Abstract
Purpose
Mycological characterisation of marine and dredged sediments consists of the isolation of vital and culturable fungal strains as well as their identification and analysis, in order to increase knowledge of sediment mycobiota and isolated species that can be employed in biotechnological processes. Our study undertook the mycological characterisation of sediments from six different port environments: marine bottom sediments from the ports of Genoa (Liguria, Italy) and Centuri (Corsica, France), dredged sediments (sediments removed from the sea bottom) from landfill sites (contaminated land sites where dredged sediments are deposited) of the ports of Leghorn (Tuscany, Italy) and Cagliari (Sardinia, Italy), bottom muds from the brackish environment of the navigable Navicelli Canal of Pisa (Tuscany, Italy) and dredged marine sediments from a temporary storage site in the port of Toulon (Var, France).
Materials and methods
At each site, 30 kg of sediment was sampled for physical, chemical and mycological analyses. They were analysed in terms of grain size composition, organic and inorganic content, metal concentration and hydrocarbon and polychlorinated biphenyl concentration. Fungi were then isolated from sediments by a modified dilution plate technique from 1:10 up to 1:100. Fungal identification was carried out using a morphological and molecular polyphasic approach.
Results and discussion
Forty-six fungal species belonging to 20 genera were isolated. The highest biodiversity was found in Leghorn (14 species), Genoa (11) and Cagliari (11) sediments, while very low numbers of species were isolated from the ports of Centuri (3) and Toulon (4). Similarly, the number of colony-forming units (CFUs), calculated on the dry weight of the sediments, followed this order: Genoa (3,765 CFUs*g-1) > Leghorn (1,370 CFUs*g-1) > Pisa (1,190 CFUs*g-1) > Cagliari (410 CFUs*g-1) > Toulon (380 CFUs*g-1) > Centuri (220 CFUs*g-1). The most represented genera were Penicillium, Aspergillus and Trichoderma. Some halotolerant species known for their biotechnological properties were isolated: Emericellopsis maritima, Cladosporium halotolerans and Aspergillus micronesiensis. A potential marine pathogenic fungus was found: Aspergillus sydowii.
Conclusions
This work increased knowledge of fungi from marine and dredged sediments in six Mediterranean ports in the framework of the SEDITERRA Project.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fungi are ubiquitous and chemoheterotrophic microorganisms able to colonise every kind of environment, including marine, estuarine and brackish (Damare et al. 2012). Kohlmeyer and Kohlmeyer (1979) have divided and classified marine fungi by ecological criteria into obligate and facultative: the former grow and sporulate exclusively in marine or estuarine habitats, while the latter are those from freshwater or terrestrial milieus that are able to grow in marine environments. Both marine and estuarine fungi can develop several ecological strategies: they may be symbionts of algae, aquatic macrophytes (e.g. Posidonia oceanica (L.) Delile), corals and sponges; saprotrophs of dead organic matter in the bottom sediments; or obligate or opportunistic parasites of fish, corals, sponges and other organisms (Hyde et al. 1998; Das et al. 2006; Vohník et al. 2016; Greco et al. 2017; Vera et al. 2017). Some of these species also occur in seawater, freshwater or terrestrial habitats, but the term ‘marine’ now refers to all fungi that occur in the sea (Jones 2000; Pang et al. 2016). To date, a relatively small percentage of the described species of fungi are associated with marine environments, with ∼1,100 species retrieved exclusively from such habitats (Amend et al. 2019); moreover, there is little knowledge of the role of fungi in the marine and dredged sediments of ports (Hyde et al. 1998). Therefore, new studies are needed to overcome this lack of knowledge.
In the Mediterranean Sea, several studies have investigated mycobiota, mainly focusing on algae, sponges and seagrasses (Garzoli et al. 2015; Gnavi et al. 2017; Bovio et al. 2019), thereby describing fungal communities living in association with marine organisms. Other works have investigated the biotechnological properties of some marine fungal strains for pharmaceutical purposes (Agrawal et al. 2018; Marchese et al. 2020) and with regard to their bioremediation properties (Salvo et al. 2005; Abdel-Azeem et al. 2015; Garzoli et al. 2015; Cecchi et al. 2019, 2020; Youssef et al. 2019; Maamar et al. 2020). By contrast, few studies have focused on the biodiversity of fungi in Mediterranean ports’ sediments (Abdel-Azeem et al. 2015; Greco et al. 2018, 2020; Maamar et al. 2020). The general concept of port sediments can involve numerous different environments: strictly marine bottom sediments, dredged sediments, sediments from dry landfill sites and estuarine waterway sediments. Mycological investigations and characterisations of these environmental sectors are important not only to enrich knowledge about mycological diversity in these contaminated, compromised and anthropized environments, but also to isolate and select fungi for biotechnological purposes.
Every year, marine port sediments undergo dredging processes, which produce large volumes of contaminated sediments to be either disposed of or treated (OSPAR Commission 2014; Akcil et al. 2015). Dredging involves the removal of sediments from the aquatic environment (i.e. port and harbour navigation channels), berthing areas and marinas (Harrington et al. 2016). Excavation, transport and disposal of sediments are the three main stages of the dredging process (Manap and Voulvoulis 2015): the sediments are first removed from the sea bottom using a hydraulic and/or mechanical dredge (Antipov et al. 2003; Du and Li 2010; Manap and Voulvoulis 2015); the dredged sediments are then frequently transferred into hopper barges or pipelines using suction pipes, conveyor belts, bucket or grab to be transported to the disposal site (Duran Neira 2010; Manap and Voulvoulis 2015); lastly, the dredged sediments are disposed at the final site (land or sea site) or a temporary storage or treatment site. During the dredging process, the chemical and grain-size composition of sediments can change, for example due to the loss of fine material during the different phases of excavation and deposition (Nayar et al. 2007; Cutroneo et al. 2013), the loss or degradation of the sediment’s organic content (Graca et al. 2004; Nayar et al. 2007), the reduction in concentration of contaminants that are released into the water during the mechanical removal of sediments from the bottom, or the sediment’s exposure to atmospheric agents in landfill (EPA 2004). All these processes make the final sediments very different from the original, greatly influencing the selection of sediment mycoflora.
