Abstract
Potentially toxic elements (PTEs), especially arsenic in drinking water, pose significant global health risks, including cancer. This study evaluates the groundwater quality in Giresun province on the Black Sea coast of Türkiye by analyzing twelve groundwater resources. The mean concentrations of macronutrients (mg/L) were: Ca (10.53 ± 6.63), Na (6.81 ± 3.47), Mg (3.39 ± 2.27), and K (2.05 ± 1.10). The mean levels of PTEs (µg/L) were: Al (40.02 ± 15.45), Fe (17.65 ± 14.35), Zn (5.63 ± 2.59), V (4.74 ± 5.85), Cu (1.57 ± 0.81), Mn (1.02 ± 0.76), As (0.93 ± 0.73), Cr (0.75 ± 0.57), Ni (0.41 ± 0.18), Pb (0.36 ± 0.23), and Cd (0.10 ± 0.05). All PTE levels complied with WHO drinking water safety guidelines, and overall water quality was excellent. The heavy metal evaluation index (HEI < 10) and heavy metal pollution index (HPI < 45) indicate low pollution levels across all stations. Irrigation water quality was largely adequate, as shown by the magnesium hazard (MH), sodium adsorption ratio (SAR), Na%, and Kelly's ratio (KR). The total hazard index (THI) values consistently remained below 1, indicating no non-carcinogenic health risks. However, at station 10 (city center), the cancer risk (CR) for adults due to arsenic was slightly above the threshold (1.44E-04). Using principal component analysis (PCA), positive matrix factorization (PMF), and geographic information system (GIS) mapping, the study determined that most PTEs originated from natural geological formations or a combination of natural and human sources, with minimal impact from human activities. These findings highlight the safety and reliability of the groundwater sources studied, emphasizing their potential as a long-term, safe water supply for nearby populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is a vital natural resource with significant social and economic importance. The increasing scarcity of water poses a substantial threat to human survival. Both surface water and groundwater serve as primary sources of drinking water globally (Chidiac et al. 2023; Tokatli et al. 2022). Groundwater, in particular, plays a crucial role as a freshwater source, fulfilling diverse human needs in various regions worldwide (Adimalla et al. 2022). Approximately 43% of the world's water for agricultural purposes is sourced from groundwater. Moreover, groundwater serves as a substantial source of water for domestic activities in numerous regions (Wang et al. 2024; Krishnamoorthy et al. 2023).
Industrialization, agricultural activities, urbanization, and other human activities have led to significant increases in pollution levels of raw water, including groundwater, especially in developing countries (Varol and Tokatlı 2022; Wang et al. 2023). Therefore, the assessment of groundwater quality stands as a critical aspect of environmental science, crucial for comprehending the ecological health and long-term sustainability of underground aquifers (World Water Quality Alliance 2021; Ustaoğlu et al. 2021).
Certain metals are indispensable for life forms, categorized as micronutrients (e.g., Se, Cu, Zn, Cr, Mn, Mo, Fe, and Co) and macronutrients (e.g., Mg, Ca, P, S, and Na). Yet, elevated concentrations of these metals can exert toxic effects on various organisms, including humans, disrupting vital processes such as reproduction, biotransformation, and growth (Rashid et al. 2023; Yüksel et al. 2021). However, lead, cadmium, mercury, and antimony are among the toxic metals frequently employed in industrial activities, and pose a significant environmental health risk (Topaldemir et al. 2023). Sources of contamination can be both geological and human-induced. Potentially toxic metals and chemical ions, along with other organic and inorganic contaminants, are linked to pollution in drinking water sources (Ullah et al. 2023).
The presence of PTEs in groundwater poses significant environmental and public health risks due to potential contamination. Natural constituents of the Earth's crust, such as lead, arsenic, cadmium, and chromium, are among these elements (Nafi and Taseidifar 2022). However, anthropogenic activities such as industrial effluents, agricultural runoff, and improper waste disposal can cause these elements to leach into groundwater. Even at low concentrations, exposure to these metals can result in chronic diseases, developmental complications, and other health issues (Siddiqua et al. 2022). Their ability to persist in the environment can prolong exposure, compounding associated risks (Manisalidis et al. 2020).
Assessing metal concentrations in groundwater is crucial for ensuring the safety of drinking water supplies and evaluating water quality (Alrowais et al. 2023; Rezaei et al. 2019). Metal analysis provides essential data for regulatory compliance, hazard assessment, and effective water management strategies. Understanding and monitoring the distribution and concentrations of potentially hazardous metals in groundwater are fundamental to informed decision-making processes aimed at preventing environmental pollution and safeguarding public health (Laonamsai et al. 2023).
Water quality assessment utilizing trace elements is essential for environmental research and public health. Low levels of trace elements affect water quality, aquatic ecosystems, and human health (Tekin-Özan et al. 2024). Toxic metals should be monitored due to their toxicity and environmental persistence. At high concentrations, these elements, can affect aquatic organisms, ecosystems, and humans through the food chain (Mitra et al. 2022). Trace elements reveal pollution sources and pathways. Comprehensive water quality researches promote pollution prevention and water resource management. Water trace element assessment protects ecosystems, humans, and the environment from waterborne pollutants (Turdi and Yang 2016). Computational toxicology and water quality assessment represent innovative approaches to understanding and protecting the environment and human health (Tokatlı et al. 2023; Arcega et al. 2023). Furthermore, the quality of irrigation water varies between different countries, nations, and locations due to factors such as the method of groundwater collection and use, the intensity of rainfall, and the subsequent recharge of aquifers. In arid regions with little rainfall, the use of groundwater for agriculture causes groundwater salinity to increase, thus limiting the variety of products that can be grown. Historically, the main problems with irrigation water quality have been soil salinization and reduced agricultural productivity. In recent years, the amount of data suggesting the presence of geogenic contaminants in waters has increased. The quality of the irrigation water has a significant impact on the production of greenhouse crops. Therefore, it is imperative to evaluate the caliber of irrigation water. Assessment of irrigation water quality can be done using various factors (Aloui et al. 2023; Malakar et al. 2019; Zaman et al. 2018).
