Abstract
Introduction
Articular cartilage is the major affected tissue during the development of osteoarthritis (OA) in temporomandibular joint (TMJ). The core circadian rhythm molecule Bmal1 regulates chondrocyte proliferation, differentiation and apoptosis; however, its roles in condylar cartilage function and in TMJ OA have not been fully elucidated.
Materials and methods
TMJ OA mouse model was induced by unilateral anterior crossbite (UAC) and Bmal1 protein expression in condylar cartilage were examined by western blot analysis. To determine the role of Bmal1 in TMJ OA, we generated cartilage-specific Bmal1 conditional knockout (cKO) mice (Bmal1Agc1CreER mice) and hematoxylin and eosin staining, toluidine blue and Safranin O/fast green, immunohistochemistry, TUNEL assay, real-time PCR analysis and Western blot assay were followed.
Results
Bmal1 expression was reduced in condylar cartilage in a TMJ OA mouse model induced by UAC. The Bmal1 cKO mice displayed decreased cartilage matrix synthesis, reduced chondrocyte proliferation, increased chondrocyte hypertrophy and apoptosis as well as the upregulation of YAP expression in TMJ condylar cartilage.
Conclusions
We demonstrated that Bmal1 was essential for TMJ tissue homeostasis and loss-of-function of Bmal1 in chondrocytes leads to the development of TMJ OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is the most common form of arthritis and the temporomandibular joint (TMJ) is one of the most common sites of OA, often leading to severe pain in the orofacial region. The main tissue affected in OA is articular cartilage. Several factors, including age, endocrine factors, psychosocial factors (such as anxiety and depression), malocclusion, and some behaviors have been found might induce TMJ OA [1]. However, it remains unclear about the etiology, pathogenic processes and underlying molecular mechanisms involved in TMJ OA development. Among those animal models established to mimic the development of TMJ OA, unilateral anterior crossbite (UAC) stimulation is one of the most widely used animal model to study the etiology and pathogenic of TMJ OA [2, 3].
In recent years, many important physiological activities in body exhibit obvious circadian rhythm, and accumulative evidence has shown linkage of abnormal circadian clock system with OA [4]. Brain and muscle Arnt-like protein-1 (Bmal1) is the core circadian clock component and controls important physiological processes. The circadian clock maintains and regulates the circadian rhythm through a feedback loop: Bmal1 binds Clock to form heterodimers and initiates the transcription of target genes, resulting in suppression of transcriptional activity of Bmal1 and Clock [5].
There is considerable evidence that Bmal1 expression was in a lower level in knee joint OA chondrocytes [4, 6,7,8,9,10]. However, little is known about the effect of Bmal1 on TMJ OA cartilage. So far, only two publications reported the relation of Bmal1 to TMJ OA. One study reported that sleep rhythm disturbance downregulated Bmal1 in TMJ condylar cartilage and might induce the global circadian rhythm disruption, implicating that disrupted circadian rhythm was a risk factor for TMJ OA [11]. The other study reported that the stimulation of low-intensity pulsed ultrasound caused a reduction of Bmal1 level in a rat TMJ OA model [12]. Therefore, in this study, we crossed Aggrecan-CreER transgenic mice with Bmal1flox/flox mice to create a tamoxifen-inducible, cartilage-specific Bmal1 conditional knockout (cKO) mouse model (Bmal1Agc1CreER mice), to determine the regulatory roles of Bmal1 in the condylar cartilage during the TMJ OA initiation and progression.
Materials and methods
Unilateral anterior crossbite (UAC)-induced TMJ OA model
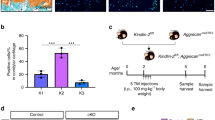
Six-week-old C57BL/6 J female mice were provided by the Animal Center of Xi’an Jiaotong University in China (N = 6). In the UAC groups, UAC was applied to mice as we previously described [2] (Fig. 1a). In the control groups, the mice underwent similar procedure but no metal tubes were bonded (Fig. 1a). All mice were killed 3 weeks after performing UAC, referencing to previous published reference [2]. All the animal experiments involved in this study were approved by the Animal Experiment Administration Committee of Xi’an Jiaotong University, China (2020-387).
