Abstract
Land-use practices can alter shallow groundwater and salinity, further impacting greenhouse gas (GHG) emissions, particularly in the hydrologically dynamic riparian zones of wetlands. Emissions of CO2, CH4, and N2O were estimated in soil cores collected from two prairie pothole region (PPR) sites with three adjacent land-use practices (i.e., annual crop = AC, pasture = PA, and short rotation willow = SRW) and treated with declining water table depths (2 to 26 cm), and salinity (S0 = control, S1 = 6 mS cm−1, and S2 = 12 mS cm−1) in a microcosm experiment. Land-use practices significantly (p < 0.001) affected GHG emissions in soils from both sites in the order of PA > AC = SRW. Compared to the control, emissions of CO2 and CH4 were significantly lower under higher salinity treatments (i.e., S1 and S2), while N2O was significantly higher (p < 0.05). Emissions under declining groundwater table depths were significantly (p < 0.001) variable and specific to each gas, indicating the impacts of shifted soil moisture regime. Overall, the CO2 and CH4 emissions increased up to week four and then decreased with declining water table depths, whereas N2O emission increased up to a maximum at week six. The soils from SRW had considerably lower global warming potential compared to AC and PA. Groundwater salinity in soils from contrasting land-use in the PPR has significant impacts on GHG emissions with potential for crucial climate feedback; however, the magnitude and direction of the impacts depend on hydrology.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agroecosystem soil C and N cycling contribute significantly to atmospheric carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) emissions and, thus, global warming (IPCC 2018; Smith et al. 2008). The North American prairie pothole region (PPR) is characterized by relatively small and highly productive wetlands embedded within an agriculture-dominated landscape. The PPR delivers essential ecosystem services such as improving soil and water quality, storing water, reducing soil erosion, and providing habitat for wildlife, especially waterfowl (Gleason et al. 2008). Salt dynamics within the PPR wetlands are driven by hydrology, which cycles seasonally and responds to land-use practice changes (Nachshon et al. 2013). Vegetation in the riparian zone pulls water from the soil and transfers it to the atmosphere via transpiration (Millar 1971), resulting in a gradual decline in the groundwater table (GWT). In turn, a declining GWT can increase soil salinity and deposit soluble salts at the soil surface (Arndt and Richardson 1989). Land-use affects both the production and consumption of GHG through its influence on wetland soil hydrology. For instance, wetland-riparian zones that are drained and cropped likely would have minimal CH4 production because this practice promotes aerobic conditions that do not favor methanogenesis (Smith et al. 2003). Conversely, the same catchment would have a higher likelihood of emitting N2O due to a combination of N-fertilizer additions and moist but unsaturated soil moisture conditions (Davidson et al. 2000). Hence, changes in land-use practice can increase the potential for greenhouse gas (GHG) emissions and diminish the capacity of PPR wetlands to deliver ecosystem services (Gleason et al. 2009).
Land-use practice can strongly influence soil-derived GHG emissions (Liebig et al. 2005; Schaufler et al. 2010; Tangen et al. 2015). In general, wetlands have a greater GHG emission potential than forestlands, croplands, and grasslands (Oertel et al. 2016); however, the amount of CO2, CH4, and N2O emitted vary depending on the type of vegetation and environmental conditions (Kayranli et al. 2009). The production of GHG in wetlands is controlled by highly variable abiotic factors that are themselves affected by land-use; these include the soil moisture and groundwater regime, the period of inundation, redox conditions, and groundwater salinity (Marton et al. 2012). The land-use practice also affects soil biological processes that regulate GHG emissions by influencing the composition of soil microbial and plant communities and the availability of organic substrates (Tangen et al. 2015). Moreover, riparian land-use practice affects the microclimate and soil properties that can influence the production/consumption and GHG emission (Moore et al. 2017). Consequently, land-use practices that affect dynamic wetland riparian zones can significantly alter the amount of GHG released into the atmosphere (Vidon 2010).
Land-use practice can alter soil organic carbon (SOC) dynamics and, in turn, GHG emissions (Kooch et al. 2016; Lang et al. 2010; Merino et al. 2004). Agroforestry is a promising land-use practice that can increase above- and below-ground C stocks, mitigate N2O and CO2 emissions, and increase the CH4 sink potential when compared to cropland; unlike cropland, agroforestry has lower losses of aboveground biomass via harvest and lower CO2 emissions from soil organic matter (SOM) decomposition (Mutuo et al. 2005). One study (Baah-Acheamfour et al. 2016) recommended that incorporating agroforestry and grassland cover into agricultural lands can reduce CH4 and N2O emissions. Parmar et al. (2015) also found that “short-rotation” forestry can contribute to GHG savings via reduced soil respiration losses. Thus, establishing perennial agroforestry systems such as short rotation willow (SRW) in the riparian zones of PPR wetlands may deliver GHG mitigation benefits. However, the effects of agroforestry practices on soil N2O and CH4 emissions are poorly understood (Albrecht and Kandji 2003). It has also been suggested that SOC could be sequestered by re-establishing permanent vegetation (i.e., grass) in PPR wetlands (Bedard-Haughn et al. 2006). However, it is unclear how the establishment of perennial SRW vegetation in the marginal riparian zones of the semi-arid PPR wetlands affects GHG emissions under dynamic soil hydrology (e.g., GWT) and salinity.
The effects of a fluctuating GWT on GHG emissions from peatlands (Berglund and Berglund 2011; Blodau et al. 2004; Updegraff et al. 2001) and riparian mineral wetlands (Mander et al. 2015) have been well studied. The effects of salinity on GHG emissions associated with a land-use change (Martin and Moseman-Valtierra 2015; Sheng et al. 2015), or depth to the GWT (Ardón et al. 2018; Mander et al. 2011) have also been studied, albeit mainly in coastal wetlands. These studies have variable results. For instance, in one microcosm experiment, artificial salinity treatments suppressed CO2 emissions under both drought and flooded conditions, CH4 emissions increased in flooded conditions only, and the impacts of salinity were conditional on hydrologic treatments for N2O (Ardón et al. 2018). In contrast, in a tidal forest soil, salinity inhibited CH4 production but increased CO2 and N2O emissions (Marton et al. 2012). In another microcosm study using semi-arid cropland soil from Australia, salinity increased N2O emissions and reduced CO2 and CH4 emissions; however, increasing soil moisture increased CO2, increased CH4 but only up to 75% water-holding capacity, and had no effect on N2O emissions (Maucieri et al. 2017). In contrast, in the riparian zones of mineral wetlands, flooding increased CH4 emissions, and CO2 and N2O emissions increased as the depth to GWT decreases (Mander et al. 2015). In constructed wetlands, CH4 emissions were reduced, and N2O emissions amplified at high salinity (> 10‰), whereas the CO2 emissions were greatest at intermediate salinity, i.e., ~ 5‰ (Sheng et al. 2015). Nevertheless, studies on the combined effects of GWT and salinity on GHG emissions under contrasting land-use practices within mineral wetlands in the PPR are scarce.
Depending on various factors, wetland soils can either be a source or sink for GHG (Beetz et al. 2013). Examining GHG emissions under the combined effects of fluctuating water table and salinity in the context of contrasting land-use practices will improve our ability to develop best management practices and mitigation strategies while advancing agricultural sustainability in the PPR. Therefore, the objective of this microcosm study was to examine the effect of a declining groundwater water table—with different groundwater salinity levels—on GHG emissions from riparian zone soils collected from different land-use practices in the PPR.
