Abstract
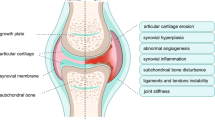
Osteoarthritis (OA) is a multi-factor disorder of sinovial joints, which characterized by escalated degeneration and loss of articular cartilage. Treatment of OA is a critical unmet need in medicine for regeneration of damaged articular cartilage in elderly. On the other hand, lubricin, a glycoprotein specifically synthesized by chondrocytes located at the surface of articular cartilage, has been shown to provide boundary lubrication of congruent articular surfaces under conditions of high contact pressure and near zero sliding speed. Lubrication of these surfaces is critical to normal joint function, while different gene expressions of lubricin had been found in the synovium of rheumatoid arthritis (RA) and OA. Moreover, mutations or lacking of lubricin gene have been shown to link to the joint disease such as camptodactyly-arthropathy-coxa vara-pericarditis syndrome (CACP), synovial hyperplasia and failure of joint function, suggesting an important role of lubricin in the pathogenesis of these joint disease. Recent studies demonstrate that administration with recombinant lubricin in the joint cavity would be effective in the prevention of cartilage degeneration in animal OA models. Therefore, a treatment with lubricin which would protect cartilage in vivo would be desirable. This article reviews recent findings with regard to the possible role of lubricin in the progression of OA, and further discusses lubricin as a novel potential biotherapeutic approaches for the treatment of OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis in humans is most commonly a multifactorial degenerative joint disease. It is incurable, costly and responds poorly to treatment. The disease process of OA is characterized by the progressive erosion of articular cartilage, leading to joint space narrowing, subchondral sclerosis, subchondral cysts, synovial inflammation, and marginal osteophyte formation [1]. A number of drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase 2 inhibitors, and steroids for the prevention and treatment of OA act by inhibiting joints degradation. However, the effectiveness of OA prevention in clinical practice is limited. Therefore, there is a great interest in finding a novel potential biotherapeutic approaches for the treatment of OA.
Lubricin, a 227.5-kDa mucinous glycoprotein which was originally identified as a lubricating glycoprotein present in synovial fluid [2], is recognized to have an important role in preventing cartilage wear and synovial cell adhesion and proliferation now [3]. Lubricin had previously been shown to be a proteoglycan specifically synthesized and expressed by articular chondrocytes of the superficial zone [4], which was known to interact with to the articular surface and function as a boundary lubricant in articular joints and reduces the coefficient of friction of the articualr cartilage surface [5]. It is possible that normal accumulation of lubricin is impaired in OA, leading to a net loss of protein. The content of this protein produced by the superficial layer of cartilage is damaged with aging and OA, and the lubricin gene is differently expressed in the synovium of RA and OA implying a possible role in the pathogenesis of these disease [6]. The role of lubricin such as lubrication and cytoprotection have been scientific investigated. Lubricin plays an important role in articualr joint physiology, and the loss of accumulation of lubricin may have a role in the pathology of OA. Therefore, a recent study by Flannery et al. [7] demonstrated the recombinant lubricin played significant disease-modifying and chondroprotective effects during the progression of animal OA model, which suggested the potential use of recombinant lubricin molecules in novel biotherapeutic approaches for the treatment of OA and associated cartilage abnormalities. This review focuses on the lubricin, emphasizing its role in joint disease and its currently in use, and potential future strategies.