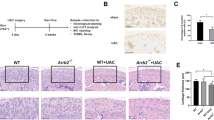
The reduction of Bmal1 correlated with TMJ OA-like lesion of mouse unilateral anterior crossbite (UAC). (a) The frontal and lateral view of anterior teeth occlusal relationship in the control (a, b) and UAC (c, d) mice at six weeks old. (b) Representative sagittal TMJ sections of HE, toluidine blue and Safranin O/fast green staining three weeks after UAC stimulation. Scale bars = 50 μm. (c) The severity of OA-like phenotype was graded by modified OARSI scoring system by the same observers blinded to the treatment category. The results demonstrated that three weeks UAC treatment induced significant TMJ OA-like defects versus the control group. **p < 0.01. (d) Representative histopathological of IHC staining of Bmal1 in TMJ condylar cartilage of UAC mice and control mice. Scale bars = 50 μm. (e) Quantification of IHC staining in Fig. 1d. *p < 0.05. (f) Western blot analysis of Bmal1 expression in TMJ condylar cartilage of UAC mice and control mice. GAPDH was used as loading control. Data shown were representative of three individual experiments. (g) Quantification of Western blot analysis of Bmal1 in Fig. 1f. **p < 0.01
The generation of Bmal1 Agc1CreER cKO mice
Bmal1-floxed mice were presented as gifts from professor Weimin Tong at the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. Agc1-CreER transgenic mice were obtained from Professor Di Chen at Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences. To generate Bmal1Agc1CreER cKO mice, Bmal1flox/flox mice were crossbred with Agc1-CreER transgenic mice. To create cartilage-specific Baml1 gene deletion after birth, the mice were intraperitoneally injected with tamoxifen at 5, 7, 9 and 11 days after birth. The mice were sacrificed 9, 13 or 17 weeks after birth for histologic analysis, referencing to the time points 3, 7, 11 weeks after UAC stimulation [2, 13]. Cre-negative littermates (Bmal1flox/flox) were used as controls (N = 6).
Histology and immunohistochemistry (IHC) and immunofluorescence (IF) staining
Skulls were dissected from Bmal1Agc1CreER mice, Cre negative control mice, UAC and C57BL/6 J control mice, respectively (N = 6). Samples were collected as previous [14] and 3 μm mid-sagittal sections at 3 different levels (50 μm apart) were cut from the lateral part of TMJ and then stained with hematoxylin and eosin (HE), toluidine blue and Safranin O/fast green and IHC staining. For IHC staining, tissue sections were incubated with primary antibodies against Bmal1 (Abcam, ab230822), YAP (Cell signaling, 14074S), Aggrecan (Abcam, ab216965), MMP13 (Abcam, ab39012), PCNA (Abcam, ab18197) at 4 °C overnight. 3 slides per mouse, 6 mice in each group were analyzed. For IF staining, tissue sections were incubated with primary antibodies against YAP (Cell signaling, 14074S), CY3 Conjugated AffiniPure Goat Anti rabbit IgG secondary antibody was used (1:100, 2 h) at 37 °C and sections were mounted with DAPI before imaging.
TUNEL assay
For TMJ condylar cartilage histological tissue sections, cell apoptosis was analyzed with Cell Death Detection Kit (Roche Diagnostics, Germany; Cat.No.11684 817910) according to the manufacturer’s instructions. Briefly, the samples were permeated with TUNEL reaction mixture and stained by DAB to distinguish the positive cells (N = 6). The apoptotic rate was calculated by proportion of the positive cells in total cells of TMJ condylar cartilage. The observer are blinded to grouping and treatment.
Real-time PCR analysis
Bmal1Agc1CreER mice and their Cre-negative control mice were euthanized and TMJ condylar cartilages were collected at age of 9 weeks (N = 3). The process of RT-PCR was performed as we previous published [14]. Bmal1 and GAPDH genes were designed as follows: Bmal1: forward, TCTCCGAGTCTGTCTTCA, reverse, AGTCTTGGCATCAATGAGT; GAPDH: forward, TGGCCTTCCGTGTTCCTAC, reverse, GAGTTGCTGTTGAAGTCGCA.