Materials and methods
Site description and collection of intact soil cores
A controlled microcosm experiment was conducted to determine the influence of groundwater salinity and declining water table level on soil-derived emissions of CO2, CH4, and N2O. Soils were collected from sites managed under three different land-use practices at two sites in the PPR. Both sites (Site A and Site B) were located near the Agriculture and Agri-Food Canada Indian Head Agroforestry Development Centre at Indian Head, Saskatchewan, Canada (N 50° 30.605′; W 103° 43.011′) (Supplementary Fig. 1). Soils at both sites were classified as Oxbow Association, non-calcareous Black Chernozems developed on loamy glacial till in a landscape with level to gentle rolling (0–10% slope) topography (Saskatchewan Soil Survey Staff 1986). The SRW treatments (Salix dasyclados Wimm, popularly known as ‘India’) were established in June 2013 in the marginal fallow riparian zones at both sites. The pasture treatment (PA) comprised of a mix of alfalfa (Medicago sativa) and bromegrass (Bromus madritensis) that had been established in 2001–2003. Both SRW and PA areas were located (Supplementary Fig. 1) adjacent to the cropped area that was seeded with oat (Avena sativa).
The soils at Site A were non-saline, with ECs ranging from 0.6 to 1.9 mS cm−1; soils at Site B were non- to slightly saline, with ECs ranging from 1.0 to 2.6 mS cm−1 (see Supplementary Table 1). Intact soil cores (n = 3) were collected from each of the three land-use treatments at Sites A and B (i.e., annual crop [AC], pasture [PA], and short-rotation willow [SRW]) in mid-August 2015. Intact soil cores were used to avoid the disturbance produced by sieving (Reichstein et al. 2005). The soil cores were collected using a truck-mounted hydraulic punch (Giddings Machine Company Ltd., Windsor, CO, USA) fitted with cylindrical (30-cm tall ⋅ 9-cm i.d.) PVC sleeves. Cores were collected three years after SRW plantation (i.e., at the end of the first rotation cycle of SRW) to capture land-use practice effects on soil. The overlying litter-fibric-humic layer and grasses were removed before collecting the soil cores from the field. All soil cores were collected from the riparian zones. For the SRW, all soil cores were collected within a 1-m radius of the root zone between two planted rows. In total, 54 soil cores (2 sites ⋅ 3 land-use practices ⋅ 9 reps) were collected and transported in coolers to the University of Saskatchewan, where they were preserved frozen (at − 20 °C) until the start of the incubation study. Additional soil cores (0–30 cm depth; 9-cm i.d.) from each sampling location were collected and analyzed to determine soil physical and chemical properties (see Supplementary Table 1). Bulk density samples were collected using a hand-held core sampler (3-cm tall ⋅ 5.4-cm i.d.).
Initial soil characterization
Soil physiochemical properties were determined before starting the microcosm experiment. Each soil was divided into three subsamples, which were processed as follows: (1) one subsample was air-dried, ground, passed through a 2-mm sieve, and analyzed for particle size distribution, cation exchange capacity (CEC), pH, electrical conductivity (EC), and ammonium acetate extractable N and P; (2) the second subsample was air-dried, finely ground with a ball mill, and analyzed for organic- and total-C and total-N; and (3) the third subsample was frozen until it was analyzed for water-extractable organic carbon (WEOC) and water-extractable organic nitrogen (WEON). Samples collected for bulk density measurement were weighed, oven-dried at 105 °C for 24 h, cooled to room temperature in a desiccator, and reweighed. Bulk density was determined by dividing the oven-dry weight of the soil by the volume (74.7 cm3) of the core sampler.
Soil physiochemical analyses were carried out using the procedures described in Soil Sampling and Methods of Analysis (Carter and Gregorich (2008). The modified pipette method (Kroetsch and Wang 2008) was used to determine soil particle size distribution. Cation exchange capacity was determined using ammonium acetate at pH 7, followed by colorimetric analysis using a Technicon Auto-Analyzer (Technicon Industrial Systems; Tarrytown, NY, USA) (Hendershot et al. 2008a). Soil pH was determined in a 1:2 (w/v) soil:deionized-water suspension using a digital pH meter (Oakton™ PC700 pH/mV/conductivity meter; Oakton Instruments, Vernon Hills, IL, USA) (Hendershot et al. 2008b). EC was determined in a same extract after 1 h of shaking with an end-over-end shaker; filtrate (No. 42, Whatman Inc., Piscataway, NJ) was measured using a digital EC meter (PC700 pH/mV/conductivity, Oakton, Vernon Hills, IL, USA) (Miller and Curtin 2008). Ammonium (\({\text{NH}}_{4}^{+}\)), nitrate (\({\text{NO}}_{3}^{-}\)), phosphate (\({\text{PO}}_{4}^{3-}\)), and sulfate (\({\text{SO}}_{4}^{2-}\)) were measured using a 1 M ammonium acetate (buffered at pH 7) extraction followed by colorimetric analysis for \({\text{NH}}_{4}^{+}\), \({\text{NO}}_{3}^{-}\), \({\text{PO}}_{4}^{3-}\) via Technicon Auto-Analyzer (Technicon Industrial Systems, Tarrytown, NY, USA), and \({\text{SO}}_{4}^{2-}\) via Microwave Plasma-Atomic Emission Spectrometer (Model 4100, Agilent Technologies, Santa Clara, CA, USA) (Simard 1993). Total soil carbon (TSC) and soil organic carbon (SOC) were determined by dry combustion—following HCl fumigation to remove carbonates—using a Leco-2000 CNS analyzer (Leco Corporation, St. Joseph, MI, USA) (Skjemstad and Baldock 2008). Total nitrogen (TN) was determined using dry combustion with a Leco C632 CNS analyzer (Leco Corporation, St. Joseph, MI, USA) (Rutherford et al. 2008). Water extractable organic C and WEON were determined by gently mixing defrosted soil (20 ± 1 g) with 30-mL of 5 mM CaCl2, filtering the suspension through a 0.45-µm polycarbonate membrane filter (Whatman Inc., Piscataway, NJ, USA), and measuring total C and N in the filtrate using a TOC-VCPN analyzer (Shimadzu Scientific Instruments, Kyoto, Japan) (Chantigny et al. 2008).
Experimental design
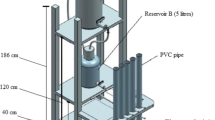
The microcosm incubation experiment was set up in the greenhouse at the University of Saskatchewan using a nested experimental design (Krzywinski et al. 2014; Schielzeth et al. 2013); the experiment was conducted over nine weeks. The 54 soil cores were arranged into following: 2 sites ⋅ 3 land-use practices ⋅ 3 groundwater salinity treatments (control = 0.3 mS cm−1, S1 = 6 mS cm−1, and S2 = 16 mS cm−1) ⋅ 3 replicates (Supplementary Fig. 2). Each experimental unit consisted of a 19-L plastic (PVC) bucket (38.1 cm tall ⋅ 30.48 cm i.d.) containing a 2.5-cm thick layer of gravel, 17-L of synthetic groundwater, and a single intact soil core—the bottom of which was wrapped in 1-mm mesh fiberglass screen to hold the soil securely (Fig. 1). The PVC cylinders housing the soil cores were drilled with a uniform series of 3-mm holes, which allowed for movement of the synthetic groundwater into and out of the soil core.
The dominant salts present in the soil and groundwater in the Prairie region of Canada and the northern United States are Na2SO4, KCl, CaCl2, and MgSO4 (Last and Ginn 2005). Thus, the synthetic groundwater treatments were prepared using a 5:2:12:14 mix of Na2SO4:KCl: CaCl2:MgSO4 salts (by weight) in distilled water; the quantity (g) of salts in S2 was double that of the S1 treatment. The control (no added salts) salinity treatment consisted of distilled water alone. Initially, the synthetic groundwater was maintained level with the surface of the soil cores (Fig. 1), with subsequent GWT drawdown achieved by manually lowering the water level by 2 cm at the end of the first week and then by 3 cm at the end of each of the next nine weeks (Fig. 1).