Both multivariate and bivariate statistical analyzes are useful tools in interpreting the sources of elements in water bodies. Principal Component Analysis (PCA), Hierarchical Cluster Analysis (HCA), Pearson Correlation Coefficient (PCC), and Positive Matrix Factorization (PMF) are statistical techniques that can provide complementary assistance in distinguishing patterns, relationships, and sources of variability in the data (Din et al. 2023).
Türkiye's Black Sea coast, known for its abundant biological variety and important strategic value, faces increasing challenges in protecting the quality of groundwater resources. Considering the increasing urbanization, industry, and especially hazelnut farming activities in the region, a comprehensive assessment of the effects of human activities on groundwater quality is of great importance. Furthermore, groundwater is a critical resource, often perceived locally as superior in taste and health benefits compared to other sources. However, public authorities in coastal regions have limited systematic analysis of groundwater quality. This study addresses this gap by analyzing groundwater samples along a 90-km coastal stretch, focusing on 12 key sampling points used for drinking and irrigation. Therefore, this study attempts to achieve several objectives: i) measurement of potentially toxic element (PTE) levels in groundwater samples; ii) use of multivariate and bivariate statistical tools to categorize the natural and anthropogenic resources and factors affecting groundwater quality; iii) assessing the quality of groundwater samples designated for drinking purposes using indices such as heavy metal evaluation index (HEI), heavy metal pollution index (HPI), and water quality index (WQI); iv) evaluation of irrigation water quality through sodium adsorption ratio (SAR), magnesium hazard (MH), Kelly's ratio (KR), and Na%; and v) assessment of health risks using the hazard quotient (HQ), hazard index (HI), and carcinogenic risk (CR) indices.
Materials and methods
The flowchart in Fig. 1 provides a brief overview of the sample collection process, the specific PTEs examined, the analytical method used, and the many indicators used to assess statistical, ecological, water quality, and health risks.
Sampling region of the research
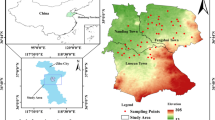
Ground water sampling was carried out from twelve stations in the coastal areas of Giresun province, overlooking the Southern Black Sea, consisting of high-sloping valleys divided by streams. Settlement was concentrated in small plains where rivers reach the sea, and provincial and district centers were established at these points. The mountain ranges running parallel to the coast in the south of the province have made passage to Central Anatolia difficult and squeezed the human settlements in the province into the narrow coastline. The amount of precipitation in the Giresun Coastal Region varies between 1300 and 1760 mm. The precipitation, which is notably elevated along the coast, diminishes progressively inland. Giresun coastal region is the second region with the highest rainfall in Turkey after Rize. Temperature fluctuations in this region range from + 9.8 °C to + 37.3 °C. The northern part, characterized by abundant precipitation, has diverse vegetation. In this region, there are hazelnut and other fruit trees up to an elevation of 600 m, as well as deciduous trees such as alder, maple, beech, hornbeam, oak, fir, linden, and chestnut. Sampling was conducted in November 2023 during the rainy season when surface runoff is abundant, resulting in significant water infiltration into the subsurface. Hence, twelve strategically chosen points along the 90-km coastal stretch of Giresun province, famous for its hazelnut farming industry in Türkiye, are positioned in the region's most frequented and densely populated areas along the coastline. These locations are crucial for evaluating the effects of human activities on groundwater quality and comprehending related health risks (Fig. 2).
Chemicals and standard solutions
Calibration plots were calculated using multi-element stock solutions obtained from VHG LABS (Manchester, NH, USA). The concentration of each element is equal to 10 mg/L. Quantification stability of the instrument was regulated by obtaining an internal standard multi-element stock solution for each element from Agilent®, USA. 65% v/v nitric acid solution (HNO3, Merck, Darmstadt, Germany) was provided to produce sample solutions and calibration standards. The validity of the test was evaluated using UME CRM 1201 Spring Water, a certified reference material (CRM) obtained from Tubitak, Türkiye. A locally based Turkish supplier then supplied argon gas with 99.999% analytical purity.
Water sample pretreatment
Following filtering of the samples using an Acrodisc® Minispike PTFE membrane with a pore size of 0.45 µm (Merck, Germany), 10 mL of the samples was mixed with an equal amount of 8% (v/v) nitric acid. Calibration standards were established by diluting a multi-element calibration stock solution obtained from VHG Labs in Manchester, NH, USA, using the appropriate amount of 4% (v/v) nitric acid. Calibration standards were prepared with concentrations of 1.0, 5.0, 10.0, 25.0, 50.0, and 100.0 µg/L. Before analysis, glassware (Analitik Kimya, Istanbul, Türkiye) was immersed in 10.0% (v/v) nitric acid solution for 24 h to reduce the possibility of cross-contamination (Yüksel and Arica 2018).
ICP-MS instrument parameters: setting and validation
An inductively coupled plasma-mass spectrometer (ICP-MS, Agilent 7700X, Agilent Corporation, USA) was used to quantify certain metals (Na, Mg, K, Ca, Al, Cr, Ni, Mn, Fe, Cu, Zn, V, As, Cd, and Pb) in groundwater samples. Direct-Q8 system (Merck-Millipore, Germany) was used to produce ultrapure water with a resistivity of 18 MΩ cm specifically for sample processing. The device was configured with the operating parameters listed below. Water samples were injected over a period of sixty seconds at a rotational rate of 0.3 revolutions per second using a Meinhard® nebulizer and a cold spray chamber. Within the specified time period, the autosampler was quickly placed in the sampling stand without the use of a flow injection valve. The forward and reflected powers of the argon gas plasma were measured as 1300 W and 7.0 W, respectively, as per the specified parameters. Additionally, the discharge rates of auxiliary, plasma, and nebulizer gases were changed to 1.0 L/min, 16.0 L/min, and 1.0 L/min, respectively. Following this, the device began to be used in peak bounce mode using nickel interface cones. Similarly, during intervals between infusions, the autosampler pump was subjected to a three-step sanitization procedure: flushed with ultrapure water for 30 s, flushed with 2% (v/v) nitric acid for 50 s, and then flushed again with ultrapure water for an additional 50 s.