Western blot
The sample from TMJ condylar cartilage (N = 6) were disrupted by RIPA buffer (Abcam, ab288006). The following processes were performed as we previous published [15]. The primary antibodies were the same to those used in IHC staining. The bands were quantitated by Image J.
Statistical analysis
Data are presented as the mean ± SD for three individual experiments for each group. Two-tailed Student’s t test was performed to compare data of two groups. P values less than 0.05 were considered statistically significant.
Results
The expression of Bmal1 was reduced in TMJ condylar cartilage in the unilateral anterior crossbite (UAC)-induced TMJ OA mouse model
UAC was performed as displayed in Fig. 1a. A representative picture of normal TMJ condylar cartilage is shown in Supplementary Fig. 1a. The results from hematoxylin and eosin (HE), toluidine blue and Safranin O staining demonstrated that the decrease in the thickness (black arrow, Supplementary Fig. 1b), the reduce of cartilage matrix, and the appearance of cell-free area (yellow arrow), which were typical OA-like lesion, were observed in the mouse TMJ cartilage layer after 3 weeks UAC induction, compared with the Cre-negative mice (Fig. 1b). UAC model was validated as a higher score than the control evaluated in TMJ condylar cartilage by modified OARSI (Fig. 1c). To examine changes in Bmal1 expression TMJ chondrocytes of the mice, IHC staining and Western blot were performed at this time points. IHC staining demonstrated that, in the TMJ condylar cartilage of control mice, Bmal1-positive cells were distributed throughout the entire cartilage zone, and brown-stained particles are deep and numerous in the proliferative zone and pre-hypertrophic cartilage zone. While in the UAC group, the number of Bmal1-positive cells significantly decreased in the fibrous zone of TMJ condylar cartilage, proliferative layer, and there were only a few positive cells in the hypertrophic layer (Figs. 1d, 1e). Consistently, the result of Western blot analysis demonstrated that Bmal1 protein levels in TMJ condylar cartilage were obviously reduced in the UAC mice versus the control mice (Fig. 1f, 1g).
Deletion of Bmal1 in chondrocyte postnatally induced TMJ OA-like lesion
To validate the specificity of Bmal1 gene deletion in chondrocyte of Bmal1 cKO mice, PCR assay, IHC staining and Western blot analysis were performed. Bmal1 mRNA and protein levels were significantly decreased in Bmal1 cKO mice at ages of 9 weeks by RT-PCR (Fig. 2a) and Western blot analysis (Fig. 2b and Supplementary Fig. 1c). Meanwhile, IHC staining showed that Bmal1-positive signals were reduced in TMJ condylar cartilage of Bmal1 cKO mice at 9 weeks of age (Fig. 2c, 2d). The toluidine blue staining displayed decreased content of cartilage matrix (Fig. 2e) and thickness of condylar cartilage (Fig. 2f). Collectively, all those data demonstrated that ablation of Bmal1 in chondrocyte at post-natal stage induced TMJ OA-like lesions.
