Abstract
Currently, there is a lack of knowledge about GHG emissions, specifically N2O and CH4, in subtropical coastal freshwater wetland and mangroves in the southern hemisphere. In this study, we quantified the gas fluxes and substrate availability in a subtropical coastal wetland off the coast of southeast Queensland, Australia over a complete wet-dry seasonal cycle. Sites were selected along a salinity gradient ranging from marine (34 psu) in a mangrove forest to freshwater (0.05 psu) wetland, encompassing the range of tidal influence. Fluxes were quantified for CH4 (range −0.4–483 mg C–CH4 h−1 m−2) and N2O (−5.5–126.4 μg N–N2O h−1 m−2), with the system acting as an overall source for CH4 and N2O (mean N2O and CH4 fluxes: 52.8 μg N–N2O h−1 m−2 and 48.7 mg C–CH4 h−1 m−2, respectively). Significantly higher N2O fluxes were measured during the summer months (summer mean 64.2 ± 22.2 μg N–N2O h−1 m−2; winter mean 33.1 ± 24.4 µg N–N2O h–1 m−2) but not CH4 fluxes (summer mean 30.2 ± 81.1 mg C–CH4 h−1 m−2; winter mean 37.4 ± 79.6 mg C–CH4 h−1 m−2). The changes with season are primarily driven by temperature and precipitation controls on the dissolved inorganic nitrogen (DIN) concentration. A significant spatial pattern was observed based on location within the study site, with highest fluxes observed in the freshwater tidal wetland and decreasing through the mangrove forest. The dissolved organic carbon (DOC) varied throughout the landscape and was correlated with higher CH4 fluxes, but this was a nonlinear trend. DIN availability was dominated by N–NH4 and correlated to changes in N2O fluxes throughout the landscape. Overall, we did not observe linear relationships between CH4 and N2O fluxes and salinity, oxygen or substrate availability along the fresh-marine continuum, suggesting that this ecosystem is a mosaic of processes and responses to environmental changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal wetlands and estuaries encompass a range of distinct landscapes, including mangroves, salt marshes, tidal saline wetlands, and fresh tidal wetlands which uniquely act as dynamic filters and as transformers of nutrients (Masselink and Gehrels 2015). Due to the high productivity of these areas relative to both freshwater and marine systems and the complex hydrodynamic and chemical regimes along the salinity gradient, coastal ecosystems provide a biogeochemical connection between the marine and terrestrial environments, serving as both a sink and source of nutrients, elements, and the resulting greenhouse gases (GHG) (Bouillon et al. 2008; Feller et al. 2010; Weston et al. 2014). The interaction of carbon (C) and nitrogen (N) contained within the surface waters, groundwater, and the sediments fuel microbial activity (e.g. methanogenesis and denitrification) resulting in the fluxes of methane (CH4) and nitrous oxide (N2O) (Chen et al. 2012; Fernandes and Bharathi 2011). Sediment gas fluxes are the result of soil microbial activity, determined by the reduction–oxidation potential (redox potential), availability of electron acceptors (organic carbon) and substrate, and the microbial community structure (Jahangir et al. 2012, 2013; Weymann et al. 2008). Adding to the complexity is the high temporal and spatial variability of the controlling factors which can create “hot spots” and “hot moments” of activity (McClain et al. 2003). Changes in the flooding regime, tidal inundation, and temperature will result in changes in the distribution of carbon and nutrient cycling and the rates of CH4 and N2O exchange between estuarine ecosystems and the atmosphere (Barendregt and Swarth 2013; Murray et al. 2015; Weston et al. 2014). Quantifying the processes can be difficult in these environments due to strong spatiotemporal variability in the key processes (i.e. tidal fluctuations) (Groffman et al. 2009; Vidon et al. 2010).
Due to varying redox conditions, coastal sediments can be either a sink or source for CH4. When oxygen is present in sediments or groundwater, aerobic respiration is possible, but as oxygen concentration decreases, suboxic and anoxic reductive processes will occur according to the available electron acceptors: denitrification (NO3 −), manganese reduction (Mn), iron reduction (Fe), sulfate reduction (SO4), and finally methanogenesis. Methane flux from the sediments to the atmosphere is the sum of two opposing processes; methanogenesis in anaerobic conditions and methane oxidation in aerobic conditions (Conrad 1996). The strength of the CH4 flux can be influenced by inter-annual and inter-seasonal changes in the concentration of organic carbon stocks within the sediments.
Recent estimates show that mangroves account for 14% of carbon sequestration by the global ocean (Alongi 2012). These systems contribute up to 10% of the global terrestrially derived particulate organic carbon (POC) and dissolved organic carbon (DOC) exported to the coastal zone (Bergamaschi et al. 2012). As water temperature is expected to rise as a result of climate change, the carbon burial and export from estuaries is predicted to increase due to an increase of benthic productivity (Maher and Eyre 2011). If mangrove carbon stocks are disturbed, resultant gas emissions may be very high (Alongi 2012). Due to the inhibitory effect of sulfate electron acceptors common in saline environments, the CH4 source strength of coastal mangroves and saline marshes is expected to be small. However, recent studies have shown that large CH4 emissions can occur even when sulfate reduction is dominant (Alongi and Brinkman 2011; Lee et al. 2008).
Likewise, nitrogen cycling and the resultant N2O and N2 fluxes are facilitated predominantly by microbial processes and constrained by sediment redox conditions (Alongi 1996). Denitrification is the anaerobic microbial mediated process which converts nitrate (N–NO3 −) to either N2O or non-reactive di-nitrogen gas (N2). Aerobic sediments can produce N2O as a by-product of nitrification, the process which transforms ammonium (N–NH4 +) to N–NO3 −. The availability of dissolved inorganic nitrogen (DIN) compounds and the redox conditions of the sediment determine the flux strength of sediments. Given the high global warming potential of N2O (310 times that of CO2), the ratio between N2 and N2O production determines whether floodplains are sources or sinks in N2O inventories (Verhoeven et al. 2006). DIN concentrations can fluctuate widely due to the location of mangroves in the intertidal zone (Meyer et al. 2008). These estuarine systems have been shown to be important zones for the input and conversion of reactive N species as well as for denitrification, particularly during times of anthropogenic inputs of nitrate and ammonium (Galloway and Cowling 2002). As much as 55% of the N loss in mangrove sediments occurs through the denitrification pathway (Chiu et al. 2004). Although mangroves have the ability to efficiently moderate elevated nutrient concentrations through denitrification, they are under-represented in global N2O inventories (Murray et al. 2015). In systems prone to elevated nutrient levels, benthic N2O production has been shown to be three orders of magnitude higher than natural production rates in estuarine sediments elsewhere (Fernandes et al. 2010).
