Abstract
Osteoarthritis (OA) of the knee is closely associated with aging; however, little is known about the age-related degeneration in the mandibular condylar cartilage (MCC) of the TMJ. Our objective was to examine whether a correlation exists between aging and degeneration of the MCC of the TMJ. Thirty-two male C57BL/6J wild-type mice were aged to 2, 12, 18, and 25 months old. The mice were euthanized by CO2 inhalation and were dissected and examined by micro-CT and histology. Sagittal sections of the condyles were stained for tartrate-resistant alkaline phosphatase, alkaline phosphatase, safranin O, picrosirius red, and toluidine blue. In addition, immunostaining for BMP2, BMP4, BMP7, PRG4, and MMP13 was performed. Bone volume fraction and tissue density significantly increased with the age of the animals. There was a significant increase in the Osteoarthritis Research Society International histopathological score and mineralization of the noncalcified cartilage in the aged animals. There was a decrease in cartilage thickness, proteoglycan distribution, and cellularity in the aged animals. Additionally, we noted increased picrosirius red staining with the increase in the age of the animals. Our protein expression showed increased BMP2, BMP4, BMP7, and MMP13, whereas there was a decrease in PRG4 expression in the aged animals. As the animal ages, there is decreased proteoglycan secretion, decreased cellularity, decreased cartilage thickness, increased fibrillation, and increased proteolytic activity. A better understanding of the basic mechanisms underlying the degeneration of the MCC in the older animals could provide novel ways to slow the development of OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is a physiological process which is accompanied by a series of cellular, molecular, and structural changes that influences the properties and function of the tissues (DiLoreto and Murphy 2015). Due to increased life expectancy over the past decades, aging-related diseases are a major issue. Aging affects all the organs and tissues of the body, including the temporomandibular joint (TMJ). Osteoarthritis (OA) has been reported as one of the major degenerative diseases accompanying aging, where both the incidence and severity of the diseases increase with age (Luder 2002; Yadav et al. 2018). Furthermore, OA is the major cause of disability and pain in the population over the age of 65.
It has been an accepted notion that OA in aging is due to an imbalance between stressors that causes damage and the mechanisms that prevent or repair the cartilage damage (Kuroda et al. 2009). Osteoarthritis can affect several joints, including the TMJ. The mandibular condylar cartilage (MCC) of the TMJ is a fibrocartilaginous tissue which contains both type I and type II collagen (Orajarvi et al. 2018; Wadhwa and Kapila 2008). The major role of the MCC is to support and distribute functional loads, allowing a frictionless motion and avoiding the breakdown of the cartilage. The critical change in the TMJ, associated with advancing age, is the replacement of cartilage with bone. The formation of calcified cartilage is due to a shift in the cellular composition, and such alterations favor the onset of degenerative disorders of the TMJ.
Aging-related quantitative and qualitative changes have been thoroughly studied in the articular cartilage of the knee, but little to no information is available on the age-related changes in the cartilage and the subchondral bone of the TMJ. In particular, nothing is known about the age-dependent changes in the expression of the proteoglycans and the pattern of the degeneration of the MCC of the TMJ. In the present study, our aim was to examine whether a correlation exists between aging and degeneration of the fibrocartilage of the TMJ. Additionally, we have investigated the protein expression of BMPs and other factors, such as MMP13 and PRG4, as they have been shown to play a major role in the OA of the TMJ (Gho et al. 2018; Hill et al. 2014; Koyama et al. 2014; Lories and Luyten 2011; Wang et al. 2013). Our hypothesis is that with aging there is increased degeneration of the osteochondral tissues of the TMJ. Although the precise etiology of the OA changes occurring in the MCC and subchondral bone of the TMJ are unknown, studying them in different age groups will help us in understanding the cellular and the molecular basis of the pathogenesis of OA in the TMJ.
Materials and methods
Ethics statement
The animals were housed in the animal facility, and all the experimental procedures involving mice were approved by the Institutional Care Committee of the University of Connecticut Health Center (UCONN Health).
Study characteristics
The experiments were designed to study the effects of normal aging on the mandibular condylar cartilage of the TMJ. The body weight of the animals was significantly different between the groups as they belong to different age groups. All experiments were approved by the Institutional Animal Care and Use Committee of UCONN Health, and all the animals were housed in a fully accredited animal care facility at UCONN Health.