Cartilage specific Bmal1 cKO mice showed TMJ OA-like lesion. (a) The relative expression of Bmal1 mRNA was detected by PCR. Independent experiments were repeated three times. **p < 0.01. (b) Western blot analysis of Bmal1 expression in TMJ condylar cartilage of Bmal1Agc1CreER mice and Cre negative mice. GAPDH was used as loading control. Data shown were representative of three individual experiments. (c) IHC staining of Bmal1 in TMJ condylar cartilage of Bmal1Agc1CreER mice and Cre negative mice. Scale bars = 50 μm. (d) Quantification of IHC staining in Fig. 2c. ***p < 0.001. (e) Representative sagittal TMJ sections of toluidine blue staining in TMJ condylar cartilage of Bmal1Agc1CreER mice and Cre negative mice at ages of 9, 13, 17 weeks. Scale bars = 100 μm. (f) Quantification of thickness of TMJ condylar cartilage in Fig. 2e. *p < 0.05, ***p < 0.001
Effect of Bmal1 deletion on TMJ condylar chondrocyte proliferation, terminal differentiation and apoptosis of 9-week-old Bmal1 cKO mice
The number of PCNA-positive cells was significantly decreased in the proliferative zone of TMJ condylar cartilage in Bmal1 cKO mice versus the Cre negative mice (Fig. 3a). In TMJ condylar cartilage of Cre negative mice, PCNA-positive signals were almost distributed throughout the entire condylar cartilage region, with a large number of brown-stained positive particles in the nucleus present in the proliferative and pre-hypertrophic chondrocyte zone, whereas only a few PCNA-positive cells were detected in the hypertrophic cartilage region; In contrast, in Bmal1 cKO mice, the number of PCNA-positive cells in the proliferative and pre-hypertrophic chondrocyte zone were significantly decreased, whereas PCNA-positive signals were almost undetectable in hypertrophic chondrocyte zone. The calculation of the percentage of PCNA-positive cells confirmed that after Bmal1 deletion the protein expression of PCNA in TMJ condylar cartilage was significantly reduced, indicating a decrease in the proliferative ability of chondrocytes (Fig. 3b). Consistently, Western blot analysis demonstrated that PCNA protein levels in the TMJ condylar cartilage was obviously reduced in Bmal1 cKO mice versus Cre-negative mice (Fig. 3c and Supplementary Fig. 1d).
Loss of Bmal1 inhibited the proliferation, but promotes the apoptosis and differentiation of TMJ chondrocytes. (a) Representative histopathological of IHC staining of PCNA in TMJ condylar cartilage of Bmal1Agc1CreER mice and Cre-negative mice. Scale bars = 50 μm. (b) Quantification of IHC staining of PCNA in Fig. 3a. ***p < 0.001. (c) Western blot analysis of PCNA expression in TMJ condylar cartilage of Bmal1Agc1CreER mice and Cre negative mice. GAPDH was used as loading control. Data shown were representative of three individual experiments. (d) IHC staining of MMP13 in TMJ condylar cartilage of Bmal1Agc1CreER mice and Cre negative mice. Scale bars = 50 μm. (e) Quantification of IHC staining of MMP13 in Fig. 3d. **p < 0.01. (f) Western blot analysis of MMP13 expression in TMJ condylar cartilage of Bmal1Agc1CreER mice and Cre negative mice. GAPDH was used as loading control. Data shown were representative of three individual experiments. (g) TUNEL assay in TMJ condylar cartilage of Bmal1Agc1CreER mice and Cre negative mice. GAPDH was used as loading control. Data shown were representative of three individual experiments. Quantification of TUNEL assay in Fig. 3 g. **p < 0.01
MMP13 is the marker for chondrocyte terminal differentiation. IHC results showed that MMP13 was specifically distributed in hypertrophic zone within the TMJ condylar cartilage (Fig. 3d). In Cre negative control mice, the brown stained area of MMP13-positive signal was limited and lighter in TMJ condylar cartilage. Whereas in Bmal1cKO mice, MMP13-positive signal was almost distributed throughout entire cartilage of TMJ condyle, and large areas of brown staining could be seen around chondrocyte and in the extracellular matrix (Fig. 3d). The results of IHC staining and western blot analysis demonstrated that MMP13 protein level in the TMJ condylar cartilage was significantly increased in Bmal1cKO mice versus control mice (Fig. 3d-f and Supplementary Fig. 1e).
In the process of endochondral ossification, chondrocytes undergo proliferation, differentiation and apoptosis, and eventually transfer from cartilage tissue to bone tissue. The same process was also thought occurred in the pathology of TMJ OA.
Therefore, we next examined whether deletion of Bmal1 affected chondrocyte apoptosis. As shown in Fig. 3g, 3h, Bmal1 ablation induced more TUNEL-positive chondrocytes in TMJ condyle of Bmal1 cKO mice than that in Cre-negative mice at 9 weeks of age. In Cre-negative mice, TUNEL-positive signals were distributed mainly in hypertrophic cartilage zone, whereas a large number of TUNEL-positive cells throughout the entire condylar cartilage were present in Bmal1cKO mice, especially in hypertrophic zone. It was concluded that after Bmal1 deletion, apoptosis of hypertrophic chondrocytes enhanced, and apoptotic chondrocytes also extended to proliferative and pre-hypertrophic zone.