Currently, there is a lack of knowledge about the GHG emissions, specifically N2O and CH4, of coastal freshwater wetlands and mangroves in Australia (Allen et al. 2007; Page and Dalal 2011). There is high variability and large uncertainties in the sediment exchange of CH4 and N2O estuaries in relation to latitude and climate (Bartlett et al. 1987; Murray et al. 2015; Reddy and DeLaune 2008). Subtropical and tropical forest soils have been acknowledged to represent significant sources of N2O emissions (Davidson and Kingerlee 1997; Kim et al. 2013; Xu et al. 2013). There is a considerable level of uncertainty for global N2O emissions, especially for the southern hemisphere, where there are fewer aquatic emission studies (Seitzinger et al. 2000). However, little research has been carried out investigating the effects of substrate pool changes (i.e. carbon and nitrate) on N2O emissions in coastal sediments from subtropical regions. Few studies have quantified and investigated CH4 and N2O fluxes in tidal wetlands (Dalal and Allen 2008; Livesley and Andrusiak 2012; Tong et al. 2013) and included the adjacent tidal oligohane and freshwater ecosystems. Yet, these adjacent wetlands and mangroves have been shown to have a significant capacity for N2O and CH4 emissions (Jennerjahn 2012; Nahlik and Mitsch 2011; Seitzinger et al. 2000). Australian mangroves cover an area of 11,500 km2 (Alongi 2002) ranging from 15°S–38°S, whereas salt marshes are more widespread, stretching from the tropics to the high latitudes of 55°N and 45°S with a distribution of >13,500 km2. Salt marshes are located in the upper tidal, hypersaline zones and can transition into freshwater wetland ecosystems. These ecosystems are a significant portion of the Australian and subtropical ecosystems and should be included when considering GHG balances. Specific to North Stradbroke Island, estuaries make up 3.4% of the island’s total area, accounting for 16.1% of all wetland types on the island (Stephens 2009). These wetlands are significant because they provide some of the best and largest representatives of southern sandy island wetlands, including a diversity of wildlife in natural conditions, and provide refuge habitat to wildlife including migratory species (Queensland 2014). The remnant ecosystems are large and well connected and have a high level of ecological integrity. Therefore, it is necessary to understand the external inputs and mechanisms driving GHG emissions in coastal estuaries along the many interfaces at the microbial, the plot and the regional scale, taking into account the temporally dynamic nature of the GHG emissions in coastal wetland ecosystems (Saha et al. 2010).
Hypotheses and objectives
The overall purpose of this study was to quantify the N2O and CH4 fluxes along a marine to freshwater gradient in an undisturbed subtropical estuary. Two main hypotheses were tested: (1) there would be a strong seasonal pattern for both CH4 and N2O fluxes, with higher fluxes measured during the summer months, and (2) there would be a strong spatial pattern, with fluxes decreasing as groundwater salinity increased, due to a change in the organic carbon and DIN availability. To test these hypotheses, the nutrient concentrations (carbon, nitrogen, phosphorous) were quantified in the local groundwater and sediments in order to follow the spatial pattern of substrate availability in one annual cycle of wet-dry seasons and compared with measured CH4 and N2O fluxes.
Methods
Study site
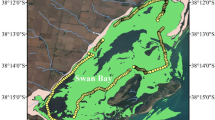
This study is located along Cooroon Cooroonphah Creek on North Stradbroke Island (NSI), (27.4°S, 153.4°E) a 27,390 ha sand mass dune-island located off the coast of southeast Queensland, Australia (Fig. 1) which forms the south-eastern boundary of Moreton Bay. The region has a subtropical climate characterized by hot, wet summers (average summer temperature of 27.9–22.4 °C; accumulated rainfall 864.4 mm) and cooler, drier winters (average winter temperature 22.5–15.5 °C; accumulated rainfall of 636.7 mm) with an annual evaporation of 1930 mm year−1 (Brisbane Aerodrome, Bureau of Meteorology, Brisbane). The geomorphology of the island is dominated by highly permeable sands resulting in high infiltration and low runoff rates even during periods of high intensity rainfall (up to 100 mm day−1) (Cox et al. 2011). A large portion (27%) of the groundwater flow on NSI is thought to flow under a semi-confining layer and to emerge as near-coastal discharge (Leach 2011).
This study site consists of a mangrove forest (co-dominated by Rhizophora stylosa Avicennia marina) followed by an oligohaline marsh (dominated by Juncae sp.) and finally a tidal freshwater wetland (dominated by Gahnia sieberiana, Empodisma minus, and Gleichenia spp). The general tidal range of these sites is given in more detail elsewhere (Specht 2011), but in short, the mangrove forest receives tides up to the mean high water spring tides (0–2 m), and the marsh and tidal wetland areas receive the extreme high water spring tides (2–5 m). Five plots were established (Fig. 1; Table 1) within the study site. At each plot, each sampling site consisted of three monitoring wells and one set of triplicate gas collars. The locations of the wells and collars were selected to minimize spatial variability within each sampling plot and the influence of vegetation.
Static chambers
Fluxes of N2O and CH4 were measured during monthly sampling campaigns conducted during the low tide period when all the sites were exposed. Gas fluxes across the sediment-atmosphere interface were measured using the closed static chamber method (Nykanen et al. 1995). At each sampling plot, triplicate static, PVC collars (diameter 150 mm, height 30 cm, total area 0.18 m2) were installed 5–10 cm into sediments, depending upon sediment texture, one month prior to the start of the study in order to minimize disturbance during sampling. During sampling, lids were placed on collars and sealed to ensure that it was gas-tight and kept closed during the low-tide period. Plants located within collars were slightly bent to fit under the chamber, but were not cut, broken or damaged during the measurement period. Gas sampling occurred through a rubber septa fitted in the top of the chamber lid. The chamber headspace was gently mixed by pumping the syringe four times prior to gas sampling. Gas samples (24 ml) were drawn from the headspace of the chambers with 60 ml polypropylene syringes (Terumo, Australia) equipped with a three-way stopcock (Connecta) and transported to the lab using pre-evacuated 12 ml extetainer vials (Labco, Bucks, UK).
Vials were kept cool and analysed within 48 h using a gas chromatograph (Agilent 7890A) fitted with a dual detector for N2O (ECD) and CH4 (FID). The standard error on standards measurements was 0.4 ± 0.2 and 2.5 ± 1.1% for N2O and CH4 respectively. The flux of N2O and CH4 were calculated from the increase or decrease in the concentration over ambient gas concentration, measured on the same day as detailed in Eq. 1.
where G1 is the gas concentration at the end of sampling and G0 is the ambient gas concentration, T is the duration of the closed chambers, and A is the surface area of the plot.
Groundwater and sediment sampling
At each sampling site, three 50 mm PVC monitoring wells were installed to a screened depth of 1.5, 2.5, and 3.5 m below the ground level. Prior to monthly sampling, all wells were purged for at least three well volumes using a low flow peristaltic pump (Eco Environmental). Temperature, electrical conductivity, pH, and reduction–oxidation potential (redox) were measured using an HQ40d meter (Hach, USA) in situ in order to limit contact with the atmosphere. Water samples were filtered through a prepared Whatman GF/F filter and analysed within 24 h for DOC (Shimadzu TOC-L CSH Total Organic Carbon Analyzer) and N–NH4 +, P–PO4 +, N–NO2 − and N–NO3 − (Lachat QuikChem8000 Flow Injection Analyser, Hach, USA).