In this study, within the framework of the European Interreg Italy-France 2014–2020 Maritime Project SEDITERRA ‘Guidelines for the sustainable treatment of dredged sediments in the maritime area’, the physico-chemical and mycological characterisation of six different port scenarios located in the Mediterranean Sea was carried out. The goals of this study were to increase knowledge of the mycoflora present in Mediterranean port environments and in different types of sediments (bottom sediments, dredged sediments, sediments exposed to atmospheric agents) and to isolate vital and culturable fungal strains employable in future biotechnological processes of dredged sediments’ mycoremediation.
2 Materials and methods
2.1 Sampling
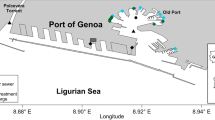
Sediments were sampled from six different locations in the Italian and French ports (Fig. 1) involved in the project, characterised by distinctive environmental characteristics. The sampling sites and details are reported in Table 1. Sediments were sampled at different times throughout the duration of the project: the first in Genoa in November 2017 and the last in Toulon and Centuri in March 2019. Sediments (30 kg) were sampled and stored in closed plastic barrels in a cool environment away from light and then analysed in the laboratory for physical, chemical and mycological characterisation. Aliquots of sediment destined for fungal community analysis were sampled immediately upon arrival at the laboratory.
2.2 Physico-chemical analyses of sediments
The sediment samples were first analysed in terms of grain size and organic and inorganic content.
The grain size analysis involved the sieving of the bulk sediment (200 g) through a 63 μm mesh with water to divide the fine fraction (Ø < 63 μm) from the coarse fraction (Ø ≥ 63 μm). The fine fraction passed through the 63 μm sieve was in turn analysed with a Coulter Counter® Multisizer 3 instrument (Beckman Coulter, Inc.) in order to discriminate the different classes of clay and silt (Ø < 4 μm for clay and 16, 30, 63 μm for silt). The coarse fraction was dried in an oven at 60 °C and subjected to dry sieving to divide the sediment into the different classes of sand and gravel (125, 250, 500, 1000 μm for sand, and Ø > 2000 μm for gravel).
Analysis of the organic and inorganic content of the sediment involved a 15 g sample, which was combusted inside an ISCO muffle (ISM320 mod.) for 3 h at 550 °C to remove the organic fraction. The uncombusted (inorganic) fraction was weighed. The organic fraction was determined by the difference between the initial weight of the sediment and that of the inorganic fraction.
A quantity (1.5 kg) of sediment from each site was chemically analysed to ascertain its metal content by Eurochem Italia s.r.l. and its hydrocarbon and polychlorinated biphenyl content by the Regional Agency for Environmental Protection of Liguria (ARPAL).
2.3 Mycological characterisation
Fungi were isolated by a modified dilution plate technique (Gams et al. 1987). Three aliquots of 1 g each of the Genoa, Leghorn, Pisa-Navicelli, Cagliari, Centuri and Toulon sediments were diluted in 10 mL of sterilised deionised water. The final dilutions were 10–1 and 10–2. In order to favour fungal growth, Rose Bengal medium was prepared using sterile seawater. The solution (1 mL) was inoculated into each plate (Ø 12 cm) and then spatulated to increase the possibility of fungal isolation. Each sample was plated in triplicate. After inoculation, the plates were incubated in the dark at 24 °C and monitored weekly. After growth, the fungal colonies and fungal morphotypes were counted, and the fungal strains were isolated in axenic cultures in test tubes.
Fungal identification was carried out using a polyphasic approach (morphological and molecular). Morphological identification was first carried out by the cultivation of each isolated fungal strain on different media following specific literature methodologies (i.e. malt extract agar, Czapek yeast agar and 25% glycerol nitrate agar for Penicillium species morphological identification; Pitt 1979; Visagie et al. 2014). The initial detection of fungal structures was carried out using a Leica Microsystems EZ4 (10–50×) stereomicroscope. Strains were then identified based on micromorphological (40–100X) analysis. Specific literature was used for the morphological and physiological identification of fungi (e.g. Pitt 1979; Samson et al. 2011; Visagie et al. 2014 for the genus Penicillium; and Raper and Fennel 1965; Klich 2002; Samson et al. 2014 for Aspergillus).
Genomic DNA was extracted from 100 mg of fresh fungal culture using a modified hexadecyltrimethylammonium bromide (CTAB) method (Doyle and Doyle 1987). The morphological identifications of the species were confirmed by amplifying the internal transcribed spacer (ITS) region using universal primers ITS1F/ITS4 (White et al. 1990; Gardes and Bruns 1993) or the β-tubulin gene using Bt2a and Bt2b primers (Samson et al. 2010) for certain strains belonging to the genera Aspergillus and Penicillium. The polymerase chain reaction (PCR) protocol was as follows: 1 × 5 min at 95 °C; 30–35 × (40 s at 94 °C; 45 s at 55 °C; 1 min at 72 °C); 1 × 10 min at 72 °C; and 1 × 10 °C for ∞. Subsequently, the PCR products were purified and sequenced by Macrogen Inc. (Seoul, Republic of Korea). Sequence assembly and editing were performed using Sequencher® (Gene Codes Corporation, version 5.2). The taxonomic assignment of the sequenced samples was carried out using the BLASTN algorithm to compare the sequences obtained against the GenBank database.