The protein levels of mechanical signaling molecule YAP were increased in TMJ condylar cartilage of 9-week-old Bmal1 cKO mice
Western blot analysis demonstrated YAP protein level was increased in Bmal1cKO mice versus Cre negative mice (Figs. 4a, 4b). IHC staining showed that, in Cre-negative control mice, YAP-positive signals were mainly distributed in pre-hypertrophic zone; however, in Bmal1 cKO mice, YAP-positive chondrocytes in hypertrophic zone were obviously enhanced, especially in the osteochondral junction area where a large amount of YAP-positive signals were detected (Fig. 4c). The results of quantitative analysis of IHC staining showed increased YAP expression in Bmal1 cKO mice versus Cre-negative control mice (Fig. 4d). And the immunofluorescence showed more positive chondrocyte localized in the nucleus in Bmal1 cKO mice versus Cre-negative mice (Fig. 4e).
Loss of Bmal1 increased the expression of YAP in chondrocytes. (a) Western blot analysis of YAP expression in TMJ condylar cartilage of Bmal1Agc1CreER mice and Cre negative mice. GAPDH was used as loading control. Data shown were representative of three individual experiments. (b) Quantification of Western blot analysis of YAP expression in Fig. 4a. *p < 0.05. (c) IHC staining of YAP in TMJ condylar cartilage of Bmal1Agc1CreER mice and Cre negative mice. Scale bars = 50 μm. (d) Quantification of IHC staining of YAP in Fig. 4c. ***p < 0.001. (e) Immunofluorescence staining of YAP in TMJ condylar cartilage of Bmal1Agc1CreER mice and Cre negative mice at 9 weeks old. The white arrow indicated the merged positive signals (purple signals). Scale bars = 50 μm
Discussion
TMJ is a load-bearing joint. Aberrant Prolonged or overloading biomechanics from abnormal dental occlusion plays a vital effect on TMJ OA development. In the current study, the result from UAC induced TMJ OA mice showed that Bmal1 was significantly reduced in condylar cartilage. This finding suggested that Bmal1 deficiency might be related to initiation or progress of TMJ OA. Therefore, we then generated cartilage-specific Bmal1 cKO mice, which was abbreviated as Bmal1Agc1CreER mice in this paper, to further shed light on the mechanisms through which Bmal1 regulates the condylar cartilage during TMJ OA progress.
Bmal1 has been confirmed to regulate chondrocyte proliferation, hypertrophy and apoptosis. In cartilage-specific Bmal1 cKO mice (Bmal1Col2CreER mice), both in growth plate as well as in articular cartilage of knee joint, the amount of proliferative chondrocytes was less while the number of apoptotic cells was more than that of the controls [6, 15], indicating that Bmal1 deletion reduced chondrocyte proliferation and promotes chondrocyte apoptosis. However, some different findings about cell proliferation were found when studying the effect of Bmal1 knockdown by culturing primary chondrocytes. One study reported a small increase in cell proliferation following knockdown of Bmal1 in cultured human cartilage chondrocytes [7] whereas another study showed no effect on cell proliferation by Bmal1 knockdown in human chondrocytes [8], due to a lack of information about Bmal1 knockdown efficiency in the second study. Two reasons may be attributed to the differences observed between the two studies. One is the different sensitivity of assays applied and the source of primary chondrocytes [4]. The other reason is that both studies are only a transient Bmal1 knockdown and it is unknown whether a prolonged Bmal1 reduction has more significant effects on chondrocyte proliferation [4]. Consistent with the above studied in growth plate and articular cartilage using Bmal1Col2CreER mice, in the current study, the anti-proliferative and pro-apoptotic effects were found in TMJ condylar cartilage of Bmal1Agc1CreER mice versus the controls. About the role of Bmal1 in chondrocyte hypertrophy, there are also some contradictory reports. It was also reported that Bmal1 expression level was lower in proliferative zone than in pre-hypertrophic or hypertrophic zone of growth plate cartilage by immunohistochemistry, implicating a dual function of bmal1 in proliferating compared with differentiating chondrocytes [16]. Consistently, we found Bmal1-positive signals in pre-hypertrophic and hypertrophic zone were more intense than those in proliferative zone. In growth plate cartilage, Col10 expression decreased in Bmal1 cKO mice, indicating that loss of Bmal1 suppressed chondrocyte hypertrophy [17]. Transient knockdown of Bmal1 in human cultured chondrocytes caused an increase of MMP13 expression in one study [7] but had no effect reported by another research involving chondrocytes isolated from patients without OA [8]. In our study, MMP13 expression was increased in TMJ condylar cartilage in Bmal1 cKO mice, suggesting that Bmal1 promoted chondrocyte terminal differentiation.