Additionally, triplicate sediment cores were taken at three depths; 0–10, 10–20 and 20–50 cm at three times during the study period. Dry weight of the sediment samples was determined by oven-drying sediments at 60 °C to constant mass. Total phosphorus was quantified with microwave digestion and analysed using ICPOES (Varian Vista Pro ICPOES) (Kingston and Walter 2007). Total carbon and total nitrogen were measured with a combustion analyser (LECO Truspec CHN at 1100 °C) (Rayment and Lyons 2011).
Statistics
Non-parametric tests (Kruskal-Wallis and Mann–Whitney U) were used to compare the dataset between plots and seasons, respectively. Spearman correlation was used to compare the gas fluxes with the environmental variables. All statistics were done with SPSS (v12).
Results
Groundwater and sediment nutrient concentrations
Based on the measured rainfall, the wet season was defined from February to August and the dry season from September to January (Fig. 2). Temperature and monthly rainfall amounts correlated positively (p < 0.05; ρ = 0.217), typical for the warm, wet summers in the subtropics. The depth to groundwater was not significantly different between the dry and wet seasons (p = 0.98 U = 906), but the highest fluctuations were measured in plots 4 (mean 10 ± 65 cm) and 5 (mean −4.7 ± 55.4 cm). Despite the large fluctuation, no significant differences were observed in the depth to groundwater between plots (p = 0.65) while the chambers were installed. However, plots 1–3 are located in the intertidal zone and have two daily tide cycles with a maximum range of approximately 2.5 m (BOM, Australia).
Significant differences between all plots were observed for pH, salinity, and redox (p < 0.01) (Fig. 3). Salinity was highest in plots 1 and 2 which were similar to marine levels of 35 psu, decreasing in the high intertidal plot (3) and reaching freshwater levels in plots 4 and 5. Plot 5 had the lowest measured groundwater pH (4.2) and highest redox potential (mean −14.7 ± 109.9 mV) when compared to all plots. No significant seasonal differences were observed (p > 0.05). Groundwater temperature was significantly higher in summer than in winter (p < 0.01, U = 308.5), but no significant differences were observed between plots.
Groundwater a redox potential, b pH, and c salinity measured during 2013–2014 dry-wet season from North Stradbroke Island. Boxes represent the 25% and 75% quartiles with hash marks showing the 90% and 10% and outliers as dark circles. Grey boxes are the summer-wet season, white boxes are the winter-dry season
When the plots were compared against each other, all measured nutrient parameters (DOC, N–NO3 −, N–NO2 −, N–NH4 +, and P–PO4 +) were significantly different (p < 0.05) (Fig. 4). This statistical difference is primarily led by the high DOC, N–NO −x , N–NH4 +, and P–PO4 + concentrations measured at plot 3 (High Intertidal), during the whole study period and especially during the winter-dry season. A significant seasonal difference (p < 0.05) was observed for the groundwater concentration of N–NO2 − (U = 589) across all study plots. Additionally, monthly rainfall amounts correlated negatively with N–NO2 − (ρ = −0.272) but positively with DOC (ρ = 0.322) (p < 0.05). N–NO3 was lower in the summer months, but not significantly, due to the high variability in plots 2 and 3 (Fig. 3). The measured sediment C:N was significantly different (p < 0.05) across all plots, with the highest C:N measured in the upper soil layers of plot 5 (29) (Table 2) . However, the N:P ratios were not significantly different between the sampling plots (p = 0.13).
N2O and CH4 fluxes
The coastal wetland system was an overall GHG source, the mean N2O and CH4 fluxes across all plots were 52.8 µg N–N2O h−1 m−2 and 48.7 mg C–CH4 h−1 m−2, respectively (Fig. 5). A significant seasonal difference was observed for N2O fluxes, but not for CH4 fluxes (p < 0.01; p = 0.156) with higher N2O fluxes measured in the wet season (mean wet 67.4 µg N–N2O h−1 m−2; dry 33.6 μg N–N2O h−1 m−2). However, measured CH4 fluxes had the largest range during the wet season, from 0.01 mg C–CH4 h−1 m−2 measured at plot 3 to 483.8 mg C–CH4 h−1 m−2 measured at plot 5. Similarly, the range of N2O fluxes was larger during the wet season, 22.8–228.3 μg N–N2O h−1 m−2. Plots were significantly different from each other for N2O fluxes (p < 0.05) and CH4 fluxes (p < 0.05). The highest N2O and CH4 fluxes throughout the year were measured in plot 5 (plot mean 59.0 ± 24.3 μg N–N2O h−1 m−2; 98.8 ± 149.1 mg C–CH4 h−1 m−2), with the peak of activity starting towards the end of the wet season (max 92.7 μg N–N2O h−1 m−2 and 483.8 mg C–CH4 h−1 m−2). In this study, large outliers were excluded from the triplicate chambers as they could represent ebullition, sampling error, or chamber leakages.
The CH4 fluxes correlated significantly (p < 0.05) with the groundwater redox potential (ρ = 0.262), P–PO4 +(ρ = −0.407), N–NH4 + (ρ = −0.273), and DOC concentrations (ρ = −0.441), while the N2O fluxes correlated positively with groundwater temperature (ρ = 0.222), redox potential (ρ = 0.225), and negatively with salinity (ρ = −0.212), and N–NO2 − concentrations (ρ = 0.223). There was no significant correlation between CH4 fluxes and N2O fluxes (p = 0.17) across all plots.
Discussion
In this study, we have quantified fluxes for both CH4 and N2O emissions across a salinity gradient in a coastal wetland environment in both wet and dry seasons. The fluxes measured during this study are higher than rates previously measured in mangroves on NSI (5–68 μg N2O h−1 m−2; 3–17,400 μg CH4 h−1 m−2) (Allen et al. 2007) but are within ranges published elsewhere for subtropical wetlands (Allen et al. 2011; Tong et al. 2013). Higher emissions from subtropical coastal wetlands can be expected than temperate coastal wetlands. The higher temperatures in the subtropics results in increased microbial activity and greater primary productivity leading to high organic inputs, sediment C contents, and subsequently high C substrate availability (Murray et al. 2015; Whalen 2005; Xu et al. 2013).
A recent study of tropospheric N2O distribution and variability demonstrated that N2O sources are concentrated in the tropics, which would suggest that subtropical wetland N2O fluxes may have a significant role in resolving the global N2O budget (Groffman et al. 2000; Kort et al. 2011; Murray et al. 2015). The ebullition effect for N2O is likely negligible (Baulch et al. 2011). The sink or source strength of coastal wetland depends on the vegetation uptake, sedimentation, sediment N load, and inundation duration (Clough et al. 2007; Weier et al. 1993).