Mice
The proposed research was carried out on male C57BL/6J wild-type mice. The mice were divided into four age groups: 2-month-old (n = 8), 12-month-old (n = 8), 18-month-old (n = 8), and 25-month-old (n = 8) age groups. The mice were euthanized by CO2 inhalation, and the mandibular condyle was harvested and fixed in 10% formalin and stored at 4 °C.
Micro-computed tomography analysis
Micro-CT was performed on mandibles by the UCONN Health Micro CT imaging facility with a μCT40 instrument (Scanco Medical AG, Bruttisellen, Switzerland). The technician performing the scans and analysis was blinded to the treatment groups. One mandible from each experimental group was dissected, cleaned, and fixed in 4% paraformaldehyde. The mandible was washed through a series of solutions and processed up to 70% alcohol. The samples (n = 8 per group) were scanned in 70% alcohol, and serial tomographic projections were acquired at 55 kV and 145 μA, with a voxel size of 6 μm, and 1000 projections per rotation were collected at 300,000 μs. The DICOM images were transferred, segmented, and reconstructed using the Mimics software (Materialise NV, Leuven, Belgium). In order to distinguish calcified tissue from noncalcified tissue, an automated algorithm using local threshold segmented the reconstructed gray scale images. The region of interest was the mushroom-shaped head of the condyle which includes the MCC and the subchondral bone. Bone volume fraction (BVF (%)) and tissue density (mg/ccmHA) were determined.
Histological evaluation and quantification
Fixed mandibular condyles were placed in 30% sucrose overnight and then embedded in cryomedium for frozen sectioning. Serial sagittal sections of mandibular condyles (n = 8 per group) were stained for safranin O (IHC WORLD, LLC; Woodstock, MD, USA) and toluidine blue (TB; IHC WORLD, LLC; Woodstock, MD, USA) to evaluate overall histology, cartilage thickness, and proteoglycan content. Sirius red staining was performed to detect collagen type I distribution (IHC WORLD, LLC; Woodstock, MD, USA). The sections were also stained for tartrate-resistant alkaline phosphatase (TRAP) using the ELF97 substrate (Life Technologies, Grand Island, NY, USA) to detect osteoclastic activity. Enzymatic mineralization within the mandibular condylar cartilage was analyzed by alkaline phosphatase (AP, IHC WORLD, LLC; Woodstock, MD, USA) staining. In addition, immunohistochemistry for MMP13 (ABCAM (39012), Cambridge, MA, USA), BMP2 (ABCAM (14933), Cambridge, MA, USA), BMP4 (ABCAM (39973), Cambridge, MA, USA), BMP7 (ABCAM (56033), Cambridge, MA, USA), and PRG4 (Novus Biologicals, CO, USA) was performed. We examined TRAP activity in the subchondral bone by counting the number of TRAP (yellow fluorescent pixels) and dividing it by the total number of pixels in the subchondral region (Adobe Photoshop, Santa Fe, CA, USA). The alkaline phosphatase distance map was measured from the outer layer of the mandibular condylar cartilage to the layer of alkaline phosphatase staining (red fluorescent staining) by using the Digimizer Image software (MedCalc Software, Ostend, Belgium). The Osteoarthritis Research Society International (OARSI) osteoarthritis cartilage histopathology assessment was performed in safranin O-stained sections following the guidelines from the OARSI histopathology initiative for mouse cartilage (Glasson et al. 2010). Distance mapping (cartilage thickness) in TB-stained sections was analyzed using Digimizer Image software (MedCalc Software, Ostend, Belgium). Measurements were calculated from the outer cellular layer of the mandibular condyle to the tidemark (in three different locations in the entire mandibular condylar cartilage). The alkaline phosphatase area and immunohistochemistry were quantified by counting the alkaline phosphatase and immunostaining-positive pixels and dividing those numbers by the total number of DAPI-positive pixels in the mandibular cartilage of each section. The number obtained was then multiplied by 100 to determine the percentage of expression (Adobe Photoshop, Santa Fe, CA, USA).
Statistical analysis
Descriptive statistics were used to examine the distribution of bone volume fraction, tissue density, histological analysis, and gene expression. A 1-sample Kolmogorov-Smirnov test was used to examine the normality of data distribution. Outcomes were compared between the different experimental groups. Statistically significant differences among means were determined by analysis of variance (ANOVA). Tukey’s honest significant difference post hoc analysis was used to compare the multiple groups and to find out exactly where the difference lies. All statistical tests were 2 sided, and a P value of < 0.05 was deemed to be statistically significant. Statistical analysis was performed using GraphPad Prism (San Diego, CA, USA).