Lubricin
Lubricin was originally identified as a lubricating glycoprotein present in synovial fluid [2], which was encoded by the Prg4 gene [8] and it is recognized to have a major protective role in preventing cartilage wear and synovial cell adhesion and proliferation recently [3]. Lubricin is also known as proteoglycan 4 (PRG4) or superficial zone protein (SZP), which was originally isolated and purified from the culture media of explants derived from the superficial zone of bovine articular cartilage [9]. In subsequent studies, lubricin and a related homologue, megakaryocyte-stimulating factor precursor protein (MSF), first detected in the urine of patients with acute thrombocytopenia undergoing bone marrow transplantation, was cloned and its primary amino acid sequence determined [10]. Besides, lubricin is also identified as an analogue with hemangiopoietin (HAPO) [11]. This protein has a molecular mass of approximately 227.5-kDa, which contains multiple protein domains that likely contribute to its diverse biologic properties. In addition, lubricin is well known to be 50% w/w O-glycosylated with β(1-3)-Gal-GalNAc and nonuniformly capped with NeuAc. The former posttranslational modification is essential to the protein’s lubricating ability [5], hydration force [12], conferring steric [13] and repulsion. The lubricin’s 1,404 amino acid sequence was analyzed and has revealed to be related to vitronectin in both the N-terminus and the C-terminus [14]. Both lubricin and vitronectin contain somatomedin B (SMB) and hemopexin-like (PEX) domains, which have approximately 60% sequence similarity in these regions. SAM and PEX domains have been suggested to regulate the complement and coagulation systems, mediate extracellular matrix attachment, and promote cell attachment and proliferation [15–17]. Purified hemopexin interacts with hyaluronan (HA) [18], suggesting that the hemopexin-like domain could also mediate lubricin binding to HA present at or near the articular surface [19]. In addition, lubricin attachment to the articular surface may be maintained by disulfide bond formation, via the unmatched cysteine residue near the C-terminus [20].
Lubricin distribution
Lubricin was first isolated based on its role as a lubricant in SF [21, 22], and this protein had previously been shown to be a proteoglycan specifically synthesized and expressed by articular chondrocytes of the superficial zone [23]. Flannery et al. [10] and Schumacher et al. [24] both found that some synovial lining cells such as synoviocytes and cells bordering the joint cavity synthesize lubricin. Another study by Schumacher et al. [25] showed lubricin was visualized in sections of bovine calf meniscus by immunohistochemistry. In their study, lubricin was detected in two regions including surfaces of meniscus and within and near cells along the radial fibers and circumferential fibers. Rees et al. [26] demonstrated that lubricin was present predominantly at the surface of fibrocartilaginous regions of tendon using immunohistochemical analyses, with the intensity of immunoreactivity in this region increasing with age. The lubricin was also present with infrapatellar fat pad (IFP) of the knee and was synthesized and secreted by IFP stromal cells, while IFP produced lubricin into synovial fluid and therefore contributed to maintain healthy joint function and homeostasis [27]. Moreover, a recent study by Sun et al. [28] demonstrated that lubricin was present in many joint tissues and for the first time they concluded there were lubricin present in the anterior cruciate ligament (ACL) and knee lateral collateral ligament (LCL). Meanwhile, they found the distribution of lubricin varied by different tissue type, and this variations in splicing and physical distribution suggested the variants of lubricin may play different roles in different locations. The distribution of lubricin in joint cavity is shown in Table 1.
Lubricin function and regulation
The discrete functional domains in lubricin suggest multiple biological functions, such as matrix binding, lubrication, cytoprotection and growth promotion. Based on biochemical composition and localization at the surface layer of articular cartilage, lubricin is known to function as a boundary lubricant in articular joints and reduces the coefficient of friction of the articualr cartilage surface [29–31]. Furthermore, mutations of PRG4 gene which located in chromosome 1q25 can cause CACP in humans [32], which is an autosomal recessive disease characterized by synovial hyperplasia with nonflammatory early-onset joint failure [33]. Expression of the mouse lubricin gene increased with progression of ectopic ossification [8]. Mice lacking the PRG4 gene showed abnormal protein deposits on the cartilage surface, disappearance of the underlying superficial zone, synovial hyperplasia, and precocious failure of joint function [3]. In addition, as the mice aged, intimal cells in the synovium surrounding the joint space became hyperplastic and further contributed to joint failure, while purified or recombinant lubricin could inhibit the growth of these synoviocytes in vitro [3]. Furthermore, as a splicing variant of lubricin, HAPO, was reported to be a novel growth factor acting on the primitive cells of both hematopoietic and endothelial cell lineage [11].