Seasonal changes
We measured strong wet-dry seasonal variability across all plots for N2O fluxes and N–NO2, with smaller trends visible for CH4 fluxes. Coastal ecosystems have been shown to demonstrate a high seasonal variability in denitrification rates, with higher rates measured in the summer (Pina-Ochoa and Alvarez-Cobelas 2006). This can be related to changing nitrite availability in the system as well as the increased temperature throughout the summer months. We observed a significant positive trend between the monthly rainfall accumulation and N–NO2 − availability, suggesting that increased precipitation could either physically transport nitrogen out of the system, or increase the N-turnover thus promoting N2O fluxes (Rajkumar et al. 2008). As previously mentioned, increasing temperature can increase the microbial activity, which supports our hypothesis that GHG fluxes will occur during the wet summer months (Hernandez and Mitsch 2007; Vicca et al. 2009). As N2O is produced under both aerobic (via nitrification) and anaerobic (via denitrification) conditions, depending on the availability of oxygen and N substrates, it is difficult to separate between the pathways of N2O production with the field measurements in this study. Anoxic conditions limit the nitrification potential, but promote denitrification, but no strong seasonal difference was observed for redox potential. Both processes can occur simultaneously in complex soil microsites with different access to O2. However, denitrification is generally considered to be the primary pathway of N2O production and consumption. The wet season associated decreased N–NO2 − concentration and increased N2O fluxes suggests that denitrification is the dominant process. In addition to oxygen and substrates, denitrification is regulated by pH, temperature, C:N ratio, electron acceptors, and the denitrifier community composition (Wallenstein et al. 2006; Zumft 1997).
The tidal freshwater wetland (plot 5) exhibited higher CH4 fluxes across all the measured plots with the highest rates during the winter-dry season, with similarly high summer-wet season rates (480 mg C–CH4 h−1 m−2). When the depth to groundwater level was very high (−252 mm), small negative rates were observed (mean = −0.39 mg C–CH4 h−1 m−2) which suggests that, while methanogenesis is the dominant process throughout the year, during dry periods the freshwater tidal wetland can act as a CH4 sink. The redox potential in plot 5 did not significantly change between the seasons, but was high enough (dry period maximum range: 61–121 mV) to possibly support aerobic conditions supportive of methantrophy. These hot spots of methanotrophic activity could be occurring at the anoxic–oxic interface, where CH4 and O2 gradients overlap (Burgin and Groffman 2012; Kettunen 2003; Kolb and Horn 2012). Small, seasonal changes in oxygen availability can alter CH4 consumption and production and hence, increase or reduce the net flux to the atmosphere (Whalen 2005).
Spatial variability
We observed strong spatial variability of both CH4 and N2O fluxes in this study with the highest fluxes measured in the freshwater wetland and the oligohaline saltwater marsh. In tidal systems, most of the information regarding C cycling comes from studies in the salt marsh areas, yet the freshwater (salinities < 0.5) and oligohaline marshes (salinity 0.5–5.0) can account for a significant fraction of the total tidal marsh area, (Barendregt and Swarth 2013; Odum 1988). These areas can be equally or more productive than the downstream salt marsh areas, which includes the removal of atmospheric CO2 via organic C accumulation in soils. The methane fluxes were generally higher in the freshwater areas compared to the salt marsh sections, as has been observed elsewhere along salinity gradients (Poffenbarger et al. 2011).
At the mid-intertidal plot located in the mangrove forest, larger CH4 fluxes were measured in areas with elevated groundwater salinity (Plot 2; mean = 36.7 ± 54.8 psu), which was surprising due to the general inhibitory effects of abundant sulphate electron acceptors and reducing bacteria generally found in saline environments (Alongi et al. 2001; van Dijk et al. 2015). Aerobic respiration and sulphate reduction are the major mechanisms of organic matter oxidation in mangrove sediments, but large CH4 fluxes have been observed when sulphate reduction is dominant (Lee et al. 2008). Ebullition can contribute a significant portion of CH4 fluxes to the atmosphere (Whalen 2005). Due to the use of chamber measurements, we cannot exclude the possibility of high ebullition, but disturbances were minimized during measurement to limit the effect. Plant stems and pneumatophores are additional direct pathways for gas fluxes, but sites were selected which did not contain pneumatophores or thick plant stems (Kreuzwieser et al. 2003).
The CH4 fluxes were best correlated with changes in salinity and redox potential across all measured sites. The overall positive relationship between CH4 fluxes and redox potential is also puzzling, since methanogens are obligate anaerobes, in which a negative relationship would be expected, as has been previously shown in other coastal wetland systems (Livesley and Andrusiak 2012). Our results are possibly skewed due to the strong salinity gradient which could be more important in controlling the methane fluxes than the oxygen gradient, resulting in an overall reduction of CH4 fluxes (Bartlett et al. 1987), causing higher fluxes to be measured in the freshwater wetland (plot 5) where salinity is low and redox potentials, while still anoxic, are higher than in the more saline plots. Increased salinity generally results in decreased rates of CH4 emissions, based on thermodynamic theory, i.e. sulphate reduction is energetically favourable over methanogenesis (Neubauer et al. 2013). In our study, CH4 emissions were generally lower in mangrove plots (mean = 13.7 ± 16.3 mg C–CH4 h−1 m−2), but we measured a high variability in the middle mangrove plot (plot 2). The salinity level of the mid-intertidal plot had the highest variation (Fig. 3; max 39.2 psu, min 0.05 psu), which could be driving the increased CH4 emissions. In the mangrove forest and salt marshes, seawater supplies sulphate (SO4 2−) which is used as the preferred pathway of anaerobic respiration and will inhibit methanogenesis, resulting in lower CH4 production and emission (Neubauer et al. 2013). As the microbial community is exposed to a wider range of salinity conditions, there could be a trade-off between methanogenesis during freshwater conditions and sulphate reduction during brackish conditions that is driven by the availability of terminal electron acceptors. The higher relative efficiency of sulphate reduction, along with the greater efficiency of other processes stimulated by saltwater intrusion could be important factors of C mineralization rates. The increased ionic strength associated with brackish water could increase C availability by desorbing previously protected labile organic matter from soil surfaces (Neubauer et al. 2013). As salinity increased, the C:N ratios decreased, but N:P ratios remained constant, suggesting that C substrate availability increased in the freshwater areas, possibly due to the increased level of root biomass associated with closed sedgeland.