YAP is a transcriptional coactivator and keeps its activity in dephosphorylation state. Once phosphorylated, YAP1/TAZ translocate to cytoplasm from the nucleus and retained in the cytoplasm, where they are ubiquitylated by E3-ubiquitin ligase BTRC and subjected to proteasome-mediated degradation [18]. Therefore, YAP exerts its transcriptional activity in dephosphorylation state. Multiple references reported YAP could regulate cell proliferation, differentiation and apoptosis [19,20,21,22,23,24]. However, among these references, reports on YAP regulation of proliferation and differentiation are controversial in vitro and in vivo. Therefore, the symphony of YAP is still not fully understood. Research has also confirmed that there is a certain connection between YAP and OA. YAP was overexpressed in OA tissues from a mouse OA model [24]. Overexpression of YAP obviously inhibited ATDC5 chondrogenic cell proliferation and reduced the gene expression related to differentiation, with these effects being reversed by YAP knockdown [24]. Consistently, in our study, anti-proliferative and pro-apoptotic effects were found in TMJ condylar cartilage of Bmal1 cKO mice versus the controls. Meanwhile, YAP expression was upregulated in condylar cartilage and the YAP positive cells were increased in hypertrophic zone, especially in the osteochondral junction area. Thus may also be related to the mechanical signaling characteristic of YAP [21]. Periodic cyclic mechanical stress can activate YAP in growth plate [25]. Matrix stiffness could induce nuclear translocation of YAP to mediate mechanics-induced fibroblast activation [26]. In hard matrix, YAP aggregated within the nucleus, promoting osteogenesis. In contrast, in the soft matrix, YAP accumulated in the cytoplasm, promoting lipid formation [27]. Moreover, our previous finding showed that complex modulus was increased in the osteochondral interface in the UAC group versus the controls [3]. This reflected the stiffness of TMJ condylar osteochondral interface was enhanced after UAC stimulation. Maybe this is why YAP positive signals were obviously increased in the osteochondral junction area in this study. It is possible that, after Bmal1 knockout, decreased extracellular matrix of condylar cartilage (Fig. 2e) enhanced the stiffness of the cartilage matrix and thus altered the mechanical signal stimulation transmitted to cells, leading to the increased expression of YAP. About the role of YAP in chondrocyte apoptosis in knee joint bone and cartilage, there are also some inconsistences. For example, in cultured prechondrocytic cell line ATDC5 in vitro, it was shown that overexpression of YAP induced chondrocyte apoptosis [24]. In contrast, it was demonstrated that, double knockouts of Yap/Taz did not affect apoptosis in vitro [23]. These inconsistences might attribute to different models used as well as different developmental stage targeted (endochondral ossification during development or regeneration after damage, or cartilage homeostasis). So far, only one study reported the effect of YAP in regulation of TMJ cartilage, which demonstrated that excessive activation of YAP promoted process of TMJ OA [28].