We took a conservative approach to species-level assignment (identity ≥ 97%) and verified the accuracy of the results by studying the macro- and micro-morphological features of the colonies. The isolated fungal strains were conserved at 4 ± 1 °C and cryopreserved at −20 °C in the culture collection ColD-UNIGE of the Mycological Laboratory of the Department of Earth, Environment and Life Sciences at the University of Genoa (http://www.mirri-it.it/index.php/associated/university-of-genoa).
2.4 Statistical analyses
The biodiversity levels of each considered sediment were evaluated in terms of species richness and Shannon’s H and Simpson’s D indexes (Shannon 1948; Simpson 1949), calculated starting from the colony-forming units (CFUs) per gram of dry sediment found for each species. Furthermore, the Sørensen’s coefficient (CC) was applied to the number of species to verify if there were any similarities between the fungal communities found in the different sediments (Sørensen 1948).
Pearson’s correlation coefficients (p value = 0.001) were calculated among the physico-chemical characteristics (grain size; inorganic and organic fraction; metals; sum of polycyclic aromatic hydrocarbons, PAHs; hydrocarbons with C > 12; and sum of polychlorinated biphenyls, PCBs) and the mycological data (number of species; CFUs; and D and H indexes) of the sediments with free R software (R Development Core Team 2019) in order to identify possible relationships.
3 Results
3.1 Physico-chemical results
Within the analysed sediments, the inorganic fraction was prevalent at all the sites (Table 2). The sediments of the port of Genoa and the landfill site of Leghorn showed the same characteristics in terms of their organic and inorganic content, with a clear prevalence of the inorganic fraction (97%). The sediments of the landfill site of Cagliari, despite the presence of vegetable fibres, contained an inorganic fraction of 95%, while those of the Navicelli Canal of Pisa were characterised by a higher percentage of organic fraction than the other sediments (12%), due to the high content in serpulids.
As regards the grain-size analyses (Table 2), the marine sediments of Genoa and Centuri with the landfill sediments of Cagliari mainly consisted of the coarse fraction (Ø > 63 μm), ranging from fine to coarse sand. Sediments of the landfill of Leghorn and the storage site of Toulon consisted of equally important coarse (Ø > 63 μm) and fine (Ø < 63 μm) fractions, while the sediments of the Navicelli Canal of Pisa were characterised by a predominant fine fraction made up of silt.
Chemical analyses (Tables S1, S2, S3) showed that the sediments of the Navicelli Canal of Pisa were characterised by more consistent contamination than the other sediments analysed, both in terms of metals and organic pollutants (PAHs, C > 12 hydrocarbons and PCBs). In the case of the Centuri and Toulon sediments, the values of organic contaminants were below the detection limit of the analysis instrument.
3.2 Fungal isolation
The data showed different fungal colonisations in the study sediments (Tables 3 and 4). The greatest specific diversity was found in the sediments from Leghorn (14), Genoa (11) and Cagliari (11), while very small numbers of species were isolated from the Centuri (3) and Toulon (4) sediments. However, the number of CFUs followed another order: Genoa (3,765 CFUs*g-1) > Leghorn (1,370 CFUs*g-1) > Pisa (1,190 CFUs*g-1) > Cagliari (410 CFUs*g-1) > Toulon (380 CFUs*g-1) > Centuri (220 CFUs*g-1) (Table 4). The different port scenarios showed opposite trends: Genoa was characterised by a large number of species and CFUs, while Centuri exhibited the lowest numbers of isolated species and CFUs. Similar behaviour was noted in the landfill sediments, where Leghorn was characterised by larger numbers of isolated species and CFUs than Cagliari. The most represented genera were Penicillium (ten species), Aspergillus (seven species) and Trichoderma (five species). No obligate marine fungi were isolated, but some halotolerant species were seen: Emericellopsis maritima, Cladosporium halotolerans and Aspergillus micronesiensis. The complete list of the fungal isolates for each sediment is shown in Table 3.
3.3 Statistical analyses
The H and D values are reported in Table 4. The highest values were observed in the sediments from the landfill sites of Leghorn and Cagliari and from the brackish environment of Pisa, highlighting the richness of fungal strains for the species found in these sediments, while the lowest values were found in the sediments from Toulon and Centuri, where small numbers of fungal species and strains of each species were isolated and counted.
According to the CC, the communities in the different sediments did not have or, at best, had a very low degree of overlap or similarity. The highest CC values were found between the Pisa and the Leghorn sediments (0.17) and between the Leghorn and the Cagliari sediments (0.17). The results of correlation are shown in Fig. 2. No correlations were found between the physico-chemical parameters of sediments and microfungal contents.