In soils where anoxia conditions occur (defined as water filled pore space greater than 60%) C:N is greater than 30, and pH is close to neutral conditions favour complete denitrification, where N2 is the final end-product (Chapuis-Lardy et al. 2007). In this study, the tidal wetland and oligohaline marsh soils were acidic and slightly below the C:N threshold needed for complete denitrification, creating the soil conditions that would prevent complete N2O reduction and increase the ratio of N2O to N2 production (Cuhel et al. 2010). Flooding of organic subtropical soils has been shown to increase the denitrification activity, resulting in an overall decrease of N2O being released to the atmosphere (Terry et al. 1981). In this study, where the depth to groundwater varies between the study sites, we expected to measure a strong response to groundwater level in N2O fluxes. However, there was no significant relationship with the depth to groundwater. Rather, in estuarine systems, where salinity alters the redox potential it could be the driving factor along with temperature regulating the biogeochemical activity within the sediments. Specific to our field site, the transition zone between mangrove and fresh marshes (Transect 3) was the highest for dissolved nutrients, but did not result in either increased N2O rates relative to the other plots. Generally, higher DIN concentrations results in higher N2O fluxes to the atmosphere, but this was not the case in transition zone (Allen et al. 2007; Chen et al. 2012). However, this study did not consider the tidal pumping of groundwater, which can contribute to high variability of N2O fluxes (Wong et al. 2013). Consumption of N2O in the top sediment layers could be offset by N2O production in the groundwater, resulting in larger N2O fluxes via groundwater pathways than through sediment-atmosphere interactions. When NH4 dominates the DIN pool, as in this study, N2O emissions may increase after sediment exposure following tidal recession, likely due to increased sediment aeration and degassing of N2O (Cheng et al. 2007; Wang et al. 2007). This potentially large effect would need a sampling strategy that includes the whole tidal cycle, and not just the low tide period, as was measured here. Alternatively, the high concentration of DIN and DOC could create ideal conditions for complete denitrification which would result in low N2O but high N2 fluxes with an increased rate of denitrification.
The soil matrices along the salinity gradient are complex with a combination of water-filled pores and gas-filled cracks, root channels, crab tunnels, and other macro pores. This creates a heterogeneous distribution of O2 in the soil. Furthermore, the strong relationship between CH4 fluxes and the NH4, PO4 and DOC concentration suggests that the fluxes are related to carbon availability. These variables did not change with season, but were related to the location within the study site. In our study, the redox potential and carbon concentration, not salinity, were the strongest correlating variables for CH4 emission. The long-term effect of saltwater intrusion on soil CH4 emission is most likely mediated through the quantity and quality of organic matter inputs to the soil. Acidic wetlands may temporarily turn into net sinks of CH4 (Jauhiainen et al. 2005; Kettunen et al. 1999), as was observed in the acidic, tidal freshwater wetland of this study (−0.4 C–CH4 h−1m−2). The groundwater pH ranged from neutral conditions in highly brackish and saline mangrove section to acidic conditions in the oligohaline and freshwater marshes. Interestingly, we measured very high DOC concentrations and elevated P–PO4 and DIN, dominated by N–NH4, concentrations and in the high intertidal zone (Fig. 4), but these were not related to an increase in CH4 fluxes. The elevated nutrient concentrations could be transported towards the coast and responsible for the elevated fluxes observed in Plot 2. As we did not measure porewater, but rather groundwater, it could be that the substrates are contained within the porewater at 0-50 cm and have a more effective relationship to the gas fluxes.
Under moderate oxygen conditions and elevated NH4 concentrations, nitrification could be the dominant process, resulting in elevated N2O fluxes. Furthermore, coupled nitrification–denitrification could result in elevated N–NO3 −, N–NO2 − concentrations within the water column that are reduced to produce high N2O fluxes. In mangroves sediments, a small addition of NO3 can significantly increase the N2O flux (Munoz-Hincapie et al. 2002; Whigham et al. 2009). Similar nutrient increases, due to land use disturbances, can increase both nitrification and denitrification rates in salt marshes (Aelion and Engle 2010). The rates of N2O consumption and production can be further controlled by the sediment water content and structure. For example, high water content will restrict gas diffusion through sediments and limit aeration, therefore increasing the residence time and opportunity for denitrification, which can reduce N2O to N2 (Fromin et al. 2010; García et al. 1998; Xu et al. 2013). In more acidic wetlands, similar to the Plot 5, the N2O:N2 ratios can be higher than in more pH neutral wetlands (Kolb and Horn 2012). We observed a significant positive correlation between salinity and N2O fluxes, but this is not a linear response, suggesting the spatial pattern reflects a combination of environmental factors.
The coastal hydrodynamics have been shown to influence carbon (C) and nitrogen (N) concentrations in coastal wetland systems (Statham 2012). Oxygen diffusion is generally only in the first few cm of sediment, but tidal fluctuations and bioturbation of benthic fauna could alter the oxygen regime, subsequently changing the balance between nitrification and denitrification in the sediments. The tidal influence on the gas fluxes cannot be ignored in coastal wetland systems. As the tide recedes, the water-area flux increases and possibility transporting higher DIN out of the systems additionally transporting dissolved N2O (Banks et al. 2011; Ferron et al. 2007). Increases in sediment moisture from tidal fluctuations can result in an increase of the anoxic soil volume, and therefore temporarily increase the heterotrophic turnover of organic matter as has been demonstrated in the tropical Pantanal wetland, where water level fluctuations controlled the N2O dynamics (Liengaard et al. 2013). Additionally, tidal pumping of groundwater can contribute to tidal variability by increasing subterranean denitrification or moving organic carbon and DIN through groundwater pathways (Wong et al. 2013).
Our results show a strong spatial variability in the groundwater sources of DIN and organic carbon, suggesting that the local groundwater could be an important transport mechanism for substrates. Although this study is limited to low-tide measurements only, we can hypothesize that the range of tidal influence could result in large daily fluctuations within the estuarine system. Our results suggest that microbial communities responsible for N2O and CH4 fluxes are regulated by a seasonal, and perhaps tidal, fluctuating water table (Clough et al. 2007; Fiedler and Sommer 2000). When estimating a large scale ecosystem based on a larger groundwater models or other large scale models, these small-scale changes could potentially be overlooked, resulting in an under-estimation of the GHG flux. Given that the salinity gradient will strongly influence redox potentials and the quality of dissolved carbon (lability), we suggest that there is a strong spatial variability in the movement of brackish water throughout the salinity gradient, resulting in the high variability of GHG emissions.
Conclusion
This study has quantified substantial N2O and CH4 fluxes along a marine to freshwater gradient in an undisturbed subtropical estuary. We observed a strong seasonal pattern for N2O and a seasonal trend for CH4 fluxes, with higher fluxes measured during the warm wet months driven by temperature and precipitation changes, partially supporting our first hypothesis. Additionally, a significant spatial pattern was observed, with fluxes decreasing as groundwater salinity increased, likely due to a change in the redox conditions and groundwater DIN availability. We did not observe linear relationships between CH4 and N2O fluxes, suggesting that this ecosystem is a mosaic of processes controlled by oxygen and substrate availability, rather than a continuum controlled by salinity. The complex hydrodynamic processes controlling the fluxes of nutrients and materials are due to contrasts between seawater and fresh groundwater, and the influences of tides and waves (Bye and Narayan 2009). The inherent heterogeneity of estuaries and the impact of hydrological interactions on in situ processes highlights the need to sample during critical events and at specific sites (Loheide and Booth 2011; Wetzel et al. 2011). The production, movement and consumption of N2O and CH4 are dependent on aquifer hydrology, redox conditions, flow paths, dissolved oxygen, nitrate availability and microbial composition. Climate change alters the supply of water, nutrients, and sediments to coastal systems (Finkl and Charlier 2003; Hamilton 2010) which will alter the abundance and diversity of the process facilitators—the microbial and vegetation communities (Banks et al. 2011; Cabezas et al. 2008, 2009; Saha et al. 2010). The results presented in this study address the paucity of GHG flux data from Australian coastal wetlands and reveals significant fluxes which further emphasize the need to include such ecosystems in global GHG budgets. Changes in coastal wetland C and N cycling in response to global change factors, such as sea-level rise and land use changes, will influence the feedback mechanisms of coastal wetlands through changes in CH4 and N2O exchange.