The EC of the synthetic groundwater was checked weekly to ensure that salinity remained constant. The volumetric soil water content (VSWC) and EC of the experimental soil cores were measured using a digital soil moisture meter (HydroSense II, Campbell Scientific Inc., Logan, UT, USA) at the time of GHG flux measurements. The temperature of the greenhouse chamber was maintained at 20 ± 1 °C; relative humidity in the greenhouse ranged from 37.73 to 67.05% (average 50.33%) during the first seven weeks of the experiment, and then from 16.05 to 43.26% (average 29.53%) during the last three weeks of the experiment (Supplementary Fig. 3).
GHG flux measurements
Greenhouse gas flux measurements were done using non-vented, static (i.e., non-steady-state) chambers (Collier et al. 2014; Rochette and Bertrand 2008) constructed using an ABS cleanout adapter (model # RLN105-030) and male plug (model # RLN106R-030) fitted with a sampling port sealed using a gas-impermeable, grey butyl rubber septum (Supelco, USA) (see Fig. 1). Gas flux measurements were made seven days after each GWT adjustment by attaching the sampling chamber to the top of the cores using a flexible coupling (model # FC-33) and sampling the headspace atmosphere immediately after the chamber was attached (t0) and again after 30 min (t30). The cores remained open to the atmosphere during the period between GWT adjustments.
Headspace gas samples were collected at t0 and t30 using a 20-mL polypropylene syringe (Monoject™, Luer lock fitting) fitted with a 25-gauge needle; samples were injected immediately into pre-evacuated 12-mL Exetainer® vials (LabCo Inc., High Wycombe, UK). Ambient air samples—used as a check on the t0 samples— were also collected on each sampling day. The gas samples were then brought to the Prairie Environmental Agronomy Laboratory in the Department of Soil Science at the University of Saskatchewan for analysis. The concentrations of CO2, CH4, and N2O in each gas sample were determined using gas chromatography (Farrell and Elliott 2008). Sample analyses were performed using a Bruker 450 GC (Bruker Biosciences Corporation, USA) equipped with a thermal conductivity detector (TCD), flame ionization detector (FID), and electron capture detector (ECD) for the detection and quantification of CO2, CH4, and N2O, respectively. Samples were introduced into the GC using a CombiPAL auto-sampler (CTC Analytics AG, Switzerland); data processing was completed using Varian Star Chromatography Workstation (ver. 6.2) software. The GHG fluxes were calculated from the change in concentration measured during the 30-min chamber deployment using Eq. 1:
where F is the GHG flux at time zero (mg m−2 d−1); ΔC is the change in concentration (mg CO2, CH4, or N2O L−1 min−1) measured during the 30-min deployment period; V is the volume of the chamber headspace (0.6089 L); A is the surface area of the soil cores (0.0064 m2); and kt is the time constant (1440 min d−1). For the correction of potential gas losses through leaks and sampling removal, net GHG fluxes were calculated by subtracting the respective blank (sample from ambient air collected at the time of GHG sampling) values from the values for the soil cores.
Cumulative GHG emissions for each land-use were calculated using linear interpolation (Eq. 2) as described in Pennock et al. (2010). This assumes that emissions were constant both throughout the day of the measurement and during the seven days since the previous water table adjustment.
where CF is the cumulative GHG flux (mg m−2); FW is the daily flux rate measured at the end of each week (weeks 1 through 9; total 57 days of incubation); 7 is the number of days in a week. For the first week (Fw1), the calculation only includes GHG emissions for the day of sampling. Global warming potential (GWP) for each land-use practice were calculated for a 100-year time scale using conversion factors for CO2 = 1, CH4 = 25, N2O = 298 after adjusting by mass to obtain carbon dioxide equivalent (i.e., CO2-eq) flux (Myhre et al. 2013; Wang et al. 2017b).
Statistical analyses
Soil GHG emission data were statistically analyzed and visualized using R version 3.4.4 for Windows (R Core Team 2018). The Shapiro-Wilk test and histogram were used to assess the normality and Levene’s test was used to check the homogeneity of variances using “car” package. The relationships among CO2, CH4, and N2O emissions, VSWC, EC, and initial soil parameters were measured by Spearman rank-order correlation and visualized using “corrplot” package. Assumptions of both univariate and multivariate analysis of variance (ANOVA) normality were fulfilled by adding a positive constant number (+ 2) during the transformation (Logarithmic with base 10) to manage negative CH4 and N2O values in the dataset. Significant differences among land-use practices, groundwater salinity treatments, and water table depths were compared parametrically by univariate ANOVA with nested design and linear mixed-effects models (Zuur et al. 2009) using " lmerTest.“ Pairwise multiple comparison procedures (Tukey’s HSD method) were used as a post-hoc test. The permutation multivariate ANOVA (PERMANOVA) and analysis of similarities (ANOSIM) were used to assess significant differences (multivariate hypothesis testing) in GHG emission among land-use practices, groundwater salinity, and GWT depths. ANOSIM was also used to calculate a matrix of dissimilarity ranks after converting the scores to find the ratio between within-group and between-group similarities. Unconstrained ordination with a non-metric multidimensional scale (NMDS) was used to plot the position in multidimensional space with a reduced number of dimensions to visualize the difference among groundwater salinity treatments, GWT depths, and land-use practices. The variation partitioning analysis (VPA) was used to determine the proportional contribution of land-use practices, groundwater salinity, and water table depth in the variation of GHG emissions. Constrained ordination with redundancy analysis (RDA) was performed to summarize the variation explained by measured soil physiochemical characteristics. The PERMANOVA, ANOSIM, NMDS, VPA, and RDA analyses were performed using the “vegan” package (Oksanen et al. 2017). All differences were considered significant at p-values ≤ 0.05 (95% confidence interval or alpha level = 0.05).
Results
Emissions of GHG in soils from contrasting land-use practices, elevated groundwater salinity, and declining groundwater table
The soils from PA land-use in both sites showed significantly (p < 0.001) higher CO2 emissions; CO2 emissions followed consistent land-use patterns in the order of PA > AC = SRW (Table 1). Cumulative CO2 emissions were higher in soils from site A than site B (Fig. 2). The CO2 emissions were significantly (p < 0.05) higher in the control (S0) compared to the elevated salinity treatments (i.e., S1 and S2) across all land-use practices from both sites (Tables 1 and 2). A significant (p < 0.001) difference in CO2 emissions was observed among the depth to GWT (Table 2). The CO2 flux initially showed an increasing trend with declining GWT depths (i.e., weeks of measurements) in soils across all land-use practices from both sites and showed a decreasing trend after four weeks (Table 1 and Supplementary Fig. 4). The mean emissions of CO2 were highest (p > 0.05) at GWT = 11-cm (week 4) in both sites, and lowest at GWT = 26-cm (week 9) in site A, and at GWT = 20-cm (week 7) in site B (Tables 1 and 2).
The CH4 emissions significantly (p < 0.001) differed among the soil from all land-use practices in both sites (Table 2). The mean CH4 emissions were significantly higher (p < 0.001) in soils from PA and showed a consistent pattern among land-use practices (PA > AC = SRW) from both sites (Table 1). Cumulative CH4 emissions were relatively low and variable between sites; emissions were negligible in site B compared to site A (Fig. 2). Groundwater salinity treatments (both S1 and S2) reduced CH4 emission compared to the control (i.e., S0) in site A (p = 0.012); however, the effect was not significant (p = 0.069) in site B (Table 2). The CH4 flux in soils from all land-use practices from both sites showed a slightly increasing trend up to week four and then decreased with a further decline in GWT depths (Table 1 and Supplementary Fig. 4). Significantly (p < 0.001) higher mean CH4 emissions were observed at GWT = 11-cm (week 4) in site A and GWT = 8-cm (week 3) at site B; CH4 emissions from both sites were lowest at GWT = 26-cm (week 9; Tables 1 and 2).