Pearson’s correlation coefficients (p value = 0.001) among the physical-chemical characteristics (grain size, inorganic and organic fraction, metals, the sum of polycyclic aromatic hydrocarbons (PAHs), hydrocarbons of C > 12 and the sum of the polychlorinated biphenyls) and mycological data (number of species, CFUs, and D and H indices) of the sediments
4 Discussion
The contrasting grain-size distributions, organic/inorganic fraction contents and contamination degrees (Table 2; Tables S1, S2, S3) found in the different sediments reflected the varied natural and anthropic pressures as well as treatments to which they were subjected (such as dredging and exposure to atmospheric agents). Generally, sediment contamination did not exceed limit values and could be considered residual contamination. However, in the case of the Navicelli Canal of Pisa, contamination touched the threshold levels, probably due to the fact that these sediments were taken directly from the bottom of a relatively closed environment (with few water changes and poor oxygenation) as well as due to the significant presence of silt-clay sediments which favour contaminant concentration.
Among the fungi isolated, Penicillium and Aspergillus were the most common genera in the sampled sediments. This agrees with what has been previously reported by Hyde et al. (1998) and Jones et al. (2019) concerning marine sediments, but at the same time, these are among the most common genera in terrestrial environments as well. This result perfectly reflects the different port scenarios sampled (marine sediments, dredged sediments, landfill sediments).
As reported in the results section, the marine bottom sediments sampled in the ports of Genoa and Centuri showed very different degrees of mycodiversity and fungal colonisation, reflecting the fact that they are very different types of ports. Indeed, the Port of Genoa is a large industrial and commercial basin (22 km of quays) overlooked by the city of Genoa (600,000 inhabitants) and affected by streams, discharges and street runoff, while the Port of Centuri is a very small marina which is part of a village of 200 inhabitants and inserted in a natural context.
Penicillium, Aspergillus and Trichoderma were the most common genera in the Genoa sediments, showing the great terrestrial input and influence on marine mycobiota, while strictly marine fungi were not isolated. To date, in the Mediterranean Sea, over 200 fungal species have been isolated from different organic and inorganic substrates (i.e. echinoderms, seawaters, deep sediments and coastal sediments), and many studies have highlighted and confirmed the frequent recurrence of the taxa Aspergillus and Penicillium (Abdel-Azeem et al. 2015; Garzoli et al. 2015; Capello et al. 2017; Gnavi et al. 2017; Barone et al. 2018; Bovio et al. 2019; Maamar et al. 2020; Marchese et al. 2020). Moreover, a preliminary study was carried out by Greco et al. (2018) in the Port of Genoa highlighted that the most common genera were Aspergillus, Fusarium, Penicillium and Trichoderma, a finding confirmed by our results. Aspergillus sydowii was also isolated in the sediments of the Port of Genoa. This opportunistic fungal pathogen of corals and gorgonians was previously isolated for the first time in the Port of Genoa by Greco et al. (2017).
By contrast, the marine sediments of Centuri comprised the lowest number of species and strains, characterised by the lowest H and D values (0.82 and 1.9, respectively). The sediments were characterised by large amounts of P. oceanica, but despite previous research by Panno et al. (2013) indicating considerable biodiversity associated with this macrophyte, only four species were isolated here. Hyphopichia burtonii is a yeast that generally grows on starch but is also isolated from lake, fishpond and marine environments (Libkind et al. 2017). Phialemoniopsis genus includes some manglicolous species (Mahanty et al. 2019). The last genus collected was Penicillium, which comprises a very large number of species worldwide and is often reported as a marine genus typical of sediment habitat (Hyde et al. 1998; Vansteelandt et al. 2012).
Among our isolate genera, very interesting fungi able to survive and tolerate high salt concentrations were collected in landfill sediments (Leghorn, Cagliari and Toulon). Emericellopsis maritima (from the Leghorn sediments) is a known marine fungus, with optimum growth at pH 6–7 and able to tolerate higher pH values and high-saline environments (Grum-Grzhimaylo et al. 2013). The black yeast Hortaea werneckii, isolated from Leghorn and Pisa (brackish environment), has been identified as the dominant fungal species in hypersaline waters on three continents (Gunde-Cimerman and Plemenitaš 2006). Hortaea werneckii represents a new model organism for studying the mechanisms of salt tolerance in eukaryotes (Gunde-Cimerman and Plemenitaš 2006). Cladosporium halotolerans (from the Cagliari sediments) is well known for its capability to colonise marine waters and marine sediments (Bovio et al. 2017). The isolation of this kind of fungi was unexpected from terrestrial sediments, but the proximity to the seaport and the marine aerosol could have influenced its mycobiota, increasing salinity. However, an ability to survive in high-salinity conditions does not always coincide with an ability to develop the full lifecycle in such conditions, rendering salt-adapted species difficult to discriminate from ‘transit’ species (Kohlmeyer and Volkmann-Kohlmeyer 2003; Grum-Grzhimaylo et al. 2013). Moreover, the Leghorn and Cagliari sediments had both been exposed to atmospheric events and factors for a long time before sampling, yet they developed different specific fungal communities. This probably owed to the different environmental conditions in these ports. Both sediments showed the presence of strictly terrestrial species (such as Alternaria and Trichoderma) and halotolerant species (such as H. werneckii and C. halotolerans). The Cagliari sediments were characterised by a lower number of CFUs than Leghorn (410 and 1,370, respectively). Cagliari is characterised by a typical Mediterranean climate with an annual temperature of 16.2 °C and annual precipitation of 419 mm y–1 (Trabucco et al. 2018); in 2017 annual precipitation reached a minimum of 220 mm y-1 (www.sardegna-clima.it). Leghorn is characterised not only by a lower annual mean temperature (14 °C) and by higher average annual precipitation (840 mm y–1), but also by exceptional rainfall events, such as those that occurred in 2017 and 2018 (921.2 and 969 mm y-1, respectively; Puppio et al. 2018; www.sir.toscana.it). These events were able to influence the mycodiversity of the sediments, increasing the number of strictly terrestrial species. However, both these sediments were characterised by the highest H (Leghorn 2.16 and Cagliari 2.29) and D values (6.79 and 8.97, respectively), indicating that the microfungi were adapted to the sediments and showed a similar distribution of numbers and strains of species despite the contrasting environmental conditions. The latter finding was confirmed by the CC results showing a weak positive correlation (0.17).