The synthesis and secretion of lubricin were determined by a complex combination of chemical and mechanical stimuli. Schmidt et al. [34] found that inclusion of fetal bovine serum and ascorbate in culture media up-regulated lubricin secretion levels. Later in their recent study, they demonstrated lubricin expression by chondrocytes near the articular surface was markedly stimulated by TGF-β1, decreased by IL-1α, but not affected by IGF-1 [35], which findings were consistent with previous studies on the regulation of lubricin by growth factors except IGF-1, who has been shown to increase the lubricin expression [36, 37]. Moreover, Niikura et al. [38] demonstrated that the regulation of lubricin accumulation by TGF-β/BMP superfamily members was regulated differently in articular chondrocytes and synoviocytes. TGF-β was a critical regulator of lubricin in both superficial zone articular chondrocytes and synoviocytes, while synoviocytes were more sensitive to BMP family members than were superficial zone articular chondrocytes. TGF-β and BMP were also found have an additive effect on the accumulation of lubricin expression [38]. In addition, various other cytokines and growth factors such as Oncostatin M (OSM), FGF-2 and PDGF were also proven to be the potent stimulators of lubricin synthesis while proinflammatory cytokines such as IL-1β and TNF-α were shown to suppress lubricin levels in monolayer chondrocytes cultures [36, 39]. Moreover, lubricin has been shown to be proteolytically susceptible to the effects of cysteine proteases, such as cathepsin B, and serine proteases, such as neutrophil elastase [40]. Mechanical stimulation can also affect the biosynthesis and secretion of lubricin. During embryonic development of the mouse elbow joint, the mRNA expression of lubricin begins at the onset of joint cavitation, suggesting that lubricin expression might be induced by the intiation of relative motion between the articular surfaces [3]. The certain mechanical stimuli including load-induced flow by interstitial fluid [41], cyclical tension [42], articular surface motion [43, 44] and chondrocyte subpopulations surface motion [45, 46] can up-regulate chondrocyte lubricin mRNA expression by chondrocyte-seeded cartilaginous constructs. Nugent et al. [47, 48] found that both static and dynamic shear stimulation increased cartilage secretion of lubricin, and the dynamic shear stimulation increased lubricin’s secretion to 3–4 times that of unloaded controls and statically compressed samples [48]. The number of cells expressing lubricin was higher in dynamically sheared cartilage and lubricin expressed at the depths of 200–400 μm from the articualr surface where were lubricin-negative in normal cartilage [48]. Furthermore, they suggested there would be a possible feedback mechanism for mechanical regulation of the production of lubricin molecule in vivo, that the tissue strains imparted to cartilage and depleted boundary lubricant molecules at the surface of articular simultaneously signal the nearby chondrocytes to secrete more lubricin [48]. Another study by Das et al. [49] demonstrated that stretch led to an up-regulation of lubricin in gene expression in primary chondrocytes. The regulation of lubricin is shown in Table 2.
Role of lubricin in articular cartilage
Lubricin is secreted predominatly by superficial zone chondrocytes, while cells from the middle and deep zone synthesize very low amounts of this protein. The biosynthesis of lubricin by articular chondrocytes has therefore been used to demarcate the superficial zone of cartilage [4]. As a surface-active mucin glycoprotein that physiosorbs to surfaces, lubricin thus providing joint lubrication, antiadhesive properties, chondroprotective and prevention of wear of articular cartilage [3]. Lubricin has been proposed to be a key factor for joint lubrication, a in vitro study had found that treatment with lubricin reduced the adhesive strength by more than 10-fold, and lubricin at a concentration necessary for boundary lubrication markedly reduced cartilage-cartilage integration [50]. Recent work revealed a correlation between lubricin expression level at the articular surface and short-term friction coefficient at the cartilage-glass interface [31]. Moreover, DuRaine et al. [51] demonstrated that the localization of lubricin in different zone would affect the friction coefficient of articular cartilage. During normal joint articulation, expression of lubricin has an important role for both preventing cell attachment to the articular surface as well as maintaining lubrication properties at the cartilage-synovial fluid interface [10]. Loss of lubricin influences the functional properties of synovial joints and could have a role in the pathogenesis of cartilage degeneration [52]. Jay and coworkers have demonstrated that lubricin provides essential chondroprotective properties to articular cartilage, and can interact with HA in synovial fluid to enhance its chondroprotective properties via the dissipation of shear-induced energy [40, 53]. Nevertheless, in addition to its function as a boundary lubricant, other studies found that lubricin appeared to have a detrimental effect in damaged joints by coating the surfaces of damaged cartilage and inhibiting integrative repair of articular cartilage lacerations [54].