It is known that determining trace element concentrations in natural water samples is difficult due to the presence of matrix elements such as Mg, Na, Cl, K and Ca in water. (Yüksel et al. 2021). Consequently, a 10 mL volume of 8% (vol/vol) nitric acid was added to the water samples to reduce the potential impact of additional substances that may be present in the samples. As a result, the use of 205Tl at the high end, 89Y in the middle range, and 7Li with a low mass facilitated the production of the most robust signal for multi-element quantification, particularly at very low concentrations. In accordance with the ISO/IEC 17025 standard, 11 measurements of UME CRM 1201 Spring Water (Tubitak, Türkiye) were taken into account to validate the ICP-MS test in terms of precision, accuracy, bias, recovery, matrix effect, and limit of detection. The methodology used to determine precision was coefficient of variation, as described in previous studies (Öncü et al. 2024; Bozalan et al. 2019; Arica et al. 2018), while relative error was used to convey accuracy. The results of the validation study, as shown in Table S1, confirm that the methodology is accurate and precise.
Analysis of indicators for water quality
Water quality assessment was carried out based on heavy metal pollution index (HPI), water quality index (WQI), and heavy metal evaluation index (HEI). Similarly, the irrigation water quality of the study samples was evaluated using sodium adsorption ratio (SAR), sodium percentage (%Na), Kelley's ratio (KR), and magnesium hazard (MH) measures.
Heavy metal pollution index (HPI)
Application of the Heavy Metal Pollution Index (HPI) is a key methodology for assessing the collective impact of multiple heavy metal indicators on water quality as a whole. As a result, the HPI metric is employed by scientists to provide a comprehensive assessment of the overall water quality impact from heavy metal pollution. (Hemachandra and Sewwandi 2023; Ahmed et al. 2023; Tokatli and Ustaoğlu 2020). HPI calculation performed in accordance with the formulas (1–3) outlined by Mohan et al. (1996).
The variable "Wi" represents the unit weight of metals, "Qi" represents the sub-index of each metal, "Ci" represents the detected concentration value of metals, WHO (2011) approved standard values for Si parameters in drinking water, and "k" represents a constant value of 1. When the HPI drops below 45, the pollution level is considered low. Similarly, a Health Pollution Index (HPI) score between 45 to 90 indicates a moderate level of pollution, as mentioned in previous articles (Agwu et al. 2023; Rahman et al. 2022).
Water quality index (WQI)
WQI is a very effective classification method because it takes into account the combined effect of multiple water quality parameters on overall water quality (Lukhabi et al. 2023). WQI is a quantitative measure that documents the suitability of water for various purposes, such as domestic, agricultural, industrial, and others (Nihalani and Meeruty 2021). It therefore provides a comprehensive and accurate assessment of the quality of water. Water quality control researchers have actively utilized the WQI since its establishment in the United States by Horton (1965). With respect to the Water Quality Index (WQI), water quality is divided into five different groups: WQI values equal to or greater than 300 indicate that the water is undrinkable; WQI values between 200 and 300 indicate very bad water quality; WQI values between 100 and 200 indicate poor water quality; WQI values between 50 and 100 indicate fair water quality; and WQI values below 50 indicate outstanding water quality (Xiao et al. 2019).
Considering that groundwater is the main source of drinking water in many developing countries globally, ensuring the quality of groundwater is vital for the sustainable and long-term use of this resource for various purposes, including drinking and irrigation (Krishnamoorthy et al. 2023). Water Quality Index (WQI) is a pragmatic and applicable approach to assess the overall quality of surface and groundwater as well as its suitability for human consumption (Ustaoğlu et al. 2021). Consequently, it has been widely used in water quality assessment research during the last decade (Wang et al. 2017; Varol and Tokatli 2021; Şener et al. 2023). Water Quality Index (WQI) was calculated using the formula (4):
Relative weight (Wi) is calculated by dividing the weight (wi) by the sum of all weights (Σwi), as shown in Table 1. As determined in a previous article (Ustaoğlu and Aydin 2020), the Wi value varies between 1 and 5 and reflects the relative importance of factors to human health and water quality. Ci represents the concentrations of variables included in the calculation, while Si represents standard values for drinking water according to WHO (2011) guidelines.
Heavy metal evaluation index (HEI)
Evaluation of water pollution caused by heavy metals was carried out using the HEI index (Varol and Tokatli 2023; Edet and Offiong 2002). This facilitates a comprehensive understanding of the extent of water pollution. As a result, HEI was calculated using the formula given below.
The equation assigns the symbol Hc to indicate the value found for each component, whereas Hmac represents the maximum acceptable concentration (MAC) for all variables according to the World Health Organization (WHO 2011). Within the MAC framework, increased metal concentrations are responsible for the decline in water quality (El-Degwy et al. 2023). Typically, it is recommended not to consume water that exceeds the MAC value for a given metal, indicated by an HEI greater than 10. When metal concentrations are below or above MAC limits, water quality is compromised with additional consequences. As a result, HEI is divided into three specific categories: significant contamination is indicated by a value greater than 20 HEI, moderate contamination is believed to be between 10 to 20 HEI, and minimal contamination is indicated by a value less than 10 HEI (Saleh et al. 2019).
Comprehensive analysis of irrigation water quality
The quality of irrigation water directly affects the protection of the environment, the abundance and diversity of agricultural crops, and the general health of the soil. Therefore, this research evaluated the irrigation water quality in the coastal groundwater of Giresun Province by determining the parameters KR, SAR, %Na, and MH using the formulas (6–9) as provided in a previous article (Ravikumar et al. 2013).
Health risk overview
Heavy metals enter the human body through the epidermis through contact with freshwater and digestion. The potential health consequences of ingestion and cutaneous exposure, including both non-carcinogenic and carcinogenic consequences, can be evaluated using experimental models. The current study used the health risk assessment methodology recommended by USEPA (2004). As stated by Wang et al. (2017), data on toxicological properties of specific metals can be found in Table 2. Formulas 10 and 11 were used to calculate the average daily dose (ADD) by skin absorption (ADD cutaneous) and direct ingestion (ADD ingestion), respectively (Saleem et al 2019; Zeng et al. 2015).