The N2O emissions were significantly (p < 0.001) higher in soils from PA and followed a similar land-use pattern to the other GHGs (PA > AC = SRW) for both sites (Tables 1 and 2). The cumulative N2O emissions were higher (p < 0.01) under both groundwater salinity treatments (i.e., S1 and S2) compared to the control in soils from both sites (Tables 1 and 2). Overall, the cumulative N2O emission was relatively low and variable between sites; however, higher in soils from site A than site B (Fig. 2). The lowest mean N2O emission was observed at GWT = 2-cm (week 1) in both sites, whereas the highest emission was at GWT = 17-cm (week 6) in site A and GWT = 23-cm (week 8) in site B (Tables 1 and 2).
Multivariate unconstrained ordination (NMDS analysis) of soil GHG emission data (stress value for site A is 0.0670, and site B is 0.0724) differed considerably among land-use practices in both sites, indicating the land-use practice type was a key factor driving the variability (Fig. 3A, B, D, and E). The NMDS ordination also showed a distinct clustering of GHG emissions based on land-use practices in both sites (stress values below 0.10 provide a fair representation of data in reduced dimension), indicating a robust land-use effect of PA soil.
The multivariate permutation analysis of variance (PERMANOVA) test confirmed the significant difference in GHG emissions among land-use practices (p = 0.001), salinity (p = 0.001) and depth to GWT (p = 0.001) in both sites (Table 3). The VPA test exhibited that the land-use practice alone has the highest contribution to the variation of soil GHG emissions in both sites (site A = 79.3% and site B = 69.6%), followed by depth to GWT (i.e., measurement week) and salinity treatments (Supplementary Fig. 5).
Soil physiochemical characteristics and their relationships with GHG
Physiochemical characteristics of experimental soil
The physiochemical properties of soils used for the microcosm experiment are presented in Table 4. No significant differences were observed in soil physiochemical properties among land-use practices and between sites except SOC, TN, and \({\text{SO}}_{4}^{2-}\) content (ANOVA results are not shown here). The SOC and TN were significantly (p < 0.05) higher in soils from PA compared to other land-use practices in the order of PA > SRW = AC in both sites (Table 4). No significant differences (p > 0.05) were found in SOC and TN content between sites. The \({\text{SO}}_{4}^{2-}\) content was approximately eight times higher in soils from site B than site A (Supplementary Table 1). By land-use practice, the soil \({\text{SO}}_{4}^{2-}\) contents were SRW > PA = AC in site A and SRW = AC > PA in site B, suggesting no consistent land-use patterns between sites.
Relationships of GHG with soil physiochemical characteristics
Overall, the relationships between soil GHG (CO2, CH4, and N2O) and soil clay content, SOC, TN, and C/N ratio were positive, whereas bulk density, initial EC, WEOC, and \({\text{SO}}_{4}^{2-}\) were negative (Fig. 4). Significant positive relationships (p < 0.05) between soil GHG and clay content, SOC, and C/N ratio were observed; however, SOC vs. CH4, and C/N vs. N2O were non-significant (p > 0.05). The relationships between all GHG emissions and bulk density were negative (p < 0.05) (Fig. 4). Correlations between soil \({\text{PO}}_{4}^{3-}\), \({\text{SO}}_{4}^{2-}\), and WEOC content with CO2 and CH4 were negative (p < 0.05) except for N2O (p > 0.05). None of the correlations between other initial soil physiochemical properties and soil GHG emissions were statistically significant (p > 0.05) (Fig. 4).
Redundancy analysis (RDA) between soil physiochemical characteristics and GHG
Redundancy analysis (RDA) was performed to determine the relationships among soil physiochemical properties and GHG emissions, as shown in Fig. 3C and F. The first two component axes explained 86.23 and 12.43% of site A (Fig. 3C), 85.87 and 12.83% (Fig. 3F) of site B of soil GHG. The vector lines of SOC, TN, VSWC, EC from site A and site B were statistically significant (p < 0.05), showing that SOC and TN played a crucial role in explaining soil GHG emissions in both sites. There was a significant positive correlation (p < 0.05) between SOC, TN, and soil GHG emissions in both sites A and B (Fig. 3C and F).
Relationships of GHG with VSWC and EC measured during the microcosm experiment
Groundwater salinity manipulation resulted in a statistically significant difference (p < 0.05) in soil EC among different salinity treatment levels (in S1 and S2 compared to control) in both sites (Tables 1 and 2). Similarly, water table manipulation resulted in a significant difference (p < 0.05) in observed VSWC among groundwater table depths in both sites. We did not find any significant difference (p > 0.05) in VSWC or EC among land-use practices from site A (p > 0.05). However, we found a significant difference (p < 0.05) in soil EC and VSWC in both sites because of groundwater salinity and water table manipulation (Tables 1 and 2). We also observed a significant (p < 0.05) positive relationship between soil EC and VSWC in both sites (Supplementary Figs. 5 and 7) during the incubation experiment.
Global warming potential
The effects of different land-use practices from two sites on the GWP of CO2, CH4, and N2O were calculated (Table 4) based on CO2-eq during the incubation period. The GWP was significantly affected (p < 0.05) by the origin of the soil from three different land-use practices and sites. The GWP was significantly higher in soils from PA, followed by AC and SRW land-use practices in both sites, whereas site A showed significantly higher GWP than site B.
Discussion
Effects of land-use, salinity and groundwater table on GHG emissions
Land-use effects
In our study, CO2 emissions were significantly affected by contrasting land-use practices, suggesting that land-use was a significant driver of CO2 emission by influencing the heterotrophic respiration of SOC (Oertel et al. 2016). The highest mean and cumulative CO2 emission in our experiment was seen from PA soils, followed by AC and SRW, respectively. Enrichment of SOC can trigger microbial activities that result in the emission of CO2, CH4, N2O; microbial activities are themselves subject to various proximal and distal drivers in soil (Oertel et al. 2016). Land-use practices control SOC accumulation due to the influence of tillage (or lack thereof) and the morphological and biochemical traits of the vegetation; therefore, any changes in land-use practices can change the potential for GHG emissions (Liebig et al. 2005).
We observed a significant positive relationship between CO2 and both SOC and TN in both sites. Hence, elevated CO2 emissions were perhaps triggered by higher SOC content and turnover rates from root biomass in PA soils from both sites. Like this study, others have found the C/N ratio was positively correlated with CO2 and CH4 emissions (Shi et al. 2014; Weslien et al. 2009). Likewise, Lang et al. (2010) found that the SOC and C/N ratio dominate CO2 and N2O emissions from soil. Restoring cropland to pasture has been shown to increase SOC in the PPR; however, quantifying SOC associated with land-use can be difficult in the short term, given the high degree of variability of both biotic and abiotic factors controlling SOC sequestration over time (Tangen et al. 2015). For example, Follett et al. (2012) found that PA soil can be a significant source or sink of C and N. Similar to our experiment, Parmar et al. (2015) found reduced GHG emissions in soil cores collected from short rotation forestry. In contrast, Lang et al. (2010) found significantly higher CO2 emissions in the forest than in PA soils, with the reverse relationship for N2O emissions.
Several factors may have contributed to the variable CH4 emissions in our experimental soils. Soil CH4 emissions are generally related to moist environments where methanogenesis can occur (Bridgham et al. 2013; Levy et al. 2012), although the C/N ratio can also influence the CH4 emission (Gundersen et al. 2012). Using stable C isotope, Wu et al. (2018) observed high CH4 uptake following afforestation, which they attributed to increased SOC and microbial biomass carbon, lower C/N ratio and less inorganic N. In a meta-analysis of 5000 chamber measurements collected from a range of land-use types, Levy et al. (2012) observed low emissions or a lower rate of net uptake of CH4 in mineral soils and high emissions from organic soils; SOC, VSWC, and pH were the best sub-set of explanatory variables. Hence, higher SOC in our soils from PA land-use perhaps caused higher CH4 emissions compared to AC and SRW. Similarly, Lang et al. (2010) found that PA soils were a weak source of CH4 emission, whereas forest soils were a weak sink of CH4. However, we also found higher background \({\text{SO}}_{4}^{2-}\) content in site B than site A and higher \({\text{SO}}_{4}^{2-}\) content under SRW compared to PA land-use practices; \({\text{SO}}_{4}^{2-}\) content was negatively correlated with CO2 and CH4 fluxes in soil. Conceivably the high \({\text{SO}}_{4}^{2-}\) content inhibited the CH4 emission even under wet conditions (Ardón et al. 2018), resulting in the lower CH4 emissions from SRW.