Results
Increased bone volume and density in the mandibular condyle of aged mice
Micro-CT analysis revealed a progressive increase in bone volume and density in the mandibular condyle as the mice aged (Fig. 1). There was a significant increase in bone volume in mice aged 12, 18, and 25 months in comparison to 2-month-old mice (Fig. 1a, b). Although bone volume seemed to be positively correlated with age, no statistical significance was observed between the 12-, 18-, and 25-month-old groups (Fig. 1b). Regarding bone density, the same trend was noticed, with increased density for mice aged 12, 18, and 25 months old in comparison to the younger group (2 months old; Fig. 1a, c).
Increased bone volume and density at the mandibular condyle of mice as the animal aged. Coronal micro-CT images of condyles of 2-, 12-, 18-, and 25-month-old mice (a). Quantification of bone parameters: b BVF—bone volume fraction, c tissue density. Histograms (b and c) represent means ± SD for n = 8 per group. #: statistically significant difference between groups 2M and 12M, 18M, and 25 M; no significant difference between 12M, 18M, and 25M groups. #: p < 0.05. Scale bar = 500 μm
Decreased osteoclast activity and modified alkaline phosphatase distribution in the mandibular condyle of aged mice
We observed a dynamic TRAP expression (indicator of osteoclast activity) in the MCC and the subchondral bone of 2-month-old mice, whereas the activity dropped significantly as the mice aged, becoming only limited to the subchondral bone (Fig. 2a, c). This finding suggests a reduction in bone remodeling with aging. Alkaline phosphatase (AP) is an enzymatic marker of mineralization. We analyzed the overall AP expression in the MCC and the distance map in relation to the outer layer of the cartilage. We found a similar distribution and distance map of AP in the MCC of 2-, 12-, and 18-month-old mice (Fig. 2b, d, e). However, at 25 months of age, there was a significant shift of expression with the AP staining (mineralization) layer moving towards the outer layer (Fig. 2b, d, e), suggesting the mineralization was migrating towards the superficial layer of the cartilage, invading the unmineralized portion of the cartilage.
Decreased bone remodeling and shift in mineralization marker location in the mandibular condyle of mice with aging. Sagittal sections of mandibular condyles of 2-, 12-, 18-, and 25-month-old mice stained for TRAP (a) and alkaline phosphatase (AP, red staining) with DAPI (blue staining) nuclear staining (b). White dotted lines in a delimitate the subchondral bone (Sub. Bone) and mandibular cartilage (MC) regions. Quantification of percentage of TRAP-positive pixels (yellow pixels, c) in the subchondral bone area. Quantification of AP (red staining) distance map (d) and area (e). Histograms (c, d, and e) represent means ± SD for n = 8 per group. Statistically significant difference between groups: c #: significant difference between groups 2M and 12M, 18M, 25M; no significant difference between 12M, 18M, and 25M groups. d and e #: significant difference between groups 25M and 2M, 12M, 18M; no significant difference between 2M, 12M and 18M groups. #: p < 0.05. Scale bar = 100 μm (a) and 50 μm (b)
Decreased proteoglycan secretion and cartilage thickness in the mandibular condyle of aged mice
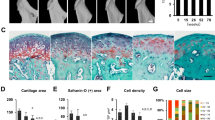
Safranin O and toluidine blue (TB) staining illustrated robust proteoglycan distribution, cartilage thickness, and cellularity in the mandibular condyle of 2-month-old mice (Fig. 3a, b). However, disruption of the proteoglycan layer integrity and a decrease in cartilage thickness and cellularity were noticed as the mice aged (Fig. 3a, b). The safranin O and TB staining showed that proteoglycan distribution was decreased from 12 to 18 months and the cartilage integrity was severely disrupted with the increase in the age of the animal. The OARSI osteoarthritis cartilage histopathology assessment, performed in safranin O-stained sections, revealed a significant increase in the scores as the mice aged (Fig. 3d). Quantification of cartilage thickness in TB-stained sections confirmed those observations; there was a significant difference between 2- and 12-, 18- and 25-month-old mice (Fig. 3e). In addition, the 25-month-old group presented with the most significant reduction in relation to the other groups (Fig. 3e).