The variables ADDingestion and ADDdermal reflect average daily dosages received via ingestion and dermal route, respectively. Both variables are represented in micrograms per kilogram per day (μg/kg/d). The variable "Cwater" denotes the concentration of heavy metals in freshwater, measured in micrograms per liter (μg/L). "IR" is the rate at which something is ingested, measured in liters per day. In this research, the ingestion rate was determined as 2 in adults and 0.64 in children. The results of study show that adults were exposed to the stimulus for a period of 70 years, while children for a period of 6 years. Additionally, the exposure frequency was appeared to be 365 days per year. Kp represents the cutaneous permeability coefficient in water, measured in centimeters per hour. The research found that daily exposure while bathing and showering was0.58 h. The unit conversion factor, CF, is equal to 1 L per 1,000 cubic centimeters. The mean body weight (in kg) of the participants in this research was 70 for adults and 20 for children. In addition, the average day length for newborns was 2,190 and for adults was 25,550. The exposed skin area (cm2) is represented as SA, with a value of 6,600 for infants and 18,000 for adults. The gastrointestinal tract has been shown to contain ABSg, an absorption factor that has no dimensions (Xiao et al. 2019). The hazard quotient (HQ) and hazard index (HI) were calculated using Eqs. 12 and 13 to quantify the possible non-carcinogenic impacts of heavy metals absorbed or penetrated into the epidermis.
A Hazard Quotient (HQ) of less than 1 means that the likelihood of experiencing adverse health consequences is low. However, if the Hazard Index (HI) is greater than 1, this indicates that exposure to heavy metals may have non-carcinogenic effects. Carcinogenic Risk (CR) measures an individual's lifetime risk of developing cancer (LCR) as a result of exposure to substances likely to cause cancer. This risk is determined using the formula (14):
CSF is often known as the cancer slope factor. Consequently, the calculation of cancer risk in this study was determined by the level of arsenic present. Finally, the magnitudes of CSF are 0.0015 and 0.00366 μg/kg/day for ingestion and dermal penetration, respectively (Gao et al. 2019).
Multivariate/bivariate statistics
Quantification of elements in water samples was evaluated through the application of various statistical methodologies. Potential source of metals was determined using PCC and EPA PMF analyses. HCA was then used to investigate the correlation between metals. PCA was ultimately integrated with EPA PMF to reduce data sets and identify new components. Statistical analysis was performed using SPSS® Version 22.0 and Origin Pro® 2022. In addition, the ArcGIS 10.2 program was applied to create a map showing the distribution of PTEs across the sampling stations (Cüce et al. 2022).
Results and discussion
Evaluation of PTE Levels in groundwater
Various approaches can be used to determine the metal contents of water samples. ICP-MS continues to be widely used due to its ability to perform multi-element analysis (Yüksel and Arica 2018). A significant portion of environmental research fails to provide sufficient transparency about the accuracy of its methods. The accuracy and precision of the ICP-MS method was validated before determining metal concentrations in groundwater samples, thus increasing the importance of this research. Certified reference materials were used to validate the technique of this research. The ranges of bias, coefficient variation, relative error, and recovery were as follows: bias ranged from 0.94 to 1.02, coefficient variation ranged from 1.35% to 7.50%, relative error ranged from 0.19% to 6.15%, and recovery ranged from 93.80% to 102.10% (Table S1).
Based on the mean value of twelve stations, macronutrient concentrations in groundwater samples were expressed in mg/L in decreasing order as Ca (10.53 ± 6.63) > Na (6.81 ± 3.47) > Mg (3.39 ± 2.27) > K (2.05 ± 1.10). Similarly, PTE levels were provided in ug/L in decreasing order as Al (40.02 ± 15.45) > Fe (17.65 ± 14.35) > Zn (5.63 ± 2.59) > V (4.74 ± 5.85) > Cu (1.57 ± 0.81) > Mn (1.02 ± 0.76) > As (0.93 ± 0.73) > Cr (0.75 ± 0.57) > Ni (0.41 ± 0.18) > Pb (0.36 ± 0.23) > Cd (0.10 ± 0.05). An in-depth investigation of metal levels in groundwater was carried out, as shown in Table 3, and it is noteworthy that all analyzed metal concentrations were found below the established regulatory limits. This observation has important implications for overall water quality and its potential use for domestic, agricultural, and industrial purposes. According to Turkish Standards (TS 2662005), World Health Organization (WHO 2011), and European Union Council Guidelines (EC 1998), the levels of metals examined in groundwater samples taken from coastal regions of Giresun Province are within safe limits for consumption.
In addition, spatial distribution of elements (Na, Mg, K, Ca, Al, Cr, Ni, Mn, Fe, Cu, Zn, Pb, As and Cd) was performed using geographic information system (GIS) mapping (Fig. 3). Previously, several studies have examined the spatial distribution and associated hazards with heavy metal contaminants in groundwater (Long et al. 2021), providing complementary aid in the health risk assessment of arsenic and other PTEs. Therefore, the application of spatial interpolation in GIS technology has proven to be effective in assessing and predicting of groundwater contamination (Adimalla and Qian 2023).
The absence of metal concentrations exceeding regulatory thresholds is consistent with established water quality standards and confirmed the suitability of underground water for consumption and other essential uses. Such findings are particularly reassuring for communities that rely on these underground sources for their drinking water needs (Abanyie et al. 2023; Varol and Tokatli 2023). Furthermore, the results of this study contribute valuable information to local authorities, water resource managers, and policymakers tasked with ensuring the safety and sustainability of water supplies. The absence of high metal levels means there is a reduced risk of adverse health effects associated with metal exposure through water consumption. This supports the idea that the studied groundwater resource can be considered a reliable and safe reservoir that improves the welfare of society. While the current study provides an optimistic assessment of the groundwater quality, continuous monitoring and periodic assessments are recommended to track possible changes over time. It also underlines the importance of considering local geological and environmental factors that may affect water quality.