In a controlled laboratory experiment to assess the effects of land-use and climate (particularly soil temperature and moisture) on the potential GHG emission from intact soil cores collected from 13 European sites, Schaufler et al. (2010) found higher N2O emission from grasslands compared to croplands, forests, and wetlands. Similarly, we found significantly higher N2O emissions from PA soil, followed by AC and SRW in both sites. High available C and N content in our PA soil likely stimulated microbial activity leading to high N2O emission, as observed by Follett et al. (2012). Research has shown that heterotrophic nitrifying bacteria can denitrify with low \({\text{NO}}_{3}^{-}\) under aerobic conditions given sufficiently high SOC content (Wrage-Mönnig et al. 2018). The quality and availability of SOM input from different land-use practices are likely a key driver (Chantigny 2003) because most of the dissolved organic matter is directly involved in many soil microbial processes (Bolan et al. 2011). In a pot experiment under field conditions, Qiu et al. (2015) observed that management practices that were adding plant-derived dissolved organic matter to the soil increased microbial biomass and were responsible for a significant increase in CO2 and N2O emissions. Hence, Wu et al. (2019) suggested that GHG fluxes from the soils are rigorously controlled by the labile components of SOM, such as dissolved organic C and N, as well as inorganic N.
In a field-scale study within a Canadian prairie agroecosystem, Baah-Acheamfour et al. (2016) observed that agroforestry could reduce CH4 and N2O emissions to a greater extent than grassland, providing potential to mitigate climate change. In a field-scale study in a humid temperate region of southern Europe, Merino et al. (2004) also observed that afforestation could significantly increase SOC content relative to annual cropland, while also decreasing N2O emission and increasing CH4 uptake. Likewise, we observed significantly lower GWP (CO2-eq) in soils from SRW than AC and PA in our experiment. Hence, SRW can be a promising land-use practice in the fallow marginal riparian zones of the PPR agroecosystem (Amichev et al. 2014). However, it is often challenging to generalize the impact of agroforestry on the GHG budget without a better understanding of the plant types, soil, and climatic drivers that control the GHG emissions (Benanti et al. 2014).
Salinity effects
Salinity treatments significantly decreased CO2 and CH4 emissions except for the CH4 emission from site B (p = 0.069); however, it significantly increased the N2O emission in soils from both sites. A microcosm experiment performed on the coastal forested wetlands (Ardón et al. 2018) found that salinity can suppress CO2 emission under both flooded and drought conditions. Similar to our experiment, an incubation study by Maucieri et al. (2017) examined short-term effects of irrigation water salinity on soil GHG emissions from semi-arid Australian soil; CO2 emissions were reduced by 19% at 5-mS cm−1 and 28% at 10-mS cm−1, whereas N2O emissions increased 60%, and CH4 emissions were not affected by increased salinity, only by soil water. Setia et al. (2011) also found a significant decrease in CO2 emission with increasing salinity ranged from 1 to 5 mS cm−1.
The salt concentration and water content regulate the osmotic potential in the soil; at both high salinity and low water content, soil microorganisms can tolerate the high osmotic potentials by synthesizing osmolytes, which lets them continue metabolism (Yan and Marschner 2013). Consequently, perhaps both salinity and hydrology controlled N2O emissions during our experiment. Fluctuating aerobic-anaerobic conditions and environments low in oxygen can promote N2O production by nitrifier denitrification (Wrage-Mönnig et al. 2018). Dang et al. (2017) likewise observed enhanced N2O production in an incubation experiment under higher soil salinity and suggested that the addition of available carbon (glucose) and nitrogen (nitrate) created favorable conditions for denitrification. Comparably, in our experimental soil, SOC and TN acts as significant drivers that controlled the GHG emission. Increased N2O emission through denitrification with increased salinity is likely (Marton et al. 2012). Tsuneda et al. (2005) observed that increased salt concentrations could substantially influence N2O emission by inhibiting nitrous oxide reductase activity. Enhanced N2O emissions may be triggered by inhibited nitrous oxide reductase impeding the kinetic balance between N2O production and consumption under salt stress conditions (Han et al. 2019).
Increased soil \({\text{NH}}_{4}^{+}\) and dissolved organic carbon were observed with increased salinity in a laboratory incubation experiment with core soils collected from freshwater tidal marshes in southeast China (Wang et al. 2017a). In contrast to our experiment, (Wang et al. 2017a) observed stimulated CO2 emission at intermediate salinities (i.e., 5 to 7.5‰) but inhibited at ≥ 15‰, CH4 emissions were unaffected up to 7.5‰ but declined substantially at salinity ≥ 10‰, whereas salinity did not affect the N2O emission. Similarly, in a review, Poffenbarger et al. (2011) observed that the CH4 emission decreased with increasing salinity in tidal marshes. We got a very low CH4 emission or some cases uptake in site B and site A except under PA land-use practice. In these soils, higher salinity increases \({\text{SO}}_{4}^{2-}\) availability; \({\text{SO}}_{4}^{2-}\) acts as an alternative terminal electron acceptor under anaerobic conditions and can shift microbial metabolism towards more energetically favorable processes (Bridgham et al. 2013). A significant inverse correlation has been observed between the CH4 emission and \({\text{SO}}_{4}^{2-}\) content in PPR wetland soils (Pennock et al. 2010). Our experimental soil from site B had very high \({\text{SO}}_{4}^{2-}\) content and had a highly negative correlation with CH4; the lowest CH4 emissions were observed from those soils with high \({\text{SO}}_{4}^{2-}\) content in the soil. Similarly, the presence of high \({\text{SO}}_{4}^{2-}\) in soil inhibited the CH4 emission in an incubation experiment (Ardón et al. 2018) and riparian areas of PPR wetlands (Dunmola et al. 2010).
Water table effects
The GWT significantly controlled the soil GHG emissions from our experimental soil cores. Soil water content controls microbial activity and processes and is the single most crucial soil parameter that regulates GHG emissions (Oertel et al. 2016). Overall, higher CO2 emission can occur from the rapid decomposition of C in well-drained areas (Freeman et al. 2001), N2O emissions are most likely between strict aerobic and anaerobic conditions (Davidson et al. 2000), and CH4 is produced via the reduction of CO2 in a strictly anaerobic microbial process known as methanogenesis (Bridgham et al. 2013). Conversely, soils can also be a sink of atmospheric CH4 through microbial oxidation under aerobic conditions (Thangarajan et al. 2013).
In our experiment, the CO2 emission rate was variable and, to some extent, dependent on VSWC in both sites, whereas CH4 emissions decreased with declining water table depths and as the VSWC decreased. Similarly, an incubation experiment with peat cores from central and eastern Canada (Blodau et al. 2004) found a lower water table depth increased CO2 production through soil respiration and microbial biomass, whereas CH4 production and emissions decreased. Additionally, in a lysimeter experiment using undisturbed peat soil columns, higher CO2 emissions were observed at the low water table depth (40 cm below surface) compared to the greater depths (80 cm below surface), CH4 emissions were very low or negative (Berglund and Berglund 2011). Studies of flooding effects on GHG observed significant CH4 emission in both forested and non-forested soils (Mander et al. 2015; Wang and Bettany 1997); however, Wang and Bettany (1997) also found that 80–90% of the CH4 was taken up within a week under non-flooded conditions.