Role of lubricin in joint disease
Justen et al. [6] have demonstrated that PRG4 gene expressed differently in the synovium of rheumatoid arthritis and osteoarthritis, which implied a possible role in the pathogenesis of these disease. SF lubricin concentrations were significantly reduced at an early stage following anterior cruciate ligament (ACL) injury when compared with those in the contralateral joint [40], and within 12 months, the lubricin concentration in the injured knee approached that in the contralateral knee, which did not change with time [40]. Therefore the decrease in SF lubricin concentrations following ACL injury may place the joint at an increased risk of wear-induced damage as a consequence of lack of boundary lubrication, potentially leading to secondary OA. In a rabbit knee injury model, Elsaid and coworkers found that the articular cartilage degradation were associate with the loss of boundary-lubricating ability of SF, while the lubricin concentrations in SF were markedly decreased after injury [55]. They also found that the association existed in patients with acute knee injuries or progressive chronic inflammatory arthritis. Another study by Jay et al. [56] demonstrated that SF lacking lubricin failed to reduce friction in the boundary mode, and joints of lubricin-null mice showed early and higher friction than joints from their wild-type counterparts, while friction was coupled with wear at the cartilage surface in vivo. They suggested that acquired lubricin degradation occurring in inflammatory joint disease predisposed the cartilage to damage [56]. Additionally, decreasing lubricin synthesis by synovial fibroblasts and superficial zone chondrocytes were found in a rat antigen-induced arthritis model [57], while the authors suggested restoring chondroprotection and preventing potential wear-induced cartilage degradation may require lubricin supplementation in synovial fluid. Lubricin also expressed in the cartilaginous deposits and osteoarthritic cartilage in patients with advanced OA [58]. All these results indicated that lubricin was involved in the joint disease and may play a beneficial role against the degradation of articular cartilage.
Potential biotherapeutic approaches for osteoarthritis
The current pharmaceutical treatments for OA include NSAIDs, cyclooxygenase 2 inhibitors, and HA-based injectables, which are promoted for symptomatic relief during the process of OA. These drugs have focused mainly on the broadspectrum targeting of matrix metalloproteinase activities, however, such inhibitors have been proven ineffective or due to several side effects such as soft tissue fibroplasia, that restricted the use of these drugs in clinical [59].
Based on the function of lubricin in the joint cavity (i.e., articular cartilage) and joint disease, lubricin would be as a potential therapeutic agent in the joint disease such as OA. The synthesis and localization of lubricin have been found down-regulated in rat, sheep, and guinea pig [52, 60] models of OA, which further suggesting that augmentation of lubricin levels in OA joints could be a potential therapeutic for OA. Additionally, the stimulation of lubricin expression in chondrocytes near the articular surface may be useful for creating tissue engineered cartilage from isolated subpopulation with a surface that is bioactive and functional in lubrication [61]. In fact, recent study by Flannery and colleagues demonstrated that intraarticular treatment with recombinant lubricin could prevent the degeneration of cartilage in a rat OA model [7]. In their study, a novel recombinant lubricin construct named LUB:1 was expressed in Chinese hamster ovary (CHO) cells and isolated at high purity, and LUB:1 represented a modified version of human lubricin and exhibited ~70% amino acid sequence identity to rat lubricin. Also, this novel recombinant protein contained important domains as lubricin, and in ex vivo functional assays, LUB:1 were found efficiently binded and localized to articular cartilage surfaces. Moreover, LUB:1 was found significantly improved cartilage boundary lubrication in a custom friction testing apparatus, and LUB:1 was also shown to inhibit the binding/adhesion of synovial sarcoma cells, which was similar to the full-length lubricin [7]. After intraarticular treatment with this recombinant in the rat OA model either 3 times per week or once per week over the subsequent 4 weeks, the tibial cartilage degeneration scores, total joint scores, and cartilage lesion and degeneration width measurements were significantly lower than that PBS-treated animals, suggesting that lubricin had significant disease-modifying and chondroprotective effects during the progression of OA.