References
Aelion CM, Engle MR (2010) Evidence for acclimation of N cycling to episodic N inputs in anthropogenically-affected intertidal salt marsh sediments. Soil Biol Biochem 42:1006–1008. doi:10.1016/j.soilbio.2010.02.015
Allen DE, Dalal RC, Rennenberg H, Meyer RL, Reeves S, Schmidt S (2007) Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biol Biochem 39:622–631. doi:10.1016/j.soilbio.2006.09.013
Allen D, Dalal RC, Rennenberg H, Schmidt S (2011) Seasonal variation in nitrous oxide and methane emissions from subtropical estuary and coastal mangrove sediments. Aust Plant Biol 13:126–133. doi:10.1111/j.1438-8677.2010.00331.x
Alongi DM (1996) The dynamics of benthic nutrient pools and fluxes in tropical mangrove forests. J Mar Res 54:123–148. doi:10.1357/0022240963213475
Alongi DM (2002) Present state and future of the world’s mangrove forests. Environ Conserv 29:331–349. doi:10.1017/s0376892902000231
Alongi DM (2012) Carbon sequestration in mangrove forests. Carbon Manag 3:313–322. doi:10.4155/cmt.12.20
Alongi DM, Brinkman R (2011) Hydrology and Biogeochemistry of Mangrove Forests. In: Levia DF, CarlyleMoses D, Tanaka T (eds) Forest Hydrology and Biogeochemistry: Synthesis of Past Research and Future Directions, vol 216. Ecological Studies-Analysis and Synthesis. pp 203-219. doi:10.1007/978-94-007-1363-5_10
Alongi DM et al (2001) Organic carbon accumulation and metabolic pathways in sediments of mangrove forests in southern Thailand. Mar Geol 179:85–103. doi:10.1016/s0025-3227(01)00195-5
Banks EW, Brunner P, Simmons CT (2011) Vegetation controls on variably saturated processes between surface water and groundwater and their impact on the state of connection. Water Resour Res 47:W11517
Barendregt A, Swarth CW (2013) Tidal freshwater wetlands: variation and changes. Estuar Coasts 36:445–456. doi:10.1007/s12237-013-9626-z
Bartlett KB, Bartlett DS, Harriss RC, Sebacher DI (1987) Methane emissions along a salt-marsh salinity gradient. Biogeochemistry 4:183–202. doi:10.1007/bf02187365
Baulch HM, Schiff SL, Maranger R, Dillon PJ (2011) Nitrogen enrichment and the emission of nitrous oxide from streams. Glob Biogeochem Cycles. doi:10.1029/2011gb004047
Bergamaschi BA, Krabbenhoft DP, Aiken GR, Patino E, Rumbold DG, Orem WH (2012) Tidally driven export of dissolved organic carbon, total mercury, and methylmercury from a mangrove-dominated estuary. Environ Sci Technol 46:1371–1378. doi:10.1021/es2029137
Bouillon S et al (2008) Mangrove production and carbon sinks: a revision of global budget estimates. Glob Biogeochem Cycles. doi:10.1029/2007gb003052
Burgin AJ, Groffman PM (2012) Soil O2 controls denitrification rates and N2O yield in a riparian wetland. J Geophys Res-Biogeosci. doi:10.1029/2011jg001799
Bye JAT, Narayan KA (2009) Groundwater response to the tide in wetlands: observations from the Gillman Marshes, South Australia. Estuar Coast Shelf Sci 84:219–226. doi:10.1016/j.ecss.2009.06.025
Cabezas A, Gonzalez E, Gallardo B, Garcia M, Gonzalez M, Comin F (2008) Effects of hydrological connectivity on the substrate and understory structure of riparian wetlands in the Middle Ebro River (NE Spain): implications for restoration and management. Aquat Sci 70:361–376. doi:10.1007/s00027-008-8059-4
Cabezas A, Comin FA, Walling DE (2009) Changing patterns of organic carbon and nitrogen accretion on the middle Ebro floodplain (NE Spain). Ecol Eng 35:1547–1558. doi:10.1016/j.ecoleng.2009.07.006
Chapuis-Lardy L, Wrage N, Metay A, Chotte JL, Bernoux M (2007) Soils, a sink for N2O? A review. Glob Change Biol 13:1–17. doi:10.1111/j.1365-2486.2006.01280.x
Chen GC, Tam NFY, Ye Y (2012) Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biol Biochem 48:175–181. doi:10.1016/j.soilbio.2012.01.029
Cheng XL et al (2007) CH(4) and N(2)O emissions from Spartina alterniflora and Phragmites australis in experimental mesocosms. Chemosphere 68:420–427. doi:10.1016/j.chemosphere.2007.01.004
Chiu CY, Lee SC, Chen TH, Tian GL (2004) Denitrification associated N loss in mangrove soil. Nutr Cycl Agroecosyst 69:185–189. doi:10.1023/b:fres.0000035170.46218.92
Clough TJ, Addy K, Kellogg DQ, Nowicki BL, Gold AJ, Groffman PM (2007) Dynamics of nitrous oxide in groundwater at the aquatic-terrestrial interface. Glob Change Biol 13:1528–1537. doi:10.1111/j.1365-2486.2007.01361.x
Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60:609–640
Cox M, James A, Hawke A, Specht A, Raiber M, Taulis M (2011) North Stradbroke Island 3D hydrology: Surface water features, settings and groundwater links. Proc R Soc Qld 117:47–64
Cuhel J, Simek M, Laughlin RJ, Bru D, Cheneby D, Watson CJ, Philippot L (2010) Insights into the effect of soil pH on N2O and N-2 emissions and denitrifier community size and activity. Appl Environ Microbiol 76:1870–1878. doi:10.1128/aem.02484-09
Dalal RC, Allen DE (2008) Greenhouse gas fluxes from natural ecosystems. Aust J Bot 56:369–407. doi:10.1071/bt07128
Davidson E, Kingerlee W (1997) A global inventory of nitric oxide emissions from soils. Nutr Cycl Agroecosyst 48:37–50. doi:10.1023/A:1009738715891
Feller IC, Lovelock CE, Berger U, McKee KL, Joye SB, Ball MC (2010) Biocomplexity in mangrove ecosystems. Annu Rev Mar Sci 2:395–417. doi:10.1146/annurev.marine.010908.163809
Fernandes SO, Bharathi PAL (2011) Nitrate levels modulate denitrification activity in tropical mangrove sediments (Goa, India). Environ Monit Assess 173:117–125. doi:10.1007/s10661-010-1375-x
Fernandes SO, Bharathi PAL, Bonin PC, Michotey VD (2010) Denitrification: an important pathway for nitrous oxide production in tropical mangrove sediments (Goa, India). J Environ Qual 39:1507–1516. doi:10.2134/jeq2009.0477
Ferron S, Ortega T, Gomez-Parra A, Forja JM (2007) Seasonal study of dissolved CH4CO2 and N2O in a shallow tidal system of the bay of Cadiz (SW Spain). J Mar Syst 66:244–257. doi:10.1016/j.jmarsys.2006.03.021
Fiedler S, Sommer M (2000) Methane emissions, groundwater levels and redox potentials of common wetland soils in a temperate-humid climate. Glob Biogeochem Cycles 14:1081–1093. doi:10.1029/1999GB001255