Evaluation of irrigation water quality
Assessing the quality of irrigation water obtained from groundwater is vital for the long-term sustainability of agriculture; because groundwater serves as a common and essential source of water for irrigation. The quality of groundwater has a significant impact on crop productivity, soil condition, and the overall effectiveness of agricultural activities (Mukherjee et al. 2022). For this reason, an analysis was made using the MH, SAR, %Na, and KR indicators to evaluate the irrigation water quality in the coastal areas of Giresun Province, (Table S2). In addition, Fig. 4A, B, C, and D show graphical representations of SAR, Na%, MH, and KR across stations. KR is acceptable for all stations except station 5. Similarly, only station 5 marginally exceeded the permissible Na% values. Although no MH levels were detected at the sampling stations, all stations demonstrated outstanding SAR performance. As a result of evaluating the suitability of groundwater samples for agricultural irrigation using parameters such as KR, Na%, SAR, and MH, it was determined that a significant amount of the water met the quality requirements.
An assessment of groundwater quality for irrigation water supply in an agro-economic region of the Lower Ganga Basin (India) reveals that almost the entire region is unsuitable for groundwater irrigation based on results from irrigation water quality indices (Mukherjee et al. 2022). On the contrary, a recent study conducted in the Gokpinar Basin of Denizli Province, Türkiye, identified several characteristics of spring water samples—SAR and Na%—that make them highly suitable for irrigation purposes (Taşdelen 2021). Unlike the Lower Ganga Basin in India (Mukherjee et al. 2022), our results presented high quality of irrigation water, consistent with the Gokpınar Basin in Denizli, Türkiye (Taşdelen 2021).
Assessing water quality
Water quality index (WQI)
The WQI calculation in this study is based on thirteen water quality parameters (Ca, Na, Mg, K, Fe, Zn, Cu, Mn, As, Cr, Ni, Pb, and Cd) presented in Table S2. The mean WQI value was found to be 5.78 ± 4.45, which indicates excellent water quality as it is < 50. Figure 5(A) shows the graphical representation of the WQI over the sampling stations. Comparing the quality of groundwater across different locations allows for an examination of global management approaches, common assessments, and challenges in achieving sustainable management. Utilizing the WQI to evaluate environmental health indicators in various areas is essential for formulating groundwater sustainability plans across diverse geopolitical contexts (Karadeniz et al. 2024). Moreover, WQI values of spring water samples in Iraq were found to be between 17.10 and 20.45 in the monsoon season and 10.76 to 18.13 in the desert season (Ameen 2019). Water quality index values in western Nepal ranged from substandard to outstanding in all areas evaluated (Gurung et al. 2019). Study findings assessing the quality of spring water in the Anantnag region of the Kashmir Himalayas showed that the water quality index (WQI) of the samples ranged from satisfactory to outstanding (Bhat et al. 2022). Groundwater quality in Burdur, Turkey was evaluated using the Water Quality Index (WQI), and WQI findings varied between 17,440 and 110,755 in the dry season and between 17,266 and 84,110 in the rainy season (Varol and Davraz 2015). In comparison, our WQI results (5.78 ± 4.45 < 50) were relatively lower than other reports mentioned above which shows that the groundwater samples were evaluated as excellent water quality.
Assessing HPI and HEI
HPI and HEI indices are widely used in groundwater quality assessment because they systematically evaluate the quality of groundwater by taking into account the amounts of various heavy metals (Chorol and Gupta 2023). HPI requires collecting water samples from various locations and then analyzing the heavy metal content in these samples. The results obtained are then compared to well-defined groundwater quality standards (Sirajudeen and Pravinkumar 2021). Pollution levels in groundwater are assessed by calculating the average HEI, which is determined by assigning unique pollution indices to each heavy metal based on their concentration. HEI complements HPI by taking into account additional features, such as bioavailability and toxicity of heavy metals. This provides more comprehensive information about the potential threats to both the environment and human health that may arise from groundwater pollution (Rajkumar et al. 2020). Both indices assist environmental scientists and policymakers in informed decision-making, facilitating the formulation of effective solutions to manage and mitigate the effects of heavy metal pollution in groundwater (Chaturvedi et al. 2018). This approach not only provides an accurate assessment of current pollution levels, but also aids ongoing surveillance efforts to monitor changes over time and ensure the resilience of groundwater resources.
In this study, HPI and HEI assessed the combined effect of elements (Fe, Zn, Cu, Mn, As, Cr, Ni, Pb, and Cd) in groundwater samples. The HPI and HEI were calculated using internationally accepted standard values from the World Health Organization (WHO 2011). Therefore, the mean HEI values were 0.44 ± 0.42. Considering that the average HEI is less than 10, the result only highlights low contamination. Similarly, the average HPI values of the sampling sites were 3.86 ± 2.81, indicating that all the stations exhibited minimal levels of pollution. Figure 5(B) and (C) show graphical depictions of the HEI and HPI at stations, respectively.
In previous studies evaluating water quality in Giresun Province and its surrounding, HPI values for nearby rivers varied between 59.68 in Gelevera Stream, 69.43 in Yağlıdere Stream (Ustaoğlu and Aydın 2020), and ranged from 5.66 to 38.71 in Çavuşlu Stream (Yüksel et al. 2021). In addition, the HEI value was recorded as 1.94 in Yağlıdere Stream and 2.76 in Aksu Stream (Ustaoğlu and Aydın 2020). Furthermore, Çavuşlu Stream has documented HEI values of 0.76 and 19.91 (Yüksel et al. 2021). Groundwater samples from the remote city Ayfonkarahisar/Turkey had low HPI and HEI values and were classified as having generally poor water quality (Şener 2023). In short, groundwater quality can vary depending on complex parameters such as geology, climate and agricultural fertilizer use.
Risk assessment for human health
The human health risk assessment of potentially toxic metals in groundwater requires a systematic investigation of the potential hazards, routes of exposure, and the associated health effects resulting from the presence of such metals in groundwater (Shi et al. 2022). First, the presence of PTEs (i.e., lead, arsenic, cadmium, mercury, and chromium) is determined, and then exposure routes such as drinking water, skin contact, and smoke inhalation are identified (Yüksel et al. 2023). To determine exposure levels, metal concentrations in water samples should be measured and these should then be compared to appropriate reference doses. Toxicological data for each metal, including bioavailability and cumulative effect, are examined to understand its effects on health. People at risk, such as children and pregnant women, are identified. The final step is to analyze the total risk, considering uncertainties, and communicating the results to stakeholders. Monitoring and risk management systems protect human health from toxic metal groundwater pollution (Myers et al. 2023; Radfard et al. 2023; OECD 2018; Adesiyan et al. 2018).