We observed low N2O emissions under the higher water table level as the experimental soil cores were near-saturated; however, emissions increased once the moisture condition became ideal. This occurred at the midpoint of groundwater table treatments, similar to what was observed by (Berglund and Berglund 2011) in their peat core experiment. Similarly, a laboratory incubation study found high N2O emission with adequate but unsaturated soil water availability, indicating that water-filled pore spaces and C availability primarily controlled the denitrification process and thus N2O emissions (Gillam et al. 2008). Substantially higher N2O emissions can occur when intermittently flooded soils are exposed to air, enhancing combined nitrification-denitrification; however, drying also initiates suboptimal conditions for complete denitrification as enhanced oxygen supply inhibits N2O reductase (Knowles 1982). Therefore, as the GWT is lowered (in the absence of standing water), the N2O reductase enzyme that catalyzes the reduction of N2O to N2 is inhibited by oxygen under suboxic conditions; N2O emissions are most likely the by-product of denitrification, and N2O diffusion to the atmosphere is unrestricted by porewater, increasing N2O fluxes (Pinto et al. 2021).
A study within the PPR agricultural landscape observed that GHG hotspots are predominantly driven by soil moisture and SOC availability (Dunmola et al. 2010). We collected intact soil cores from the annual crop, pasture, and short rotation willow plantation; however, we did not measure GHG emissions directly in the field. Although we might not specifically compare our results with field-scale studies, we observed that the VSWC in our experimental cores largely controlled the GHG emissions. When soils dry out, the substrate supply becomes increasingly limited for microbes as the water drains out from soil pores, and water films around the soil aggregates become thinner and disconnected (Yan et al. 2015). However, we should consider that lowering the water table may also expose new layers in soil containing substrate for microbial decomposition and that soil physical properties at depth might have distinct impacts on the emissions rate (Berglund and Berglund 2011).
Conclusions
Our results showed that adjacent contrasting riparian land-use practices significantly influenced GHG emissions within the PPR agroecosystems. We observed significantly higher CO2, CH4, and N2O emissions from PA land-use practice. Changes in soil properties, particularly organic C and N, evidently shaped the observed difference in soil GHG emissions because of contrasting land-use practices. Conceivably, high background SO42− concentration in soils collected from SRW land-use practices cut the CH4 emission and subsequently contributed less towards the GWP.
We saw that lowering the water table decreased CH4 emissions with the reduction of VSWC but resulted in higher N2O emissions under an intermediate water table position when VSWC in the cores reached suitable conditions for denitrification. We also noticed variable CO2 emissions, with an initial increase with the lowering of the water table followed by a decrease as the VSWC diminished. With elevated groundwater salinity, we observed a decrease in CO2 and CH4 emissions, but a significant escalation in N2O emissions.
The GWP of SRW was significantly lower than AC and PA, suggesting this is a potentially promising land-use practice in those fallow marginal riparian zones of the PPR that are not suitable for crop production due to the higher salinity. Overall, our experiment showed a decrease in GHG emissions with increasing salinity and varying responses to GWT based on GWT depth and the GHG in question.
Cumulative GHG emissions from core soils with different groundwater salinity treatments from soils collected from three land-use practices from A site A, and B site B. Error bar stands for standard deviations (± SD). GHG greenhouse gas, S0 control, S1 6 mS cm−1, S2 12 mS cm−1, AC annual crop, PA pasture, SRW short rotation willow
Non-metric multidimensional scaling (NMDS) test of soil GHG emissions visualized with land-use and groundwater salinity treatments, land-use and groundwater table depth treatments, and redundancy analysis (RDA) from site A (A, B, C) and site B (D, E, F). Blue vectors indicate linear correlations between the ordination and soil physiochemical properties. Directions and lengths of the vectors indicate the strength of correlations between variables and the ordination. The angles between vectors reflect their correlations (i.e., a vector pair with an angle of 20° have strong positive correlation as cos(20) = 0.94, and with an angle of 90° are uncorrelated as cos(90) = 0). *,**,***Indicate there is a statistically significant difference at p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001 level of significance, respectively; ns, is not significantly different (p > 0.05). GHG = greenhouse gas, AC = annual crop, PA = pasture, SRW = short rotation willow, S0 = control, S1 = 6 mS cm−1, S2 = 12 mS cm−1, EC electrical conductivity, SOC soil organic carbon, VSWC volumetric soil water content, TN total nitrogen
Relationship (Spearman rank-order correlation) among GHG, VSWC, EC, and physicochemical characteristics of the experimental soils. Blue circles indicate positive and red circles indicate a negative relationship. Larger circles and deeper colors indicate stronger relationships. *,**,***Indicate there is a statistically significant relationship at p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001 level of significance, respectively; and the remainder are not significant (p > 0.05). GHG greenhouse gas, VSWC soil water content, EC electrical conductivity, CEC cation exchange capacity, TSC total soil carbon, SOC soil organic carbon, WEOC water-extractable organic carbon, TN total nitrogen, WEON water-extractable organic nitrogen, C/N ratio carbon and nitrogen ratio. (Color figure online)
Data availability
All the relevant data of this manuscript has been shared through the electronic supplementary materials.
References
Albrecht A, Kandji ST (2003) Carbon sequestration in tropical agroforestry systems. Agr Ecosyst Environ 99(1):15–27
Amichev BY, Hangs RD, Konecsni SM, Stadnyk CN, Volk TA, Bélanger N, Vujanovic V, Schoenau JJ, Moukoumi J, Van Rees KCJ (2014) Willow short-rotation production systems in Canada and Northern United States: a review. Soil Sci Soc Am J 78(S1):S168–S182
Ardón M, Helton AM, Bernhardt ES (2018) Salinity effects on greenhouse gas emissions from wetland soils are contingent upon hydrologic setting: a microcosm experiment. Biogeochemistry 140(2):217–232
Arndt JL, Richardson JL (1989) Geochemistry of hydric soil salinity in a recharge-throughflow-discharge Prairie-Pothole wetland system. Soil Sci Soc Am J 53(3):848–855
Baah-Acheamfour M, Carlyle CN, Lim SS, Bork EW, Chang SX (2016) Forest and grassland cover types reduce net greenhouse gas emissions from agricultural soils. Sci Total Environ 571:1115–1127
Bedard-Haughn A, Jongbloed F, Akkerman J, Uijl A, de Jong E, Yates T, Pennock D (2006) The effects of erosional and management history on soil organic carbon stores in ephemeral wetlands of hummocky agricultural landscapes. Geoderma 135:296–306
Beetz S, Liebersbach H, Glatzel S, Jurasinski G, Buczko U, Höper H (2013) Effects of land use intensity on the full greenhouse gas balance in an Atlantic peat bog. Biogeosciences 10(2):1067–1082
Benanti G, Saunders M, Tobin B, Osborne B (2014) Contrasting impacts of afforestation on nitrous oxide and methane emissions. Agric For Meteorol 198–199:82–93
Berglund Ö, Berglund K (2011) Influence of water table level and soil properties on emissions of greenhouse gases from cultivated peat soil. Soil Biol Biochem 43(5):923–931