Decreased cartilage thickness in the mandibular condyle of aged mice. Sagittal sections of mandibular condyles of 2-, 12-, 18-, and 25-month-old mice stained for safranin O (a), toluidine blue (b), and sirius red (c). OARSI histopathological scores: 1 to 2: mild; 3 to 4: moderate; 5 to 6: severe (d). Quantification of cartilage thickness in toluidine blue-stained sections (e). Histogram (d and e) represents means ± SD for n = 8 per group. Statistically significant difference between groups: d #: significant difference between groups 2M and 12M, 18M, 25M; *: significant difference between groups 12M and 2M, 18M, 25M; no significant difference between 18M and 25M groups. e #: significant difference between groups 2M and 12M, 18M, 25M; ##: significant difference between groups 25M and 2M, 12M, 18M; no significant difference between 12M and 18M groups. #, ##, *: p < 0.05. Scale bar = 50 μm
Moreover, we performed picrosirius red (PR) staining to evaluate collagen type I and II distribution in the MCC and the subchondral bone of the mice as they aged. PR staining was very strong but limited to the subchondral bone of 2- and 12-month-old mice (Fig. 3c). However, at 18 and 25 months of age, a strong PR staining was observed in the MCC in addition to the subchondral bone region of the TMJ. Additionally, at 25 months of age, PR staining was even observed in the superficial layer of the MCC, which may indicate mineralization and altered extracellular matrix organization compared to 2- and 12-month-old mice (Fig. 3c).
Differential gene expression in the mandibular condyle as mice age
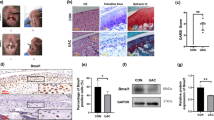
We analyzed the expression of genes relevant to cartilage degradation, chondrocyte differentiation, and protection in the mandibular cartilage of different age groups. We first examined the expression of matrix metallopeptidase 13 (MMP13), a collagenase involved in the breakdown of extracellular matrix in mandibular cartilage, promoting cartilage degradation. We observed a minimal expression of MMP13 in the younger group of mice (2-month-old); however, as the mice aged, the expression of MMP13 became pronounced (Fig. 4a, f). Next, the protein expression pattern of several members of the bone morphogenetic protein family (BMP2, BMP4, and BMP7) was analyzed. The expression of BMP2 and BMP4 was found to be relatively increased in the older groups analyzed (18- and 25-month-old) in comparison to the 2- and 12-month-old groups (Fig. 4b, c, g, h). On the other hand, the expression of BMP7 was prominent in the 18-month-old group only (Fig. 4d, i). Finally, we analyzed the protein expression of proteoglycan 4 (PRG4), which is an essential factor for joint lubrication and plays an important role in preserving the structural and cellular integrity of the MCC and subchondral bone of the TMJ. We found a significantly higher expression of PRG4 in the mandibular cartilage of the 2- and 12-month-old mice, expression that substantially declined at the 18- and 25-month-old groups (Fig. 4e, j).
Increased expression of MMP13 and BMPs and decreased expression of PRG4 at the mandibular cartilage as the mice aged. Immunostaining (fluorescent) for MMP13 (a), BMP2 (b), BMP4 (c), BMP7 (d), and PRG4 (e) in sagittal sections of mandibular condyles of 2-, 12-, 18-, and 25-month-old mice. Quantification of percentage of immunofluorescence-positive pixels (green) over DAPI-positive pixels (blue) for MMP13 (f), BMP2 (g), BMP4 (h), BMP7 (i), and PRG4 (j). Histograms (f–j) represent means ± SD for n = 8 per group. Statistically significant difference between groups: f #: significant difference between groups 2M and 12M, 18M, 25M; no significant difference between 12M, 18M, 25M groups. g and h #: significant difference between groups 18M and 2M, 12M, 25M; ##: significant difference between groups 25M and 2M, 12M, 18M; no significant difference between 2M and 12M groups. i #: significant difference between groups 18M and 2M, 12M, 25M; no significant difference between 2M, 12M, 25M groups. j Significant difference between groups 18M and 2M, 12M; ##: significant difference between groups 25M and 2M, 12M; no significant difference between 18M and 25M groups. #, ##: p < 0.05. Scale bar = 50 μm
Discussion