The presence of dissolved heavy metals in drinking water poses a threat to human health due to its association with many non-cancerous and malignant diseases. This research study investigated the non-carcinogenic and carcinogenic health hazards associated with heavy metals in both children and adults. In other words, we conducted an evaluation of the possible health consequences due to metal uptake and interaction with metals in the coastal groundwater of Giresun province, taking into account both adults and children (Table S3). Therefore, toxicological values (Wang et al. 2017; USEPA 2004) were used to determine the total hazard index (ingestion, cutaneous) and CR values for each metal. The total hazard index (THI) was calculated for non-carcinogenic health risks, and the mean THI for adult and children was 0.07 and 0.08, respectively. As seen in Fig. 6(A), since THI values are below 1, there is no non-carcinogenic risk in the stations.
The standard definition of cancer risk (CR) refers to the probability that a person will develop any type of cancer during their lifetime as a result of exposure to carcinogens (Ashraf et al. 2021; Mohammadi et al. 2019). Cancer risk (CR) for adults exposed to arsenic alone was calculated in this study using the cancer slope factor (CSF) listed in Table 2. As illustrated in Fig. 6(B), taking into consideration that arsenic is absorbed via the dermal and ingestion routes of administration, the CR value for adult subjects (Station #10) was calculated to be 1.44E-04, exceeding the USEPA's (2004) allowable range (1.00E-06 < CR < 1.00E-04). Although none of the stations exceeded the 10 µg/L permissible threshold for arsenic in drinking water, station 10 exhibited a minor carcinogenic health hazard based on the calculated CR. This result shows that hazardous metals (e.g., arsenic) are still risky even if their concentrations fall below the highest permissible levels. In other words, exposure to arsenic levels that comply with existing regulatory standards may result in debilitating conditions, such as cancer. Assessing and controlling groundwater quality is crucial in order to reduce health hazards and guarantee the long-term welfare of populations and ecosystems in the area. Overall, the result of current study shows that most of our groundwater samples are suitable for domestic use without posing any danger to human health.
The presence of inorganic arsenic in contaminated groundwater poses a significant risk to human health, especially in terms of arsenic toxicity. Groundwater pollution can occur when inorganic arsenic, a naturally-occurring element, leaches into water sources from geological formations (Yüksel et al. 2010). Long-term exposure to high amounts of inorganic arsenic has been associated with a number of adverse health consequences, including skin lesions, respiratory problems, cardiovascular disorders, and increased susceptibility to certain types of cancer, including lung and bladder cancer (Yüksel et al. 2018). The World Health Organization (WHO) has established a threshold for arsenic in drinking water, which is the highest allowable level to minimize potential health hazards. Addressing the problem of arsenic pollution in groundwater requires the use of comprehensive approaches that include consistent monitoring, adoption of water purification technology, and community education to reduce exposure and protect public health (Ramsay et al. 2021; Yüksel et al. 2015).
The current evaluation is consistent with previous scientific studies conducted in the surrounding area (Ustaoğlu et al. 2020a). In an independent study conducted in Çavuşlu Stream, Giresun Province, one of the four stations with a high lifetime cancer risk was identified. The reason for this result was the station's proximity to the waste disposal facility, which is assumed to be the source of toxic metals pollution (Yüksel et al. 2021). In contrast, most previous studies conducted near our study location did not find any evidence of long-term cancer risk based on ecotoxicological risk assessment (Ustaoğlu et al. 2020b, 2021). Additionally, in another investigation conducted in the Thrace Region of the Meric River in Türkiye, a toxicological scan of a wetland was analyzed. This research likewise found evidence that the presence of hazardous metals dissolved in water poses a long-term risk of cancer (Tokatli and Ustaoğlu 2020). Moreover, a previous study project investigated the consequences of drought on the environmental risks posed by contaminated groundwater in Poland. Researchers reported lifetime cancer risk for 90 out of 117 locations (Kubicz et al. 2021). However, assessing cancer risk alone is insufficient. Therefore, it is of great importance to identify the possible source of the pollution and provide effective solutions that protect human health and the environment.
Evaluation of contamination sources using the statistical assays
Identifying the source of dissolved metals in groundwater is a crucial component of monitoring and managing the environment (Jia et al. 2023). Principal Component Analysis (PCA) and EPA Positive Matrix Factorization (PMF) are advanced statistical methods that are very useful in identifying the sources of these pollutants. PCA facilitates reducing the complexity of data sets by transforming variables into uncorrelated principal components, thereby facilitating analysis of causes and patterns of variability in water quality (Yüksel et al. 2024; Anaman et al. 2022).
The origin of PTEs in the groundwater samples was determined by using various multivariate/bivariate data analysis techniques such as PCA, EPA PMF, HCA, and PCC. The KMO test with a value of 0.72 and Bartlett's test of sphericity with a p-value of less than 0.001 showed that the sample data collected for this study was sufficient to perform PCA. Variables that were not correlated and had a total variance of less than 0.5 were removed to increase reliability (Aydin et al. 2021). PCA found three principal components with eigenvalues greater than 1, which together explained 82.58% of the total variance. The presence of PTEs in groundwater samples can be attributed to three different sources, as shown in the component plots in rotated space (Fig. 7). As can be seen in Table S4, within principal component 1 (PC1), Al, As, and Cu elements had significant positive loading values above 0.6 and contributed 48.23% of the overall variance. On the other hand, PC2 explained 19.06 percent of the total variation in hazardous elements (Cd, Pb, Zn, and Mn) with positive loading values above 0.50. In addition, some metals, such as Ni and Cr, exhibit loading values above 0.8 relative to PC3, contributing 15.18 percent of the total variation (Fig. S1).