A recent study reported that Bmal1 induced BMSCs osteogenesis through inhibition of YAP expression [29]. In that research, Chip-Seq and RNA-Seq demonstrated Bmal1 was the upstream of YAP. Consistently, in this study, knockdown Bmal1 in cartilage led to upregulated YAP expression. Given these, our results suggested that Bmal1/YAP pathway played key role in chondrocytes proliferation, differentiation and apoptosis and lack of Bmal1 induced TMJ OA. Therefore, Bmal1/YAP may be a potential target to interfere TMJ OA.
In conclusion, in the present study, we found that UAC stimulation induced TMJ OA-like changes and decreased the expression of Bmal1. We further investigated that cartilage Bmal1 cKO mice exhibited inhibited proliferation and increased condylar chondrocyte hypertrophy and apoptosis as well as upregulated expression of YAP. These findings suggested that Bmal1 is essential for condylar cartilage homeostasis and its deficiency leads to phenotype of TMJ OA; Bmal1/YAP pathway may be involved in the initiation and progress of TMJ OA, but further studies are needed to verify the potential regulative effect of Bmal1 on YAP signaling by ex-vivo condylar chondrocyte experiments. Moreover, it is necessary to mention that this study has some limitations. Bmal1 compensatory experiments are needed to further clarify the essential role of Bmal1 to condylar cartilage homeostasis, and some detail molecular mechanisms studies and treatment strategies will be conducted.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Xu M, Zhang XY, He Y (2022) An updated view on temporomandibular joint degeneration: insights from the cell subsets of mandibular condylar cartilage. Stem Cells Dev 31:445–459
Liu YD, Liao LF, Zhang HY et al (2014) Reducing dietary loading decreases mouse temporomandibular joint degradation induced by anterior crossbite prosthesis. Osteoarthritis Cartilage 22:302–312
Zhang J, Liao L, Zhu J et al (2018) Osteochondral interface stiffening in mandibular condylar osteoarthritis. J Dent Res 97:563–570
Poulsen RC, Hearn JI, Dalbeth N (2021) The circadian clock: a central mediator of cartilage maintenance and osteoarthritis development? Rheumatology (Oxford) 60:3048–3057
Fu L, Lee CC (2003) The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer 3:350–361
Dudek M, Gossan N, Yang N et al (2016) The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. J Clin Invest 126:365–376
Snelling SJ, Forster A, Mukherjee S et al (2016) The chondrocyte-intrinsic circadian clock is disrupted in human osteoarthritis. Chronobiol Int 33:574–579
Yang W, Kang X, Liu J et al (2016) Clock gene Bmal1 modulates human cartilage gene expression by crosstalk with sirt1. Endocrinology 157:3096–3107
Soul J, Dunn SL, Anand S et al (2018) Stratification of knee osteoarthritis: two major patient subgroups identified by genome-wide expression analysis of articular cartilage. Ann Rheum Dis 77:423
Fisch KM, Gamini R, Alvarez-Garcia O et al (2018) Identification of transcription factors responsible for dysregulated networks in human osteoarthritis cartilage by global gene expression analysis. Osteoarthritis Cartilage 26:1531–1538
Chen G, Zhao H, Ma S et al (2020) Circadian rhythm protein bmal1 modulates cartilage gene expression in temporomandibular joint osteoarthritis via the MAPK/ERK pathway. Front Pharmacol 11:527744
He D, An Y, Li Y et al (2018) RNA sequencing reveals target genes of temporomandibular joint osteoarthritis in rats after the treatment of low-intensity pulsed ultrasound. Gene 672:126–136
Zhang HY, Liu YD, Yang HX et al (2015) Installing and thereafter removing an aberrant prosthesis elicited opposite remodelling responses in growing mouse temporomandibular joints. J Oral Rehabil 42:685–692
Liao L, Zhang S, Zhou GQ et al (2019) Deletion of Runx2 in condylar chondrocytes disrupts TMJ tissue homeostasis. J Cell Physiol 234:3436–3444
Ma Z, Jin X, Qian Z et al (2019) Deletion of clock gene Bmal1 impaired the chondrocyte function due to disruption of the HIF1alpha-VEGF signaling pathway. Cell Cycle 18:1473–1489