Blodau C, Basiliko N, Moore TR (2004) Carbon turnover in peatland mesocosms exposed to different water table levels. Biogeochemistry 67(3):331–351
Bolan NS, Adriano DC, Kunhikrishnan A, James T, McDowell R, Senesi N (2011) Dissolved organic matter: biogeochemistry, dynamics, and environmental significance in soils. Adv Agron 110:1–75
Bridgham SD, Cadillo-Quiroz H, Keller JK, Zhuang Q (2013) Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob Change Biol 19(5):1325–1346
Carter MR, Gregorich EG (eds) (2008) Soil sampling and methods of analysis. CRC Press and Taylor & Francis Group, Boca Raton
Chantigny MH (2003) Dissolved and water-extractable organic matter in soils: a review on the influence of land use and management practices. Geoderma 113(3–4):357–380
Chantigny MH, Angers DA, Kaiser K, Kalbitz K (2008) Extraction and characterization of dissolved organic matter. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton
Collier SM, Ruark MD, Oates LG, Jokela WE, Dell CJ (2014) Measurement of greenhouse gas flux from agricultural soils using static chambers. Journal of Visualized Experiments 90:52110
Dang DM, Macdonald B, Warneke Sr, White I (2017) Available carbon and nitrate increase greenhouse gas emissions from soils affected by salinity. Soil Res 55(1):47
Davidson EA, Keller M, Erickson HE, Verchot LV, Veldkamp E (2000) Testing a conceptual model of soil emissions of nitrous and nitric oxides. Bioscience 50(8):667
Dunmola AS, Tenuta M, Moulin AP, Yapa P, Lobb DA (2010) Pattern of greenhouse gas emission from a Prairie Pothole agricultural landscape in Manitoba, Canada. Can J Soil Sci 90(2):243–256
Farrell RE, Elliott JA (2008) Soil air. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton
Follett RF, Stewart CE, Pruessner EG, Kimble JM (2012) Effects of climate change on soil carbon and nitrogen storage in the US Great Plains. J Soil Water Conserv 67(5):331–342
Freeman C, Ostle N, Kang H (2001) An enzymic ‘latch’ on a global carbon store. Nature 409:149
Gillam KM, Zebarth BJ, Burton DL (2008) Nitrous oxide emissions from denitrification and the partitioning of gaseous losses as affected by nitrate and carbon addition and soil aeration. Can J Soil Sci 88(2):133–143
Gleason RA, Laubhan MK, Euliss NH Jr, Tangen BA, Kermes KE (2008) Ecosystem services derived from wetland conservation practices in the United States Prairie Pothole Region with an emphasis on the US Department of Agriculture Conservation Reserve and Wetlands Reserve Programs. In. US Geological Survey, Reston
Gleason RA, Tangen BA, Browne BA, Euliss NH Jr (2009) Greenhouse gas flux from cropland and restored wetlands in the Prairie Pothole Region. Soil Biol Biochem 41(12):2501–2507
Gundersen P, Christiansen JR, Alberti G, Brüggemann N, Castaldi S, Gasche R, Kitzler B, Klemedtsson L, Lobo-do-Vale R, Moldan F, Rütting T, Schleppi P, Weslien P, Zechmeister-Boltenstern S (2012) The response of methane and nitrous oxide fluxes to forest change in Europe. Biogeosciences 9(10):3999–4012
Han H, Song B, Song MJ, Yoon S (2019) Enhanced nitrous oxide production in denitrifying Dechloromonas aromatica strain RCB under salt or alkaline stress conditions. Front Microbiol 10:1203
Hendershot WH, Lalande H, Duquette M (2008) Ion exchange and exchangeable cations. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton
Hendershot WH, Lalande H, Duquette M (2008) Soil reaction and exchangeable acidity. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton
IPCC (2018) Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Cambridge, pp 1–1535
Kayranli B, Scholz M, Mustafa A, Hedmark Å (2009) Carbon storage and fluxes within freshwater wetlands: a critical review. Wetlands 30(1):111–124
Knowles R (1982) Denitrification. Microbiol Mol Biol Rev 46(1):43–70
Kooch Y, Moghimian N, Bayranvand M, Alberti G (2016) Changes of soil carbon dioxide, methane, and nitrous oxide fluxes in relation to land use/cover management. Environ Monit Assess 188:346
Kroetsch D, Wang C (2008) Particle size distribution. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton
Krzywinski M, Altman N, Blainey P (2014) Nested designs. Nat Methods 11(10):977–978
Lang M, Cai Z, Chang SX (2010) Effects of land use type and incubation temperature on greenhouse gas emissions from Chinese and Canadian soils. J Soils Sediments 11(1):15–24
Last WM, Ginn FM (2005) Saline systems of the Great Plains of western Canada: an overview of the limnogeology and paleolimnology. Saline Syst 1:10
Levy PE, Burden A, Cooper MDA, Dinsmore KJ, Drewer J, Evans C, Fowler D, Gaiawyn J, Gray A, Jones SK, Jones T, McNamara NP, Mills R, Ostle N, Sheppard LJ, Skiba U, Sowerby A, Ward SE, Zieliński P (2012) Methane emissions from soils: synthesis and analysis of a large UK data set. Glob Change Biol 18(5):1657–1669
Liebig M, Morgan J, Reeder J, Ellert B, Gollany H, Schuman G (2005) Greenhouse gas contributions and mitigation potential of agricultural practices in northwestern USA and western Canada. Soil Tillage Res 83(1):25–52
Mander Ü, Maddison M, Soosaar K, Karabelnik K (2011) The Impact of pulsing hydrology and fluctuating water table on greenhouse gas emissions from constructed wetlands. Wetlands 31(6):1023–1032
Mander Ü, Maddison M, Soosaar K, Teemusk A, Kanal A, Uri V, Truu J (2015) The impact of a pulsing groundwater table on greenhouse gas emissions in riparian grey alder stands. Environ Sci Pollut Res 22(4):2360–2371
Martin RM, Moseman-Valtierra S (2015) Greenhouse gas fluxes vary between phragmites australis and native vegetation zones in coastal wetlands along a salinity gradient. Wetlands 35(6):1021–1031
Marton JM, Herbert ER, Craft CB (2012) Effects of salinity on denitrification and greenhouse gas production from laboratory-incubated tidal forest soils. Wetlands 32(2):347–357
Maucieri C, Zhang Y, McDaniel MD, Borin M, Adams MA (2017) Short-term effects of biochar and salinity on soil greenhouse gas emissions from a semi-arid Australian soil after re-wetting. Geoderma 307:267–276
Merino A, Pérez-Batallón P, Macías F (2004) Responses of soil organic matter and greenhouse gas fluxes to soil management and land use changes in a humid temperate region of southern Europe. Soil Biol Biochem 36(6):917–925
Millar JB (1971) Shoreline-area ratio as a factor in rate of water loss from small sloughs. J Hydrol 14(3):259–284
Miller JJ, Curtin D (2008) Electrical conductivity and soluble ions. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton
Moore BD, Kaur G, Motavalli PP, Zurweller BA, Svoma BM (2017) Soil greenhouse gas emissions from agroforestry and other land uses under different moisture regimes in lower Missouri River Floodplain soils: a laboratory approach. Agrofor Syst 92:335–348
Mutuo PK, Cadisch G, Albrecht A, Palm CA, Verchot L (2005) Potential of agroforestry for carbon sequestration and mitigation of greenhouse gas emissions from soils in the tropics. Nutr Cycl Agroecosyst 71(1):43–54
Myhre G, Shindell D, Bréon FM, Collins W, Fuglestvedt J, Huang J, Koch D, Lamarque JF, Lee D, Mendoza B, Nakajima T, Robock A, Stephens G, Takemura T, Zhang H (2013) Anthropogenic and natural radiative forcing. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate Change 2013: The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, New York
Nachshon U, Ireson A, van der Kamp G, Wheater H (2013) Sulfate salt dynamics in the glaciated plains of North America. J Hydrol 499:188–199
Oertel C, Matschullat J, Zurba K, Zimmermann F, Erasmi S (2016) Greenhouse gas emissions from soils—a review. Chem Erde 76(3):327–352
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2017) R Package “vegan”. Community Ecology Package
Parmar K, Keith AM, Rowe RL, Sohi SP, Moeckel C, Pereira MG, McNamara NP (2015) Bioenergy driven land use change impacts on soil greenhouse gas regulation under Short Rotation Forestry. Biomass Bioenerg 82:40–48