Finkl CW, Charlier RH (2003) Sustainability of subtropical coastal zones in southeastern Florida: challenges for urbanized coastal environments threatened by development, pollution, water supply, and storm hazards. J Coast Res 19:934–943
Fromin N, Pinay G, Montuelle B, Landais D, Ourcival JM, Joffre R, Lensi R (2010) Impact of seasonal sediment desiccation and rewetting on microbial processes involved in greenhouse gas emissions. Ecohydrology 3:339–348. doi:10.1002/eco.115
Galloway JN, Cowling EB (2002) Reactive nitrogen and the world: 200 years of change. Ambio 31:64–71. doi:10.1639/0044-7447(2002)031[0064:rnatwy]2.0.co;2
García-Ruiz R, Pattinson SN, Whitton BA (1998) Denitrification in river sediments: relationship between process rate and properties of water and sediment. Freshw Biol 39:467–476
Groffman PM, Gold AJ, Addy K (2000) Nitrous oxide production in riparian zones and its importance to national emission inventories. Chemosph Glob Change Sci 2:291–299
Groffman P et al (2009) Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 93:49–77
Hamilton SK (2010) Biogeochemical implications of climate change for tropical rivers and floodplains. Hydrobiologia 657:19–35. doi:10.1007/s10750-009-0086-1
Hernandez ME, Mitsch WJ (2007) Denitrification in created riverine wetlands: influence of hydrology and season. Ecol Eng 30:78–88
Jahangir MMR et al (2012) Denitrification potential in subsoils: a mechanism to reduce nitrate leaching to groundwater. Agric Ecosyst Environ 147:13–23. doi:10.1016/j.agee.2011.04.015
Jahangir MMR et al (2013) Denitrification and indirect N2O emissions in groundwater: hydrologic and biogeochemical influences. J Contam Hydrol 152:70–81. doi:10.1016/j.jconhyd.2013.06.007
Jauhiainen J, Takahashi H, Heikkinen JEP, Martikainen PJ, Vasander H (2005) Carbon fluxes from a tropical peat swamp forest floor. Glob Change Biol 11:1788–1797. doi:10.1111/j.1365-2486.2005.001031.x
Jennerjahn TC (2012) Biogeochemical response of tropical coastal systems to present and past environmental change. Earth Sci Rev 114:19–41. doi:10.1016/j.earscirev.2012.04.005
Kettunen A (2003) Connecting methane fluxes to vegetation cover and water table fluctuations at microsite level: a modeling study. Glob Biogeochem Cycles 17:1051–1070
Kettunen A, Kaitala V, Lehtinen A, Lohila A, Alm J, Silvola J, Martikainen PJ (1999) Methane production and oxidation potentials in relation to water table fluctuations in two boreal mires. Soil Biol Biochem 31:1741–1749
Kim DG, Giltrap D, Hernandez-Ramirez G (2013) Background nitrous oxide emissions in agricultural and natural lands: a meta-analysis. Plant Soil 373:17–30. doi:10.1007/s11104-013-1762-5
Kingston HM, Walter PJ (2007) Test methods for evaluating solid waste, physical/chemical methods United States Environmental Protection Agency; office of solid waste; economic, methods, and risk analysis division
Kolb S, Horn MA (2012) Microbial CH(4) and N(2)O consumption in acidic wetlands. Front Microbiol 3:78. doi:10.3389/fmicb.2012.00078
Kort EA et al (2011) Tropospheric distribution and variability of N2O: evidence for strong tropical emissions. Geophys Res Lett 38:5. doi:10.1029/2011gl047612
Kreuzwieser J, Buchholz J, Rennenberg H (2003) Emission of methane and nitrous oxide by Australian mangrove ecosystems. Plant Biol 5:423–431. doi:10.1055/s-2003-42712
Leach L (2011) Hydrology and physical setting of North Stradbroke Island. Proc R Soc Qld 117:21–46
Lee R, Porubsky W, Feller I, McKee K, Joye S (2008) Porewater biogeochemistry and soil metabolism in dwarf red mangrove habitats (Twin Cays, Belize). Biogeochemistry 87:181–198. doi:10.1007/s10533-008-9176-9
Liengaard L, Nielsen LP, Revsbech NP, Priem A, Elberling B, Enrich-Prast A, Kuhl M (2013) Extreme emission of N2O from tropical wetland soil (Pantanal, South America). Front Microbiol. doi:10.3389/fmicb.2012.00433
Livesley SJ, Andrusiak SM (2012) Temperate mangrove and salt marsh sediments are a small methane and nitrous oxide source but important carbon store. Estuar Coast Shelf Sci 97:19–27. http://dx.doi.org/mur
Loheide SP, Booth EG (2011) Effects of changing channel morphology on vegetation, groundwater, and soil moisture regimes in groundwater-dependent ecosystems. Geomorphology 126:364–376. doi:10.1016/j.geomorph.2010.04.016
Maher D, Eyre B (2011) Benthic carbon metabolism in southeast Australian estuaries: habitat importance, driving forces, and application of artificial neural network models. Mar Ecol Prog Ser 439:97–115. doi:10.3354/meps09336
Masselink G, Gehrels R (2015) Introduction to Coastal Environments and Global Change. In: Coastal Environments and Global Change. Wiley, pp 1–27
McClain ME et al (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312
Meyer RL, Allen DE, Schmidt S (2008) Nitrification and denitrification as sources of sediment nitrous oxide production: a microsensor approach. Mar Chem 110:68–76. doi:10.1016/j.marchem.2008.02.004
Munoz-Hincapie M, Morell JM, Corredor JE (2002) Increase of nitrous oxide flux to the atmosphere upon nitrogen addition to red mangroves sediments. Mar Pollut Bull 44:992–996. doi:10.1016/s0025-326x(02)00132-7
Murray RH, Erler DV, Eyre BD (2015) Nitrous oxide fluxes in estuarine environments: response to global change. Glob Change Biol 21:3219–3245. doi:10.1111/gcb.12923
Nahlik AM, Mitsch WJ (2011) Methane emissions from tropical freshwater wetlands located in different climatic zones of Costa Rica. Glob Change Biol 17:1321–1334. doi:10.1111/j.1365-2486.2010.02190.x
Neubauer SC, Franklin RB, Berrier DJ (2013) Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosciences 10:8171–8183. doi:10.5194/bg-10-8171-2013
Nykanen H, Alm J, Lang K, Silvola J, Martikainen PJ (1995) Emissions of CH4, N2O and CO2 from a virgin fen and a fen drained for grassland in Finland. J Biogeogr 22:351–357. doi:10.2307/2845930
Odum WE (1988) Comparative ecology of tidal fresh-water and salt marshes. Annu Rev Ecol Syst 19:147–176. doi:10.1146/annurev.es.19.110188.001051
Page KL, Dalal RC (2011) Contribution of natural and drained wetland systems to carbon stocks, CO2, N2O, and CH4 fluxes: an Australian perspective. Soil Res 49:377–388. doi:10.1071/sr11024