In contrast, the EPA PMF is a receptor model that allocates measured metal concentrations into separate source profiles, making it easier to identify and quantify pollutant contributions from a variety of possible sources (Hristova et al. 2020). In other words, PMF is used to estimate the contribution of identified sources to all measured pollutants, while PCA is used as an explanatory tool to identify significant pollutant sources (Doumbia et al. 2023). The PMF model takes into account the uncertainties in individual data and the non-negativity of the components (loads and scores), which is a significant difference from PCA. Moreover, PMF ignores the information contained in the correlation matrix and emphasizes a technique that minimizes the sum of squares point by point (Cesari et al. 2016). Consequently, the profiles generated by the PMF model can be compared directly to the input matrix without any modification (Anaman et al. 2022). Conversely, the PCA load matrix has a singular dimension. Therefore, it is critical to include this in a multilinear regression analysis to determine precisely the extent to which the various principal components are responsible for the observed concentrations (Yang et al. 2023). EPA PMF 5.0 identified three major metal groups in this study other than Cd, Cu, and Mn (Fig. 8), which is nearly identical to the PCA results. The EPA PMF included Ni, Cr, and Mn, as well as Cd and Cu (Fig. S2). This minor difference between PCA and EPA PMF can be attributed to the inclusion of non-negative components in the PMF model as well as the consideration of individual data uncertainties (Cesari et al. 2016). Therefore, the integration of PCA with PMF basically predicted that PTEs could come from three distinct sources: geogenic (As, Al, and Fe), anthropogenic (Zn, Cu, and Ni), and mixed (Zn and Pb) in our groundwater samples.
Hierarchical Cluster Analysis (HCA) is a reliable statistical technique used to assess dissolved metal concentration in groundwater. Hierarchical clustering analysis (HCA) is an indispensable technique for identifying hidden patterns and categorizing shared features within a given dataset. Identification of different subgroups or groupings of water samples can be facilitated by comparing metal content profiles (Du et al. 2017; Aneeshkumar and Reddy 2021; Ustaoğlu et al. 2024). HCA can reveal temporal or spatial patterns in the context of dissolved metals in groundwater, allowing sample locations or periods to be clustered with comparable metal compositions. The factors that make up a given cluster exhibit comparable characteristics and are derived from similar sources of contamination. Therefore, to efficiently assess water quality, examining a single site from each cluster is sufficient; this can serve as a representative sample of water quality across the entire cluster (Tokatli 2019). Therefore, HCA was used to further validate PCA. Therefore, the dendrogram produced by hierarchical cluster analysis (HCA) and principal component analysis (PCA) was completely consistent and resulted in the identification of three distinct groups (Fig. 9).
Utilizing PCC, the strength of the linear relationship between groundwater samples and dissolved metal concentrations can be determined. The statistical measure in question determines the magnitude and orientation of the linear correlation between two variables, metal concentrations in this example (Drasovean and Murariu 2021). A PCC around + 1 indicates a robust positive correlation; This means that an increase in the concentration of one metal is typically accompanied by a corresponding increase in the concentration of another metal. On the contrary, a PCC close to -1 indicates a strong inverse relationship and indicates a negative correlation. A PCC close to zero indicates the absence or weakness of a linear correlation (Kumar and Singh 2023). By using PCC on dissolved metal content data, researchers are able to discern possible interdependencies between metal concentrations. This facilitates the detection of co-occurring patterns and provides valuable information about the underlying mechanisms governing the presence of metals in groundwater. The information provided is of great importance for understanding the intricacies of metal contamination and developing precise approaches to the management of groundwater quality (Hamuna and Wanimbo 2021). As shown in Fig. 10, the following metals exhibit significant correlations: Fe-Al (r = 0.96), Ca-Mg (r = 0.88), Fe-Pb (r = 0.78), Mg-V (r = 0.77), Al–Pb (r = 0.76), and Al-Na (r = -0.71).
In conclusion, PCA, EPA PMF, HCA, and PCC exhibited mutual potentiation due to the strong correlations observed between PTEs within the same cluster and principal component. Given the maritime nature and significant agricultural activities of the research location, it is unlikely that any human impacts will be detected. However, a recent study has shown that the main pollutants in groundwater are fertilizers and pesticides, which are often used in agricultural practices to increase crop production (Tudi et al. 2021). Due to the widespread use of hazelnut cultivation in the study area, and the widespread use of phosphate and nitrate fertilizers, it is thought that the PTEs mentioned in other components are probably related to lithogenic and geogenic origins. Consequently, under certain physical conditions, such as the exposure of rocks to heat and pressure or the flow of lava into nearby water bodies, PTEs can accumulate to very high levels in the environment. These conditions include soil layer, volcanic emissions, and evaporation. Thus, groundwater naturally contains several heavy metals (Mahipal and Rajeev 2019).
Conclusion
The rapid expansion of cities and infrastructure, combined with a growing global population, has heightened the strain on accessible potable water resources, exacerbating water scarcity issues. To address these challenges, this study focused on evaluating groundwater quality at 12 strategically selected points along the 90-km coastal stretch of Giresun province, renowned for its hazelnut farming industry in Türkiye. The study stations are situated in the most visited and densely populated areas along the coastline; these locations are pivotal for assessing the impacts of human activities on groundwater quality and understanding associated health risks. The mean level of listed potentially toxic elements (PTEs) in groundwater samples were measured in a decreasing order of Ca > Na > Mg > K > Al > Fe > Zn > V > Cu > Mn > As > Cr > Ni > Pb > Cd. Water quality, source identification, and associated health risk were assessed using a comprehensive strategy. This method involved the use of specific water quality indicators as well as advanced statistical techniques such as multivariate and bivariate analyses. The results of the current study can be listed as follows:
-
WQI analysis revealed that water quality was rated as "excellent". Additionally, HEI < 10 and HPI < 15 the values at all stations showed that pollution was low.
-
The mean amounts of all PTEs were well below the threshold values according to national and international standards.
-
Irrigation water quality is suitable according to MH, SAR and Na% (except station 5) and KR (except station 5).
-
The THI values are consistently below 1, indicating that there is no non-carcinogenic health risk. However, at station 10 the CR (1.44E-04) slightly exceeded the prescribed threshold for arsenic for adults.
-
This highlighted that exposure to arsenic levels that meet current regulatory requirements can cause incapacitating disorders including cancer.
-
Analysis of multivariate and bivariate statistics (EPA PMF, PCA, PCC, and HCA) indicated that the impact of human activities on metal levels in groundwater samples is minimal. Primary sources of potentially toxic elements (PTEs) mostly come from natural sources such as rocks or a combination of natural and human sources.