The MCC is primarily composed of 2 major elements: chondrocytes and the extracellular matrix. The chondrocytes are responsible for secretion and maintenance of the matrix and therefore are crucial for the maintenance of the homeostasis of the MCC (Aigner et al. 2002; Rahmati et al. 2017; Sandell and Aigner 2001). The principal function of the cartilage of TMJ is to adjust to the mechanical load during mastication, speech, and parafunctional movements, to absorb and distribute the compressive and shear stress to the subchondral bone (Wu et al. 2019). The load-bearing function of the cartilage is mediated by the extracellular matrix and the ability to withstand compressive loads is the direct function of the proteoglycans present in the cartilage (Mirahmadi et al. 2018; Rahmati et al. 2017). In our research, we observed a significant decrease in proteoglycans (safranin O staining) in the cartilage as the animal ages. Additionally, we observed increased breakdown of the cartilage and increased OARSI histopathological score as the animal ages. The breakdown of the cartilage is primarily due to differential synthesis and breakdown of the extracellular matrix (Loeser et al. 2014; Lotz and Loeser 2012). Furthermore, with aging, there is a decrease in the number of chondrocytes/cells and their inability to maintain the synthetic activity leads to decreased proteoglycan secretion which may lead to cartilage breakdown (Loeser et al. 2014; Lotz and Loeser 2012; Rahmati et al. 2017). Additionally, it has also been suggested that with aging chondrocytes undergo phenotypic changes, which may alter their response to mechanical stimuli thus promoting altered remodeling of the extracellular matrix (Loeser et al. 2014; Lotz and Loeser 2012).
Examination of PR staining revealed that the structure of the MCC extracellular matrix was altered in older animals when compared to 2-month-old mice. The noncalcified MCC of 2- and 12-month-old animals showed little red-orange hue, while increased intensity of red color staining was observed in older animals (18- and 25-month-old), suggesting a shift towards larger, organized collagen fibers which may lead to mineralization of the MCC (Schmitz et al. 2010).
With the decrease in number of chondrocytes in the MCC of aged animals, a decrease in cartilage thickness is expected and we observed a gradual decrease in the cartilage thickness as the animal aged. However, there was no difference in cartilage thickness between 12- and 18-month-old mice. The decrease in cartilage thickness in older mice might be due to advancement of tidemark and ultimately replacement of the cartilage by bone through endochondral ossification. Calcification/mineralization of the cartilage and migration of the mineralization front towards the uncalcified layer of the cartilage are basic signs of cartilage degeneration (Roemhildt et al. 2012). We observed both an increased area of mineralization and migration of mineralization front as the animal ages. Additionally, we noted increased bone volume (μCT) and tissue density (μCT) which may further suggest increased degeneration of the cartilage.
The superficial zone of the cartilage is most susceptible to changes with aging or injury due to applied loads (Loeser 2010; Lotz and Loeser 2012). The probable reasons are increased proteolytic activities, decreased cell number and activity, and decreased lubrication (Wang et al. 2013). We observed increased MMP13 (proteolytic activity) and decreased cellularity as the animal ages (Lotz and Loeser 2012; Rahmati et al. 2017). It has been shown that MMP13 is a major proteolytic enzyme that targets cartilage and is not only responsible for the degradation of type 1 and type 11 collagen but also the proteoglycan of the extracellular matrix (Shiomi et al. 2010; Wang et al. 2013).
The level of cartilage cellularity determines the tissue volume that is being maintained and has significant implications for cartilage repair and regeneration (Aigner et al. 2007; Martin and Buckwalter 2002). It has been shown in the articular cartilage of the knee that cellularity/cell density decreases with age and it happens most profoundly in the superficial zone of the cartilage (Lotz and Loeser 2012). Furthermore, we have observed a significant decrease in PRG4 expression in the aged animals. A decrease in PRG4 expression is linearly related with cartilage damage due to compressive loads and shear stress (Bao et al. 2011; Flannery et al. 2009; Teeple et al. 2008).