It’s worth noting that another high molecular weight polysaccharide, hyaluronic acid (HA), which has been studied for the treatment of OA over the past 2 decades, was stated played an important role during the OA treatment [62]. As an important component of synovial fluid and extracellular matrix of articular cartilage, it acts as a fluid shock absorber and it helps to maintain the structural and functional characteristics of the cartilage matrix. It also inhibits the formation and release of prostaglandins, induces proteoglycan aggregation and synthesis, and modulates the inflammatory response [63, 64]. According to Balazs and coworkers [65, 66], the injection of HA into osteoarthritic joints could restore the viscoelasticity of the synovial fluid, augment the flow of joint fluid, normalize endogenous hyaluronate synthesis, inhibit hyaluronate degradation, reduce joint pain, and improve joint function. Moreover, viscosupplementation with intra-articular HA was approved by the Food and Drug Administration (FDA) in 1997. As a novel molecular, there has been increasing interest of potential biotherapeutic approaches for the treatment of OA, and the beneficial synergy between lubricin and HA with respect to both boundary lubrication and mechanical attributes of SF has been highlighted. Schmidt et al. [67] found that lubricin and HA in combination produced a greater boundary-lubricating effect than either constituent alone. Another study by Jay et al. [53] demonstrated that lubricin can interact with HA in synovial fluid to enhance its chondroprotective properties via the dissipation of shear-induced energy.
Conclusions
Many researchers have demonstrated the role of lubricin in the joint cavity and diseases. Several studies suggested that lubricin has a major protective role in preventing cartilage wear and synovial cell adhesion and proliferation during the process of OA. Moreover, several recent studies found that treatment with recombinant lubricin could protect articular cartilage and prevent the process of OA in animal model. This suggests that lubricin is a novel potential biotherapeutic approaches to the treatment of OA. However, some concerns about technical problems for local delivery of lubricin in the joint cavity, as well as its potential side effect still remain. Further in vivo studies will increase our understanding of the ture significance of lubricin and provide the foundations for the development of effective therapy for virous joint disease, especially the OA.
References
Buckwalter JA, Mankin HJ (1998) Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect 47:487–504
Swann DA, Slayter HS, Silver FH (1981) The molecular structure of lubricating glycoprotein-I, the boundary lubricant for articular cartilage. J Biol Chem 256:5921–5925
Rhee DK, Marcelino J, Baker M et al (2005) The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest 115:622–631
Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE (1994) A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys 311:144–152
Jay GD, Harris DA, Cha CJ (2001) Boundary lubrication by lubricin is mediated by O-linked beta(1–3)Gal-GalNAc oligosaccharides. Glycoconj J 18:807–815
Justen HP, Grunewald E, Totzke G et al (2000) Differential gene expression in synovium of rheumatoid arthritis and osteoarthritis. Mol Cell Biol Res Commun 3:165–172
Flannery CR, Zollner R, Corcoran C et al (2009) Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum 60:840–847
Ikegawa S, Sano M, Koshizuka Y, Nakamura Y (2000) Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet 90:291–297
Jay GD, Cha CJ (1999) The effect of phospholipase digestion upon the boundary lubricating ability of synovial fluid. J Rheumatol 26:2454–2457
Flannery CR, Hughes CE, Schumacher BL et al (1999) Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun 254:535–541
Liu YJ, Lu SH, Xu B et al (2004) Hemangiopoietin, a novel human growth factor for the primitive cells of both hematopoietic and endothelial cell lineages. Blood 103:4449–4456
Jay GD (1992) Characterization of a bovine synovial fluid lubricating factor. I. Chemical, surface activity and lubricating properties. Connect Tissue Res 28:71–88
Zappone B, Ruths M, Greene GW, Jay GD, Israelachvili JN (2007) Adsorption, lubrication, and wear of lubricin on model surfaces: polymer brush-like behavior of a glycoprotein. Biophys J 92:1693–1708
