.ecoleng.2013.09.065
Zhao SY, Wang Q, Zhou JM, Yuan DD, Zhu GB (2018) Linking abundance and community of microbial N2O-producers and N2O-reducers with enzymatic N2O production potential in a riparian zone. Sci Total Environ 642:1090–1099. https://doi.org/10.1016/j.scitotenv.2018.06.110
Acknowledgements
We would like to thank Meng Wang, Yili Meng, Baoyue Zhou, Negar Dehghani Tafti, Scott M Pensky, and Zhuo Wei for their help with sample collection, preparation, and analysis.
Funding
This research was, in part, supported by the National Natural Science Foundation of China (Grant No.41401563, 41977322), the Liaocheng University Research Foundation (Grant No. 318011909, 318012019), the USDA National Institute of Food and Agriculture (NIFA)-Agricultural and Food Research Initiative (AFRI) (Grant #2009–65102-05975), and the USDA National Institute of Food and Agriculture Hatch Project (Grant #10138880).
Author information
Authors and Affiliations
Contributions
JZ and JJW designed the research. JZ, RX, and HD completed the experiment and sample analysis. JZ wrote and edited the original draft of the paper. JJW and RDD revised the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Weixin Ding
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Wang, J.J., Xiao, R. et al. Effect of salinity on greenhouse gas production and emission in marsh soils during the decomposition of wetland plants. J Soils Sediments 23, 131–144 (2023). https://doi.org/10.1007/s11368-022-03334-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-022-03334-5