Altered chondrocyte differentiation and extracellular matrix remodeling play a central role in age-related degeneration of the cartilage of the TMJ. Bone morphogenetic proteins (BMPs) are known to promote chondrocyte differentiation, remodeling of the matrix, and mineralization (Thielen et al. 2019; Wu et al. 2012; Zhang et al. 2019). It has been shown that increased mineralization of the cartilage can be due to increased expression of BMPs and altered signaling (Bechtold et al. 2016a; Bechtold et al. 2016b). BMP2 and BMP4 are chiefly known to stimulate chondrocyte differentiation and matrix synthesis, and also lead to increased expression of MMP13 (Thielen et al. 2019; Wu et al. 2012; Zhang et al. 2019). Remarkably, chondrocyte differentiation, elevated matrix synthesis, and elevated MMP13 expression are characteristic of OA (Thielen et al. 2019; Zhang et al. 2019). It has been shown that elevated BMP levels in degenerated/diseased cartilage can contribute to cartilage regeneration and repair by boosting matrix synthesis, but at the same time stimulate cartilage degeneration by altering chondrocyte behavior and stimulating MMP13 expression (Thielen et al. 2019; Wu et al. 2012). In our research, we observed increased BMP2, BMP4, and MMP13 expression, which might have caused increased bone volume, mineralization, and cartilage breakdown.
One of the drawbacks of our study is that we have evaluated only male animals. In our future directions, we plan to compare the development of OA in both genders.
Conclusion
Our research has documented aging-associated changes in the extracellular matrix and cells of the cartilage of the TMJ. The most important changes observed were decreased proteoglycan secretion, decreased cellularity, decreased cartilage thickness, increased fibrillation, and increased proteolytic activity. We also observed a significant decrease in PRG4 protein expression and a significant increase in BMP-related proteins as the animal ages.
References
Aigner T, Kurz B, Fukui N, Sandell L (2002) Roles of chondrocytes in the pathogenesis of osteoarthritis. Curr Opin Rheumatol 14:578–584
Aigner T, Soder S, Gebhard PM, McAlinden A, Haag J (2007) Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis--structure, chaos and senescence. Nat Clin Pract Rheumatol 3:391–399. https://doi.org/10.1038/ncprheum0534
Bao JP, Chen WP, Wu LD (2011) Lubricin: a novel potential biotherapeutic approaches for the treatment of osteoarthritis. Mol Biol Rep 38:2879–2885. https://doi.org/10.1007/s11033-010-9949-9
Bechtold TE et al (2016a) Osteophyte formation and matrix mineralization in a TMJ osteoarthritis mouse model are associated with ectopic hedgehog signaling. Matrix Biol 52-54:339–354. https://doi.org/10.1016/j.matbio.2016.03.001
Bechtold TE et al (2016b) Excess BMP signaling in heterotopic cartilage forming in Prg4-null TMJ discs. J Dent Res 95:292–301. https://doi.org/10.1177/0022034515613508
DiLoreto R, Murphy CT (2015) The cell biology of aging. Mol Biol Cell 26:4524–4531. https://doi.org/10.1091/mbc.E14-06-1084
Flannery CR et al (2009) Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum 60:840–847. https://doi.org/10.1002/art.24304
Gho WG, Choi Y, Park K-H, Huh J-K (2018) Expression of collagenases (matrix metalloproteinase-1, 8, 13) and tissue inhibitor of metalloproteinase-1 of retrodiscal tissue in temporomandibular joint disorder patients. J Korean Assoc Oral Maxillofac Surg 44:120–127. https://doi.org/10.5125/jkaoms.2018.44.3.120
Glasson SS, Chambers MG, Van Den Berg WB, Little CB (2010) The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse osteoarthritis and cartilage / OARS. Osteoarthr Res Soc 18(Suppl 3):S17–S23. https://doi.org/10.1016/j.joca.2010.05.025
Hill A, Duran J, Purcell P (2014) Lubricin protects the temporomandibular joint surfaces from degeneration. PloS One 9:e106497. https://doi.org/10.1371/journal.pone.0106497
Koyama E et al (2014) Lubricin is required for the structural integrity and post-natal maintenance of TMJ. J Dent Res 93:663–670. https://doi.org/10.1177/0022034514535807
Kuroda S, Tanimoto K, Izawa T, Fujihara S, Koolstra JH, Tanaka E (2009) Biomechanical and biochemical characteristics of the mandibular condylar cartilage osteoarthritis and cartilage / OARS. Osteoarthr Res Soc 17:1408–1415. https://doi.org/10.1016/j.joca.2009.04.025