Despite the interesting endophytic fungi isolated (A. micronesiensis, Lichtheimia ramosa and L. corymbifera; Flewelling et al. 2015; Luyen et al. 2019), the sediments of Toulon were characterised by a low number of fungal species and CFUs. It is probable that the high environmental impact and the high population density of the area impose stress conditions and negatively affect microorganism populations (Rossi and Jamet 2008). In addition, these sediments were first dredged and then deposited in the temporary storage site, where they were moved by mechanical vehicles. They subsequently underwent first handling and washout during dredging (confirmed by the very low contaminant concentrations found) and then a second handling and exposure to atmospheric agents. Therefore, the sediments of Toulon represented a highly disturbed environment that may not have given the mycoflora time to recover and develop.
An interesting environment was also represented by the Navicelli Canal of Pisa. Estuarine habitats are, in fact, characterised by a significant proportion of organisms previously observed in terrestrial environments (Gonçalves et al. 2020); this is perhaps indicative of allochthonous inputs of terrestrial fungi into the aquatic environment, or the occurrence of a resident coastal/estuarine fungal community that has close phylogenetic or historical links with terrestrial populations. In the sediments of the Navicelli Canal of Pisa, strictly terrestrial species were found, such as Alternaria chlamydospora and Fusarium oxysporum, but at the same time, the halotolerant species H. werneckii was collected (Gunde-Cimerman and Plemenitaš 2006). Fusarium oxysporum is a well-known parasitic fungus of crops and A. chlamydospora is a common soil fungus (Maciá-Vicente et al. 2008; Michielse and Rep 2009; Lombard et al. 2019).
All the port scenarios studied were characterised by the presence of both typical terrestrial and halotolerant fungal species, indicating that port and coastal habitats represent an ecotone where terrestrial, estuarine and ‘open-ocean fungi’ co-exist. However, among the analysed sediments, there were large numbers of differences in terms of fungal communities. This could indicate that the environmental conditions and anthropic pressures extant in the area and the typologies of wastewater inputs, in addition to the treatments to which the sediments were subjected, significantly influenced the fungal colonisation of the sediments.
5 Conclusion
Ports are interesting ecotone zones between open-sea and terrestrial environments, often affected by industrial and human activities and contamination. These characteristics render ports peculiar ecosystems inhabited by tolerant microorganisms that develop survival strategies and metabolisms different from those of their strictly terrestrial counterparts. Mycological investigations and characterisations of different sectors of port sediments are essential for the isolation and selection of fungi for efficient use in biotechnological activities. This work has shown how ports can act as collection basins for different culturable fungal strains, highlighting not only the great richness of interesting fungi in these environments, but that each port scenario (sea bottom, sediment landfill, brackish environment) represents a different and unique ecosystem characterised by its own physico-chemical properties, environmental characteristics and anthropic pressures, developing distinctive fungal communities as a result.
References
Abdel-Azeem AM, El-Morsy EM, Nour El-Dein MM, Rashad HM (2015) Occurrence and diversity of mycobiota in heavy metal contaminated sediments of Mediterranean coastal lagoon El-Manzala, Egypt. Mycosphere 6:228–240
Agrawal S, Adholeya A, Barrow CJ, Deshmukha SK (2018) Marine fungi: an untapped bioresource for future cosmeceuticals. Phytochem Lett 23:15–20. https://doi.org/10.1016/j.phytol.2017.11.003
Akcil A, Erust C, Ozdemiroglu S, Fonti V, Beolchini F (2015) A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J Clean Prod 86:24–36
Amend A, Burgaud G, Cunliffe M, Edgcomb VP, Ettinger CL, Gutiérrez MH, Heitman J, Hom EFY, Ianiri G, Jones AC, Kagami M, Picard KT, Quandt CA, Raghukumar S, Riquelme M, Stajich J, Vargas-Munic J, Walker AK, Yarded O, Gladfelter AS (2019) Fungi in the marine environment: open questions and unsolved problems. MBio 10:e01189–e01118. https://doi.org/10.1128/mBio.01189-18
Antipov VV, Antipov Yu V, Brakker II, Brenner VA, Naumov Yu N, Pushkarev AE, Pushkarev VA, Podkolzin AA, Zhabin AB (2003) (2003) Channel dredging method, involves cutting ground with working blades of mechanical cutting tools installed on rotary cutting head, which performs horizontal intermittent movement in both directions transversely to axis of rotation. Russian Patent RU 122595:23