Pennock D, Yates T, Bedard-Haughn A, Phipps K, Farrell R, McDougal R (2010) Landscape controls on N2O and CH4 emissions from freshwater mineral soil wetlands of the Canadian Prairie Pothole region. Geoderma 155(3–4):308–319
Pinto R, Weigelhofer G, Brito AG, Hein T (2021) Effects of dry-wet cycles on nitrous oxide emissions in freshwater sediments: a synthesis. PeerJ 9:e10767
Poffenbarger HJ, Needelman BA, Megonigal JP (2011) Salinity influence on methane emissions from tidal marshes. Wetlands 31(5):831–842
Qiu Q, Wu L, Ouyang Z, Li B, Xu Y, Wu S, Gregorich EG (2015) Effects of plant-derived dissolved organic matter (DOM) on soil CO 2 and N 2 O emissions and soil carbon and nitrogen sequestrations. Appl Soil Ecol 96:122–130
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reichstein M, Subke J-A, Angeli AC, Tenhunen JD (2005) Does the temperature sensitivity of decomposition of soil organic matter depend upon water content, soil horizon, or incubation time? Glob Change Biol 11(10):1754–1767
Rochette P, Bertrand N (2008) Soil-surface gas emissions. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton
Rutherford PM, McGill WB, Arocena JM, Figueiredo CT (2008) Total nitrogen. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton
Saskatchewan Soil Survey Staff (1986) Soil Survey Reports for Saskatchewan: The Soils of Indian Head, Rural Municipalty no 156 Saskatchewan. In. Saskatchewan Institute of Pedology Publication S202. Saskatoon, Saskatchewan
Schaufler G, Kitzler B, Schindlbacher A, Skiba U, Sutton M, Zechmeister-Boltenstern S (2010) Greenhouse gas emissions from European soils under different land use: effects of soil moisture and temperature. Eur J Soil Sci 61(5):683–696
Schielzeth H, Nakagawa S, Freckleton R (2013) Nested by design: model fitting and interpretation in a mixed model era. Methods Ecol Evol 4(1):14–24
Setia R, Marschner P, Baldock J, Chittleborough D, Smith P, Smith J (2011) Salinity effects on carbon mineralization in soils of varying texture. Soil Biol Biochem 43(9):1908–1916
Sheng Q, Wang L, Wu J (2015) Vegetation alters the effects of salinity on greenhouse gas emissions and carbon sequestration in a newly created wetland. Ecol Eng 84:542–550
Shi W-Y, Yan M-J, Zhang J-G, Guan J-H, Du S (2014) Soil CO2 emissions from five different types of land use on the semiarid Loess Plateau of China, with emphasis on the contribution of winter soil respiration. Atmos Environ 88:74–82
Simard RR (1993) Ammonium acetate-extractable elements. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis Publishers, Boca Raton
Skjemstad JO, Baldock JA (2008) Total and organic carbon. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton
Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A (2003) Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur J Soil Sci 54:779–791
Smith P, Martino D, Cai Z, Gwary D, Janzen H, Kumar P, McCarl B, Ogle S, O’Mara F, Rice C, Scholes B, Sirotenko O, Howden M, McAllister T, Pan G, Romanenkov V, Schneider U, Towprayoon S, Wattenbach M, Smith J (2008) Greenhouse gas mitigation in agriculture. Philos Trans R Soc B Biol Sci 363(1492):789–813
Tangen BA, Finocchiaro RG, Gleason RA (2015) Effects of land use on greenhouse gas fluxes and soil properties of wetland catchments in the Prairie Pothole Region of North America. Sci Total Environ 533:391–409
Thangarajan R, Bolan NS, Tian G, Naidu R, Kunhikrishnan A (2013) Role of organic amendment application on greenhouse gas emission from soil. Sci Total Environ 465:72–96
Tsuneda S, Mikami M, Kimochi Y, Hirata A (2005) Effect of salinity on nitrous oxide emission in the biological nitrogen removal process for industrial wastewater. J Hazard Mater 119(1–3):93–98
Updegraff K, Bridgham SD, Pastor J, Weishampel P, Harth C (2001) Response of CO2 and CH4 emissions from peatlands to warming and water table manipulation. Ecol Appl 11(2):311–326
Vidon P (2010) Riparian zone management and environmental quality: a multi-contaminant challenge. Hydrol Process 24(11):1532–1535
Wang C, Tong C, Chambers LG, Liu X (2017) Identifying the salinity thresholds that impact greenhouse gas production in subtropical tidal freshwater marsh soils. Wetlands 37(3):559–571
Wang FL, Bettany JR (1997) Methane emission from Canadian prairie and forest soils under short term flooding conditions. Nutr Cycl Agroecosyst 49(1):197–202
Wang H, Yu L, Zhang Z, Liu W, Chen L, Cao G, Yue H, Zhou J, Yang Y, Tang Y, He JS (2017b) Molecular mechanisms of water table lowering and nitrogen deposition in affecting greenhouse gas emissions from a Tibetan alpine wetland. Glob Change Biol 23(2):815–829
Weslien P, Kasimir Klemedtsson Å, Börjesson G, Klemedtsson L (2009) Strong pH influence on N2O and CH4 fluxes from forested organic soils. Eur J Soil Sci 60(3):311–320
Wrage-Mönnig N, Horn MA, Well R, Müller C, Velthof G, Oenema O (2018) The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol Biochem 123:A3–A16
Wu H, Xingkai X, Cheng W, Lin H (2019) Dissolved organic matter and inorganic N jointly regulate greenhouse gases fluxes from forest soils with different moistures during a freeze-thaw period. Soil Sci Plant Nutr 66(1):163–176
Wu J, Li Q, Chen J, Lei Y, Zhang Q, Yang F, Zhang D, Zhang Q, Cheng X (2018) Afforestation enhanced soil CH4 uptake rate in subtropical China: evidence from carbon stable isotope experiments. Soil Biol Biochem 118:199–206
Yan N, Marschner P (2013) Response of soil respiration and microbial biomass to changing EC in saline soils. Soil Biol Biochem 65:322–328
Yan N, Marschner P, Cao W, Zuo C, Qin W (2015) Influence of salinity and water content on soil microorganisms. Int Soil Water Conserv Res 3(4):316–323
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effect models and extensions in ecology with R. Springer, New York
Acknowledgements
We would like to thank Ron Gares, Brain Bogdan, and Derek Durun from the Agroforestry Development Centre, Agriculture and Agri-Food Canada, Indian Head; Cierra Wallington from the Applied Pedology Lab, Department of Soil Science, University of Saskatchewan, SK Canada for their assistance in soil core samples collection. We also thank Shahrima Tahsin for her support in collecting GHG samples and Frank Krijnen for assisting with GHG measurements.
Funding
This work was financially supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to ABH (RGPIN-2017-05909) and Agriculture and Agri-Food Canada (AAFC) A-Base research funding to RS (LOI 1231). Also, SS received funding support from AAFC’s Research Affiliate Program. The funding agency had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
SS conceptualized, designed, and performed the experiments, analyzed the data, prepared all figures and tables, authored and reviewed drafts of the manuscript, and made final editorial decisions regarding all text and graphs, and approved the final draft. RF contributed reagents and instrument support for GHG analyses, made critical comments, reviewed drafts of the manuscript, and approved the final draft. RS provided technical supports for soil core samples collection, reviewed drafts of the manuscript, made critical comments, and approved the final draft. AB-H provided all necessary financial support and supplies required for the greenhouse experiment, contributed and guided the fundamental ideas for this work, reviewed drafts of the manuscript, made critical comments, and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Brian Branfireun
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shahariar, S., Farrell, R., Soolanayakanahally, R. et al. Elevated salinity and water table drawdown significantly affect greenhouse gas emissions in soils from contrasting land-use practices in the prairie pothole region. Biogeochemistry 155, 127–146 (2021). https://doi.org/10.1007/s10533-021-00818-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-021-00818-3