Jay GD, Tantravahi U, Britt DE, Barrach HJ, Cha CJ (2001) Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res 19:677–687
Deng G, Curriden SA, Hu G, Czekay RP, Loskutoff DJ (2001) Plasminogen activator inhibitor-1 regulates cell adhesion by binding to the somatomedin B domain of vitronectin. J Cell Physiol 189:23–33
Schvartz I, Seger D, Shaltiel S (1999) Vitronectin. Int J Biochem Cell Biol 31:539–544
Seiffert D, Smith JW (1997) The cell adhesion domain in plasma vitronectin is cryptic. J Biol Chem 272:13705–13710
Hrkal Z, Kuzelova K, Muller-Eberhard U, Stern R (1996) Hyaluronan-binding properties of human serum hemopexin. FEBS Lett 383:72–74
Nugent-Derfus GE, Chan AH, Schumacher BL, Sah RL (2007) PRG4 exchange between the articular cartilage surface and synovial fluid. J Orthop Res 25:1269–1276
Jones AR, Gleghorn JP, Hughes CE et al (2007) Binding and localization of recombinant lubricin to articular cartilage surfaces. J Orthop Res 25:283–292
Swann DA, Silver FH, Slayter HS, Stafford W, Shore E (1985) The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J 225:195–201
Jay GD, Haberstroh K, Cha CJ (1998) Comparison of the boundary-lubricating ability of bovine synovial fluid, lubricin, and Healon. J Biomed Mater Res 40:414–418
Su JL, Schumacher BL, Lindley KM et al (2001) Detection of superficial zone protein in human and animal body fluids by cross-species monoclonal antibodies specific to superficial zone protein. Hybridoma 20:149–157
Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB (1999) Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res 17:110–120
Schumacher BL, Schmidt TA, Voegtline MS, Chen AC, Sah RL (2005) Proteoglycan 4 (PRG4) synthesis and immunolocalization in bovine meniscus. J Orthop Res 23:562–568
Rees SG, Davies JR, Tudor D et al (2002) Immunolocalisation and expression of proteoglycan 4 (cartilage superficial zone proteoglycan) in tendon. Matrix Biol 21:593–602
Lee SY, Nakagawa T, Reddi AH (2008) Induction of chondrogenesis and expression of superficial zone protein (SZP)/lubricin by mesenchymal progenitors in the infrapatellar fat pad of the knee joint treated with TGF-beta1 and BMP-7. Biochem Biophys Res Commun 376:148–153
Sun Y, Berger EJ, Zhao C, An KN, Amadio PC, Jay G (2006) Mapping lubricin in canine musculoskeletal tissues. Connect Tissue Res 47:215–221
Jay GD, Elsaid KA, Zack J et al (2004) Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol 31:557–564
Kumar P, Oka M, Toguchida J et al (2001) Role of uppermost superficial surface layer of articular cartilage in the lubrication mechanism of joints. J Anat 199:241–250
Neu CP, Khalafi A, Komvopoulos K, Schmid TM, Reddi AH (2007) Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis Rheum 56:3706–3714
Marcelino J, Carpten JD, Suwairi WM et al (1999) CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet 23:319–322
Bahabri SA, Suwairi WM, Laxer RM, Polinkovsky A, Dalaan AA, Warman ML (1998) The camptodactyly-arthropathy-coxa vara-pericarditis syndrome: clinical features and genetic mapping to human chromosome 1. Arthritis Rheum 41:730–735
Schmidt TA, Schumacher BL, Klein TJ, Voegtline MS, Sah RL (2004) Synthesis of proteoglycan 4 by chondrocyte subpopulations in cartilage explants, monolayer cultures, and resurfaced cartilage cultures. Arthritis Rheum 50:2849–2857
Schmidt TA, Gastelum NS, Han EH, Nugent-Derfus GE, Schumacher BL, Sah RL (2008) Differential regulation of proteoglycan 4 metabolism in cartilage by IL-1alpha, IGF-I, and TGF-beta1. Osteoarthr Cartil 16:90–97
Khalafi A, Schmid TM, Neu C, Reddi AH (2007) Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res 25:293–303
Darling EM, Athanasiou KA (2005) Growth factor impact on articular cartilage subpopulations. Cell Tissue Res 322:463–473
Niikura T, Reddi AH (2007) Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum 56:2312–2321
Jones AR, Flannery CR (2007) Bioregulation of lubricin expression by growth factors and cytokines. Eur Cell Mater 13:40–45 discussion 45
Elsaid KA, Fleming BC, Oksendahl HL et al (2008) Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum 58:1707–1715
Buschmann MD, Kim YJ, Wong M, Frank E, Hunziker EB, Grodzinsky AJ (1999) Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch Biochem Biophys 366:1–7