Pina-Ochoa E, Alvarez-Cobelas M (2006) Denitrification in aquatic environments: a cross-system analysis. Biogeochemistry 81:111–130
Poffenbarger HJ, Needelman BA, Megonigal JP (2011) Salinity influence on methane emissions from tidal marshes. Wetlands 31:831–842. doi:10.1007/s13157-011-0197-0
Queensland (2014) Resources, WetlandInfo
Rajkumar AN, Barnes J, Ramesh R, Purvaja R, Upstill-Goddard RC (2008) Methane and nitrous oxide fluxes in the polluted Adyar River and estuary, SE India. Mar Pollut Bull 56:2043–2051. doi:10.1016/j.marpolbul.2008.08.005
Rayment GE, Lyons DJ (2011) Soil chemical methods—Australasia. Australian soil and land survey handbooks series. CSIRO Publishing
Reddy KR, DeLaune RD (2008) Biogeochemistry of wetlands: science and applications. CRC Press, Boca Raton
Saha AK, Sternberg LDO, Ross MS, Miralles-Wilhelm F (2010) Water source utilization and foliar nutrient status differs between upland and flooded plant communities in wetland tree islands. Wetl Ecol Manag 18:343–355. doi:10.1007/s11273-010-9175-1
Seitzinger SP, Kroeze C, Styles RV (2000) Global distribution of N2O emissions from aquatic systems: natural emissions and anthropogenic effects. Chemosph Glob Change Sci 2:267–279
Specht R (2011) Plant communities of North Stradbroke Island: development of structure and species richness. Proc R Soc Qld 117:181–191
Statham PJ (2012) Nutrients in estuaries—An overview and the potential impacts of climate change. Sci Total Environ 434:213–227. doi:10.1016/j.scitotenv.2011.09.088
Stephens KM (2009) The flora of North Stradbroke Island. The State of Queensland, Environmental Protection Agency, Brisbane
Terry RE, Tate RL III, Duxbury JM (1981) The effect of flooding on nitrous oxide emissions from an organic soil. Soil Sci 132:228–232
Tong C, Huang JF, Hu ZQ, Jin YF (2013) Diurnal variations of carbon dioxide, methane, and nitrous oxide vertical fluxes in a subtropical estuarine marsh on neap and spring tide days. Estuar Coasts 36:633–642. doi:10.1007/s12237-013-9596-1
van Dijk G, Smolders AJP, Loeb R, Bout A, Roelofs JGM, Lamers LPM (2015) Salinization of coastal freshwater wetlands; effects of constant versus fluctuating salinity on sediment biogeochemistry. Biogeochemistry 126:71–84. doi:10.1007/s10533-015-0140-1
Verhoeven JTA, Arheimer B, Yin CQ, Hefting MM (2006) Regional and global concerns over wetlands and water quality. Trends Ecol Evolut 21:96–103
Vicca S, Janssens IA, Flessa H, Fiedler S, Jungkunst HF (2009) Temperature dependence of greenhouse gas emissions from three hydromorphic soils at different groundwater levels. Geobiology 7:465–476. doi:10.1111/j.1472-4669.2009.00205.x
Vidon P et al (2010) Hot spots and hot moments in Riparian zones: potential for improved water quality management. J Am Water Res Assoc 46:278–298. doi:10.1111/j.1752-1688.2010.00420.x
Wallenstein MD, Myrold DD, Firestone M, Voytek M (2006) Environmental controls on denitrifying communities and denitrification rates: insights from molecular methods. Ecol Appl 16:2143–2152
Wang DQ et al (2007) Summer-time denitrification and nitrous oxide exchange in the intertidal zone of the Yangtze Estuary. Estuar Coast Shelf Sci 73:43–53. doi:10.1016/j.ecss.2006.11.002
Weier KL, Doran JW, Power JF, Walters DT (1993) Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Sci Soc Am J 57:66–72. doi:10.2136/sssaj1993.03615995005700010013x
Weston NB, Neubauer SC, Velinsky DJ, Vile MA (2014) Net ecosystem carbon exchange and the greenhouse gas balance of tidal marshes along an estuarine salinity gradient. Biogeochemistry 120:163–189. doi:10.1007/s10533-014-9989-7
Wetzel PR et al (2011) Biogeochemical processes on tree islands in the greater everglades: initiating a new paradigm. Crit Rev Environ Sci Technol 41:670–701. doi:10.1080/10643389.2010.530908
Weymann D et al (2008) Groundwater N2O emission factors of nitrate-contaminated aquifers as derived from denitrification progress and N2O accumulation. Biogeosciences 5:1215–1226. doi:10.5194/bg-5-1215-2008
Whalen SC (2005) Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ Eng Sci 22:73–94. doi:10.1089/ees.2005.22.73
Whigham DF, Verhoeven JTA, Samarkin V, Megonigal PJ (2009) Responses of Avicennia germinans (black mangrove) and the soil microbial community to nitrogen addition in a hypersaline wetland. Estuar Coasts 32:926–936. doi:10.1007/s12237-009-9184-6
Wong WW, Grace MR, Cartwright I, Cardenas MB, Zamora PB, Cook PLM (2013) Dynamics of groundwater-derived nitrate and nitrous oxide in a tidal estuary from radon mass balance modeling. Limnol Oceanogr 58:1689–1706. doi:10.4319/lo.2013.58.5.1689
Xu Y, Xu Z, Cai Z, Reverchon F (2013) Review of denitrification in tropical and subtropical soils of terrestrial ecosystems. J Soils Sedim 13:699–710. doi:10.1007/s11368-013-0650-1
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616
Acknowledgements
The authors would like to acknowledge the traditional landowners, the Quandamooka People, for their permission to undertake this research. Additionally, we want to acknowledge J.Canard, C. Woodward, and S. Felder for their help and expertise in the field along with the support of the staff at the Moreton Bay Research Station located on N. Stradbroke Island. The authors would like to thank P. Martikainen and J. Pumpanen for their comments on an earlier version of this manuscript. This research was funded through the National Centre for Groundwater Research and Training. Additional support to N. Welti was provided through the Academy of Finland (Grant Number 258875: Mechanisms and atmospheric importance of nitrous oxide uptake in soils) for the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Maren Voss.
Rights and permissions
About this article
Cite this article
Welti, N., Hayes, M. & Lockington, D. Seasonal nitrous oxide and methane emissions across a subtropical estuarine salinity gradient. Biogeochemistry 132, 55–69 (2017). https://doi.org/10.1007/s10533-016-0287-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-016-0287-4