Barone G, Rastelli E, Corinaldesi C, Tangherlini M, Danovaro R, Dell’Anno A (2018) Benthic deep-sea fungi in submarine canyons of the Mediterranean Sea. Prog Oceanogr 168:57–64. https://doi.org/10.1016/j.pocean.2018.09.011
Bovio E, Gnavi G, Prigione V, Spina F, Denaro R, Yakimov M, Calogero R, Crisafi F, Varese GC (2017) The culturable mycobiota of a Mediterranean marine site after an oil spill: isolation, identification and potential application in bioremediation. Sci Total Environ 576:310–318. https://doi.org/10.1016/j.scitotenv.2016.10.064
Bovio E, Sfecci E, Poli A, Gnavi G, Prigione V, Lacour T, Mehiri M, Varese GC (2019) The culturable mycobiota associated with the Mediterranean sponges Aplysina cavernicola, Crambe crambe and Phorbas tenacior. FEMS Microbiol Lett 366:fnaa014. https://doi.org/10.1093/femsle/fnaa014
Capello M, Carbone C, Cecchi G, Consani S, Cutroneo L, Di Piazza S, Greco G, Tolotti R, Vagge G, Zotti M (2017) A mycological baseline study based on a multidisciplinary approach in a coastal area affected by contaminated torrent input. Mar Pollut Bull 119:446–453. https://doi.org/10.1016/j.marpolbul.2017.03.070
Cecchi G, Vagge G, Cutroneo L, Greco G, Di Piazza S, Faga M, Zotti M, Capello M (2019) Fungi as potential tool for polluted port sediment remediation. Environ Sci Pollut Res 26:35602–35609. https://doi.org/10.1007/s11356-019-04844-5
Cecchi G, Cutroneo L, Di Piazza S, Vagge G, Capello M, Zotti M (2020) From waste to resource: mycoremediation of contaminated marine sediments in the SEDITERRA Project. J Soils Sediments 20:2653–2663. https://doi.org/10.1007/s11368-019-02527-9
Cutroneo L, Castellano M, Ferranti MP, Povero P, Tucci S, Capello M (2013) Use of optical and acoustic instruments to study the turbid plumes generated by three different types of dredges during dredging activities inside and outside of a port. J Soils Sediments 13:1645–1654. https://doi.org/10.1007/s11368-013-0756-5
Damare S, Singh P, Raghukumar S (2012) Biotechnology of marine fungi. In: Raghukumar C (ed) Biology of marine fungi. Prog Mol Subcell Biol 53:277–297. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-23342-5_14
Das S, Lyla PS, Khan SA (2006) Marine microbial diversity and ecology: importance and future perspectives. Curr Sci 90:1325–1335 www.jstor.org/stable/24091982
Doyle JL, Doyle JJ (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Du Y, Li H (2010) Mechanical technology for dyke reinforcement by sediment discharge in the lower reaches of the Yellow River. Yellow River Conservancy Press, Chengdong Lu
Duran Neira C (2010) Closed-circuit water system for suction dredgers, drives water accumulated in compartment through spout pipe towards dredging head of suction pipe for recirculating aspirated water. Spanish patent ES PCT/ES2010/000138
EPA (2004) Evaluating environmental effects of dredged material management alternatives - a technical framework. EPA842-B-92-008. https://frtr.gov/matrix/documents/Environmental-Dredging/2004-Evaluating-Environmental-Effects-of-Dredged-Material-Management-Alternatives.pdf. Accessed 27 Apr 2020
Flewelling AJ, Currie J, Gray CA, Johnson JA (2015) Endophytes from marine macroalgae: promising sources of novel natural products. Curr Sci 109:88–111 https://www.jstor.org/stable/24905694
Gams W, Van der Aa HA, Van der Plaats-Niterink AJ, Samson RA, Stalpers JA (1987) CBS course of mycology (No. Ed. 3). Centraalbureau voor schimmelcultures, Baarn, pp 136
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Garzoli L, Gnavi G, Tamma F, Tosi S, Varese GC, Picco AM (2015) Sink or swim: updated knowledge on marine fungi associated with wood substrates in the Mediterranean Sea and hints about their potential to remediate hydrocarbons. Progr Ocean 137:140–148. https://doi.org/10.1016/j.pocean.2015.05.028
Gnavi G, Garzoli L, Poli A, Prigione V, Burgaud G, Varese GC (2017) The culturable mycobiota of Flabellia petiolata: first survey of marine fungi associated to a Mediterranean green alga. PLoS One 12:e0175941. https://doi.org/10.1371/journal.pone.0175941
Gonçalves MF, Vicente TF, Esteves AC, Alves A (2020) Novel halotolerant species of Emericellopsis and Parasarocladium associated with macroalgae in an estuarine environment. Mycologia 112:154–171. https://doi.org/10.1080/00275514.2019.1677448
Graca B, Burska D, Matuszewska K (2004) The impact of dredging deep pits on organic matter decomposition in sediments. Water Air Soil Pollut 158:237–259
Greco G, Capello M, Cecchi G, Cutroneo L, Di Piazza S, Zotti M (2017) Another possible risk for the Mediterranean Sea? Aspergillus sydowii discovered in the Port of Genoa (Ligurian Sea, Italy). Mar Pollut Bull 122:470–474. https://doi.org/10.1016/j.marpolbul.2017.06.058
Greco G, Cecchi G, Di Piazza S, Cutroneo L, Capello M, Zotti M (2018) Fungal characterisation of a contaminated marine environment: the case of the Port of Genoa (North-Western Italy). Webbia 73:97–106