Wong M, Siegrist M, Goodwin K (2003) Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone 33:685–693
Grad S, Lee CR, Gorna K, Gogolewski S, Wimmer MA, Alini M (2005) Surface motion upregulates superficial zone protein and hyaluronan production in chondrocyte-seeded three-dimensional scaffolds. Tissue Eng 11:249–256
Nugent-Derfus GE, Takara T, O’Neill JK et al (2007) Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4. Osteoarthr Cartil 15:566–574
Li Z, Yao S, Alini M, Grad S (2007) Different response of articular chondrocyte subpopulations to surface motion. Osteoarthr Cartil 15:1034–1041
Grad S, Lee CR, Wimmer MA, Alini M (2006) Chondrocyte gene expression under applied surface motion. Biorheology 43:259–269
Nugent GE, Schmidt TA, Schumacher BL et al (2006) Static and dynamic compression regulate cartilage metabolism of PRoteoGlycan 4 (PRG4). Biorheology 43:191–200
Nugent GE, Aneloski NM, Schmidt TA, Schumacher BL, Voegtline MS, Sah RL (2006) Dynamic shear stimulation of bovine cartilage biosynthesis of proteoglycan 4. Arthritis Rheum 54:1888–1896
Das RH, Jahr H, Verhaar JA, van der Linden JC, Van Osch GJ, Weinans H (2008) In vitro expansion affects the response of chondrocytes to mechanical stimulation. Osteoarthr Cartil 16:385–391
Schaefer DB, Wendt D, Moretti M et al (2004) Lubricin reduces cartilage–cartilage integration. Biorheology 41:503–508
DuRaine G, Neu CP, Chan SM, Komvopoulos K, June RK, Reddi AH (2009) Regulation of the friction coefficient of articular cartilage by TGF-beta1 and IL-1beta. J Orthop Res 27:249–256
Young AA, McLennan S, Smith MM et al (2006) Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther 8:R41
Jay GD, Torres JR, Warman ML, Laderer MC, Breuer KS (2007) The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci USA 104:6194–6199
Englert C, McGowan KB, Klein TJ, Giurea A, Schumacher BL, Sah RL (2005) Inhibition of integrative cartilage repair by proteoglycan 4 in synovial fluid. Arthritis Rheum 52:1091–1099
Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO (2005) Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum 52:1746–1755
Jay GD, Torres JR, Rhee DK et al (2007) Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum 56:3662–3669
Elsaid KA, Jay GD, Chichester CO (2007) Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum 56:108–116
Zhang D, Johnson LJ, Hsu HP, Spector M (2007) Cartilaginous deposits in subchondral bone in regions of exposed bone in osteoarthritis of the human knee: histomorphometric study of PRG4 distribution in osteoarthritic cartilage. J Orthop Res 25:873–883
Skotnicki JS, DiGrandi MJ, Levin JI (2003) Design strategies for the identification of MMP-13 and Tace inhibitors. Curr Opin Drug Discov Dev 6:742–759
Teeple E, Elsaid KA, Fleming BC et al (2008) Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res 26:231–237
Klein TJ, Schumacher BL, Schmidt TA et al (2003) Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthr Cartil 11:595–602
Creamer P, Hochberg MC (1997) Osteoarthritis. Lancet 350:503–508
Frizziero L, Govoni E, Bacchini P (1998) Intra-articular hyaluronic acid in the treatment of osteoarthritis of the knee: clinical and morphological study. Clin Exp Rheumatol 16:441–449
Adams ME, Atkinson MH, Lussier AJ et al (1995) The role of viscosupplementation with hylan G-F 20 (Synvisc) in the treatment of osteoarthritis of the knee: a Canadian multicenter trial comparing hylan G-F 20 alone, hylan G-F 20 with non-steroidal anti-inflammatory drugs (NSAIDs) and NSAIDs alone. Osteoarthr Cartil 3:213–225
Balazs EA, Denlinger JL (1993) Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol 39:3–9
Rydell N, Balazs EA (1971) Effect of intra-articular injection of hyaluronic acid on the clinical symptoms of osteoarthritis and on granulation tissue formation. Clin Orthop Relat Res 80:25–32
Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL (2007) Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum 56:882–891
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bao, Jp., Chen, Wp. & Wu, Ld. Lubricin: a novel potential biotherapeutic approaches for the treatment of osteoarthritis. Mol Biol Rep 38, 2879–2885 (2011). https://doi.org/10.1007/s11033-010-9949-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-9949-9