Takarada T, Kodama A, Hotta S et al (2012) Clock genes influence gene expression in growth plate and endochondral ossification in mice. J Biol Chem 287:36081–36095
Bekki H, Duffy T, Okubo N et al (2020) Suppression of circadian clock protein cryptochrome 2 promotes osteoarthritis. Osteoarthritis Cartilage 28:966–976
Zhao B, Li L, Tumaneng K et al (2010) A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev 24:72–85
Chuang LSH, Ito Y (2021) The multiple interactions of runx with the Hippo-YAP pathway. Cells 10:2925
Deng Y, Wu A, Li P et al (2016) Yap1 regulates multiple steps of chondrocyte differentiation during skeletal development and bone repair. Cell Rep 14:2224–2237
Yang K, Wu Y, Cheng P et al (2016) YAP and ERK mediated mechanical strain-induced cell cycle progression through RhoA and cytoskeletal dynamics in rat growth plate chondrocytes. J Orthop Res 34:1121–1129
Goto H, Nishio M, To Y et al (2018) Loss of Mob1a/b in mice results in chondrodysplasia due to YAP1/TAZ-TEAD-dependent repression of SOX9. Development. https://doi.org/10.1242/dev.159244
Vanyai HK, Prin F, Guillermin O et al (2020) Control of skeletal morphogenesis by the Hippo-YAP/TAZ pathway. Development. https://doi.org/10.1242/dev.187187
Zhang Q, Fang X, Zhao W et al (2019) The transcriptional coactivator YAP1 is overexpressed in osteoarthritis and promotes its progression by interacting with Beclin-1. Gene 689:210–219
Jing X, Ye Y, Bao Y et al (2018) Mechano-growth factor protects against mechanical overload induced damage and promotes migration of growth plate chondrocytes through RhoA/YAP pathway. Exp Cell Res 366:81–91
Li S, Li C, Zhang Y et al (2019) Targeting mechanics-induced fibroblast activation through CD44-RhoA-YAP pathway ameliorates crystalline silica-induced silicosis. Theranostics 9:4993–5008
Zhong W, Li Y, Li L et al (2013) YAP-mediated regulation of the chondrogenic phenotype in response to matrix elasticity. J Mol Histol 44:587–595
Qi H, Zhang Y, Xu L et al (2023) Loss of RAP2A aggravates cartilage degradation in TMJOA via YAP signaling. J Dent Res 102:302–312
Cha S, Wang J, Lee SM et al (2022) Clock-modified mesenchymal stromal cells therapy rescues molecular circadian oscillation and age-related bone loss via miR142-3p/Bmal1/YAP signaling axis. Cell Death Discov 8:111
Acknowledgements
The authors are grateful to Miss. Xiao Ma and Xiaomin Kang (Center for Translational Medicine, the First Affiliated Hospital of Xi’an Jiaotong University) for their help in experimental techniques.
Funding
National Natural Science Foundation of China, Grant/Award Number: 81700997; Xi'an Innovation Ability Strong Foundation Program—Medical Research Project, Grant/Award: 21YXYJ0124.
Author information
Authors and Affiliations
Contributions
Lifan Liao and Yang Lin: conceptualization; methodology; writing—original draft; writing—review and editing. Yu Li: methodology; data curation; supervision. Jiale Hu: methodology; validation; formal analysis. Huang Lu and Zhaoli Meng: methodology; supervision. Huan Liu and Jiahao Huang: methodology; validation. Jianfei Liang: software; supervision. Di Chen and Longlong He: visualization, resources; supervision. Qin Zhou: project administration; supervision. Shufang Wu and Xiaofeng Chang: project administration; resources; funding acquisition; writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
The current study was approved by the Animal Ethics Committee of our institute (protocol number: 2020-387). Extensive efforts were made to minimize both the number of animals and their respective suffering in the experiments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Liao, L., Yang, L., Li, Y. et al. Deletion of Bmal1 in aggrecan-expressing cells leads to mouse temporomandibular joint osteoarthritis. J Bone Miner Metab (2024). https://doi.org/10.1007/s00774-024-01524-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00774-024-01524-4