Greco G, Cutroneo L, Di Piazza S, Capello M, Zotti M (2020) Trapping of marine-derived fungi on wooden baits to select species potentially usable in mycoremediation. Ital J Mycol 49:101–115. https://doi.org/10.6092/issn.2531-7342/10769
Grum-Grzhimaylo AA, Georgieva ML, Debets AJ, Bilanenko EN (2013) Are alkalitolerant fungi of the Emericellopsis lineage (Bionectriaceae) of marine origin? IMA Fungus 4:213. https://doi.org/10.5598/imafungus.2013.04.02.07
Gunde-Cimerman N, Plemenitaš A (2006) Ecology and molecular adaptations of the halophilic black yeast Hortaea werneckii. In: Amils R, Ellis-Evans C, Hinghofer-Szalkay H (eds) Life in extreme environments. Springer, Dordrecht, pp 177–185. https://doi.org/10.1007/978-1-4020-6285-8_11
Harrington J, Murphy J, Coleman M, Jordan D, Szacsuri G (2016) Financial modelling and analysis of the management of dredged marine sediments–development of a decision support tool. J Ship Trade 1:1–10. https://doi.org/10.1186/s41072-016-0010-6
Hyde KD, Jones EBG, Leanä E, Pointing SB, Poonyth AD, Vrijmoed LLP (1998) Role of fungi in marine ecosystems. Biodivers Conserv 7:1147–1161
Jones EBG (2000) Marine fungi: some factors influencing biodiversity. Fungal Divers 4:53–73
Jones EG, Pang KL, Abdel-Wahab MA, Scholz B, Hyde KD, Boekhout T, Ebel R, Rateb ME, Henderson L, Sakayaroj J, Suetrong S, Dayaratne MC, Kumar V, Raghukumar S, Sridar KR, Bhakali AHA, Gleason FH, Norphanphoun C (2019) An online resource for marine fungi. Fungal Divers 96:347–433. https://doi.org/10.1007/s13225-019-00426-5
Klich MA (2002) Identification of common Aspergillus species. Centraalbureau voor schimmelcultures, Utrecht, p 116
Kohlmeyer J, Kohlmeyer E (1979) Marine mycology and the higher fungi. Academic Press, New York
Kohlmeyer J, Volkmann-Kohlmeyer B (2003) Fungi from coral reefs: a commentary. Mycol Res 107:386–387
Libkind D, Buzzini P, Turchetti B, Rosa CA (2017) Yeasts in continental and seawater. In: Buzzini P, Lachance MA, Yurkov A (eds) Yeasts in Natural Ecosystems: Diversity. Springer, Cham. https://doi.org/10.1007/978-3-319-62683-3_1
Lombard L, Sandoval-Denis M, Lamprecht SC, Crous PW (2019) Epitypification of Fusarium oxysporum–clearing the taxonomic chaos. Persoonia: Mol Phylogenet Evol Fungi 43:1–47. https://doi.org/10.3767/persoonia.2019.43.01
Luyen ND, Huong LM, Ha TTH, Cuong LH, Yen DTH, Tai BH, Nhiem NX, Van Kiem P (2019) Polyoxygenated polyketides from the marine-derived fungus Aspergillus micronesiensis. Vietnam J Chem 57:654–660. https://doi.org/10.1002/vjch.201900036
Maamar A, Lucchesi ME, Debaets S, Nguyen van Long N, Quemener M, Coton E, Bouderbala M, Burgaud G, Matallah-Boutiba A (2020) Highlighting the crude oil bioremediation potential of marine fungi isolated from the Port of Oran (Algeria). Diversity 12:196. https://doi.org/10.3390/d12050196
Maciá-Vicente JG, Jansson HB, Abdullah SK, Descals E, Salinas J, Lopez-Llorca LV (2008) Fungal root endophytes from natural vegetation in Mediterranean environments with special reference to Fusarium spp. FEMS Microbiol Ecol 64:90–105. https://doi.org/10.1111/j.1574-6941.2007.00443.x
Mahanty S, Bakshi M, Ghosh S, Chatterjee S, Bhattacharyya S, Das P, Das S, Chaudhuri P (2019) Green synthesis of iron oxide nanoparticles mediated by filamentous fungi isolated from Sundarban Mangrove ecosystem, India. BioNanoScience 9:637–651. https://doi.org/10.1007/s12668-019-00644-w
Manap N, Voulvoulis N (2015) Environmental management for dredging sediments–the requirement of developing nations. J Environ Manag 147:338–348. https://doi.org/10.1016/j.jenvman.2014.09.024
Marchese P, Garzoli L, Gnavi G, O’Connell E, Bouraoui A, Mehiri M, Murphy GM, Varese GC (2020) Diversity and bioactivity of fungi associated with the marine sea cucumber Holothuria poli: disclosing the strains potential for biomedical applications. J Appl Microbiol 129:612–625. https://doi.org/10.1111/jam.14659
Michielse CB, Rep M (2009) Pathogen profile update: Fusarium oxysporum. Mol Plant Pathol 10:311
Nayar S, Miller DJ, Hunt A, Goh BP, Chou LM (2007) Environmental effects of dredging on sediment nutrients, carbon and granulometry in a tropical estuary. Environ Monit Assess 127:1–13
OSPAR Commission (2014) OSPAR Guidelines for the management of dredged material at sea. Draft Summary Record - EIHA, Annex 7, pp 34. https://dredging.org/media/ceda/org/documents/guidance/ospar/ospar-dredged-materialguidelines_for%20london-2014.pdf. Accessed 27 Apr 2020
Pang KL, Overy DP, Jones EG, da Luz Calado M, Burgaud G, Walker AK, Johnson JA, Kerr RG, Cha HJ, Bills GF (2016) ‘Marine fungi’ and ‘marine-derived fungi’ in natural product chemistry research: toward a new consensual definition. Fungal Biol Rev 30:163–175. https://doi.org/10.1016/j.fbr.2016.08.001
Panno L, Bruno M, Voyron S, Anastasi A, Gnavi G, Miserere L, Varese GC (2013) Diversity, ecological role and potential biotechnological applications of marine fungi associated to the seagrass Posidonia oceanica. New Biotechnol 30:685–694. https://doi.org/10.1016/j.nbt.2013.01.010