Loeser RF (2010) Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med 26:371–386. https://doi.org/10.1016/j.cger.2010.03.002
Loeser RF, Gandhi U, Long DL, Yin W, Chubinskaya S (2014) Aging and oxidative stress reduce the response of human articular chondrocytes to insulin-like growth factor 1 and osteogenic protein 1. Arthritis Rheumatol 66:2201–2209. https://doi.org/10.1002/art.38641
Lories RJ, Luyten FP (2011) The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol 7:43–49. https://doi.org/10.1038/nrrheum.2010.197
Lotz M, Loeser RF (2012) Effects of aging on articular cartilage homeostasis. Bone 51:241–248. https://doi.org/10.1016/j.bone.2012.03.023
Luder HU (2002) Factors affecting degeneration in human temporomandibular joints as assessed histologically. Eur J Oral Sci 110:106–113
Martin JA, Buckwalter JA (2002) Human chondrocyte senescence and osteoarthritis. Biorheology 39:145–152
Mirahmadi F et al (2018) Mechanical stiffness of TMJ condylar cartilage increases after artificial aging by ribose. Arch Oral Biol 87:102–109. https://doi.org/10.1016/j.archoralbio.2017.12.010
Orajarvi M et al (2018) Changes in type I and type II collagen expression in rat mandibular condylar cartilage associated with aging and dietary loading. J Oral Facial Pain Headache 32:258–265. https://doi.org/10.11607/ofph.1581
Rahmati M, Nalesso G, Mobasheri A, Mozafari M (2017) Aging and osteoarthritis: central role of the extracellular matrix. Ageing Res Rev 40:20–30. https://doi.org/10.1016/j.arr.2017.07.004
Roemhildt ML, Beynnon BD, Gardner-Morse M (2012) Mineralization of articular cartilage in the Sprague-Dawley rat: characterization and mechanical analysis osteoarthritis and cartilage / OARS. Osteoarthr Res Soc 20:796–800. https://doi.org/10.1016/j.joca.2012.04.011
Sandell LJ, Aigner T (2001) Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res 3:107–113. https://doi.org/10.1186/ar148
Schmitz N, Laverty S, Kraus VB, Aigner T (2010) Basic methods in histopathology of joint tissues osteoarthritis and cartilage / OARS. Osteoarthr Res Soc 18(Suppl 3):S113–S116. https://doi.org/10.1016/j.joca.2010.05.026
Shiomi T, Lemaitre V, D'Armiento J, Okada Y (2010) Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases. Pathol Int 60:477–496. https://doi.org/10.1111/j.1440-1827.2010.02547.x
Teeple E, Elsaid KA, Fleming BC, Jay GD, Aslani K, Crisco JJ, Mechrefe AP (2008) Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient Guinea pig knee. J Orthop Res 26:231–237. https://doi.org/10.1002/jor.20492
Thielen NGM, van der Kraan PM, van Caam APM (2019) TGFbeta/BMP signaling pathway in cartilage homeostasis cells 8. https://doi.org/10.3390/cells8090969
Wadhwa S, Kapila S (2008) TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Educ 72:930–947
Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, Chen D (2013) MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther 15:R5. https://doi.org/10.1186/ar4133
Wu L, Huang X, Li L, Huang H, Xu R, Luyten W (2012) Insights on biology and pathology of HIF-1alpha/-2alpha, TGFbeta/BMP, Wnt/beta-catenin, and NF-kappaB pathways in osteoarthritis. Curr Pharm Des 18:3293–3312. https://doi.org/10.2174/1381612811209023293
Wu Y et al (2019) Effect of sustained joint loading on TMJ disc nutrient environment. J Dent Res 98:888–895. https://doi.org/10.1177/0022034519851044
Yadav S, Yang Y, Dutra EH, Robinson JL, Wadhwa S (2018) Temporomandibular joint disorders in older adults. J Am Geriatr Soc 66:1213–1217. https://doi.org/10.1111/jgs.15354
Zhang W, Robertson WB, Zhao J, Chen W, Xu J (2019) Emerging trend in the pharmacotherapy of osteoarthritis. Front Endocrinol (Lausanne) 10:431. https://doi.org/10.3389/fendo.2019.00431
Acknowledgments
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institute of Health under the award number KO8DE025914 and by the American Association of Orthodontic Foundation and startup funds provided to SY.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Chen, PJ., Dutra, E.H., Mehta, S. et al. Age-related changes in the cartilage of the temporomandibular joint. GeroScience 42, 995–1004 (2020). https://doi.org/10.1007/s11357-020-00160-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00160-w