Pitt JI (1979) The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, New York 634 pp
Puppio ML, Sara N, Sassu M (2018) Failure evidences of reduced span bridges in case of extreme rainfalls the case of Livorno. Frattura e Integrità Strutturale 12:190–202. https://doi.org/10.3221/FIGF-ESIS.46.18
R Development Core Team (2019) R: A language and environment for statistical computing (version 3.1. 2). R Foundation for Statistical Computing, Vienna, Austria, p 2014
Raper KB, Fennel DI (1965) Aspergillus terreus group. The genus Aspergillus. The Williams & Wilkins Co., Baltimore, pp 293–344
Rossi N, Jamet JL (2008) In situ heavy metals (copper, lead and cadmium) in different plankton compartments and suspended particulate matter in two coupled Mediterranean coastal ecosystems (Toulon Bay, France). Mar Pollut Bull 56:1862–1870. https://doi.org/10.1016/j.marpolbul.2008.07.018
Salvo VS, Gallizia I, Moreno M, Fabiano M (2005) Fungal communities in PAH-impacted sediments of Genoa-Voltri Harbour (NW Mediterranean, Italy). Mar Pollut Bull 50:553–559. https://doi.org/10.1016/j.marpolbul.2005.01.001
Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B (2010) Food and indoor fungi. CBS Laboratory Manual Series. Westerdijk Fungal Biodiversity Institute, Utrecht, p 390
Samson RA, Yilmaz N, Houbraken J, Spierenburg H, Seifert KA, Peterson SW, Varga J, Frisvad JC (2011) Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud Mycol 70:159–183
Samson RA, Visagie CM, Houbraken J, Hong SB, Hubka V, Klaassen CHW, Perrone G, Seifert KA, Susca A, Tanney JB, Varga J, Kocsubè S, Szigeti G, Yaguchi T, Frisvad JC (2014) Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol 78:141–173. https://doi.org/10.1016/j.simyco.2014.07.004
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423
Simpson EH (1949) Measurement of diversity. Nature 163(4148):688–688. https://doi.org/10.1038/163688a0
Sørensen TA (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol Skar 5:1–34
Trabucco A, Sušnik J, Vamvakeridou-Lyroudia L, Evans B, Masia S, Blanco M, Roson R, Sartori M, Alexandri E, Brouwer F, Spano D, Damiano A, Virdis A, Sistu G, Pulinu D, Statzu V, Madau F, Strazzera E, Mereu S (2018) Water-food-energy nexus under climate change in Sardinia. In Multidisciplinary Digital Publishing Institute Proceedings 2: 609. https://doi.org/10.3390/proceedings2110609
Vansteelandt M, Kerzaon I, Blanchet E, Tankoua OF, Du Pont TR, Joubert Y, Monteau F, Le Bizec B, Frisvad JC, Pouchus FY, Grovel O (2012) Patulin and secondary metabolite production by marine-derived Penicillium strains. Fungal Biol 116:954–961. https://doi.org/10.1016/j.funbio.2012.06.005
Vera J, Gutiérrez MH, Palfner G, Pantoja S (2017) Diversity of culturable filamentous Ascomycetes in the eastern South Pacific Ocean off Chile. World J Microbiol Biotechnol 33:157. https://doi.org/10.1007/s11274-017-2321-7
Visagie CM, Houbraken J, Frisvad JC, Hong SB, Klaassen CHW, Perrone G, Seifert KA, Varga J, Yaguchi T, Samson RA (2014) Identification and nomenclature of the genus Penicillium. Stud Mycol 78:343–371. https://doi.org/10.1016/j.simyco.2014.09.001
Vohník M, Borovec O, Kolařík M (2016) Communities of cultivable root mycobionts of the seagrass Posidonia oceanica in the northwest Mediterranean Sea are dominated by a Hitherto undescribed Pleosporalean dark septate endophyte. Microb Ecol 71:442–451. https://doi.org/10.1007/s00248-015-0640-5
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: a guide to methods and applications. Academic Press Inc, New York, pp 315–322
Youssef FS, Ashour ML, Singab ANB, Wink M (2019) A comprehensive review of bioactive peptides from marine fungi and their biological significance. Mar Drugs 17:559. https://doi.org/10.3390/md17100559
Acknowledgements
The authors would like to thank all the Partners of the SEDITERRA Project (Département du Var, Institut National de Sciences Appliquées de Lyon - INSA, Istituto Superiore per la Protezione e la Ricerca Ambientale - ISPRA, Provincia di Pisa, Regione Autonoma della Sardegna, Collectivité de Corse) who have made possible the realisation of the project activities. Moreover, the authors would like to thank the Port Authority of Genoa for allowing the SEDITERRA Project to take place within the Port of Genoa.
Funding
This study was funded by the European Interreg Italy-France 2014-2020 Maritime Project SEDITERRA “Guidelines for the sustainable treatment of dredged sediments in the Maritime area” (CUP I42F17000010006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jos Brils
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cecchi, G., Cutroneo, L., Di Piazza, S. et al. Culturable fungi from dredged and marine sediments from six ports studied in the framework of the SEDITERRA Project. J Soils Sediments 21, 1563–1573 (2021). https://doi.org/10.1007/s11368-021-02884-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-021-02884-4