-
Spatial distribution of PTEs using GIS provided complementary assistance for assessing potential risks on stations.
-
Therefore, it is recommended to conduct periodic analyses of groundwater collected from fountains in order to reduce potential public health problems.
-
To enhance water quality, it is crucial to reduce anthropogenic activities such as industrial discharges and agricultural runoff
-
The results of this study contribute positively to the groundwater quality discourse by showing that metal levels in the analyzed samples remained below legal limits.
-
The findings provide a baseline for the safety and reliability of groundwater source studied, highlighting its potential to be a sustainable and safe water source for the surrounding community.
Data availability
The complete set of data that was produced or examined throughout this investigation has been incorporated into this published article and its supplementary materials.
References
Abanyie SK, Apea OB, Abagale SA, Amuah EEY, Sunkari ED (2023) Sources and factors influencing groundwater quality and associated health implications: A review. Emerg Contam 9(2):100207. https://doi.org/10.1016/j.emcon.2023.100207
Adesiyan IM, Bisi-Johnson M, Aladesanmi OT, Okoh AI, Ogunfowokan AO (2018) Concentrations and Human Health Risk of Heavy Metals in Rivers in Southwest Nigeria. J Health Pollut 8(19):180907. https://doi.org/10.5696/2156-9614-8.19.180907
Adimalla N, Qian H (2023) Groundwater quality and contamination: an application of GIS. Environ Earth Sci 82(18):423
Adimalla N, Manne R, Zhang Y, Xu P, Qian H (2022) Evaluation of groundwater quality and its suitability for drinking purposes in semi-arid region of Southern India: an application of GIS. Geocarto Int 37(25):10843–10854
Agwu EJ, Odanwu SE, Ezewudo BI, Odo GE, Nzei JI, Iheanacho SC, Islam S (2023) Assessment of water quality status using heavy metal pollution indices: A case from Eha-Amufu catchment area of Ebonyi River, Nigeria. Acta Ecol Sin. https://doi.org/10.1016/j.chnaes.2023.02.003
Ahmed RS, Abuarab ME, Ibrahim MM, Baioumy M, Mokhtar A (2023) Assessment of environmental and toxicity impacts and potential health hazards of heavy metals pollution of agricultural drainage adjacent to industrial zones in Egypt. Chemosphere 318:137872. https://doi.org/10.1016/j.chemosphere.2023.137872
Aloui S, Zghibi A, Mazzoni A, Elomri A, Triki C (2023) Groundwater resources in Qatar: A comprehensive review and informative recommendations for research, governance, and management in support of sustainability. J Hydrol: Reg Stud 50:101564. https://doi.org/10.1016/j.ejrh.2023.101564
Alrowais R, Abdel daiem MM, Li R, Maklad MA, Helmi AM, Nasef BM, Said N (2023) Groundwater Quality Assessment for Drinking and Irrigation Purposes at Al-Jouf Area in KSA Using Artificial Neural Network, GIS, and Multivariate Statistical Techniques. Water 15(16):2982. https://doi.org/10.3390/w15162982
Ameen HA (2019) Spring water quality assessment using water quality index in villages of Barwari Bala, Duhok, Iraq. Appl Water Sci 9:176. https://doi.org/10.1007/s13201-019-1080-z
Anaman R, Peng C, Jiang Z, Liu X, Zhou Z, Guo Z, Xiao X (2022) Identifying sources and transport routes of heavy metals in soil with different land uses around a smelting site by GIS based PCA and PMF. Sci Total Environ 823:153759. https://doi.org/10.1016/j.scitotenv.2022.153759
Aneeshkumar N, Reddy AGS (2021) Hierarchical Cluster Analysis as an Indicative of the Hydrogeochemical Evolution of Ground Water in a Shallow Aquifer System. Int J Environ Sci Nat Res 26(5):556197. https://doi.org/10.19080/IJESNR.2021.26.556197
Arcega RD, Chen RJ, Chih PS, Huang YH, Chang WH, Kong TK, Lee CC, Mahmudiono T, Tsui CC, Hou WC, Hsueh HT, Chen HL (2023) Toxicity prediction: An application of alternative testing and computational toxicology in contaminated groundwater sites in Taiwan. J Environ Manage 328:116982. https://doi.org/10.1016/j.jenvman.2022.116982
Arica E, Yuksel B, Yener I, Dolak I, Gok E, Yilmaz E (2018) Icp-ms determination of lead levels in autopsy liver samples: an application in forensic medicine. At Spectrosc 39:62–66. https://doi.org/10.46770/AS.2018.02.002
Ashraf I, Ahmad F, Sharif A, Altaf AR, Teng H (2021) Heavy metals assessment in water, soil, vegetables and their associated health risks via consumption of vegetables, District Kasur, Pakistan. SN Appl Sci 3:552. https://doi.org/10.1007/s42452-021-04547-y
Aydin H, Ustaoğlu F, Tepe Y, Soylu EN (2021) Assessment of water quality of streams in northeast Turkey by water quality index and multiple statistical methods. Environ Forensic 22(1–2):270–287
Bhat SU, Nisa AU, Sabha I, Mondal NC (2022) Spring water quality assessment of Anantnag district of Kashmir Himalaya: towards understanding the looming threats to spring ecosystem services. Appl Water Sci 12:180. https://doi.org/10.1007/s13201-022-01691-7
Bozalan M, Türksoy VA, Yüksel B, Güvendik G, Söylemezoğlu T (2019) Preliminary Assessment of Lead Levels in Soft Plastic Toys by Flame Atomic Absorption Spectroscopy. Turk Hij Den Biyol Derg 76(3):243–254. https://doi.org/10.5505/TurkHijyen.2019.58234
Cesari D, Amato F, Pandolfi M, Alastuey A, Querol X, Contini D (2016) An inter-comparison of PM10 source apportionment using PCA and PMF receptor models in three European sites. Environ Sci Pollut Res Int 23(15):15133–48. https://doi.org/10.1007/s11356-016-6599-z
Chaturvedi A, Bhattacharjee S, Singh AK, Kumar V (2018) A new approach for indexing groundwater heavy metal pollution. Ecol Ind 87:323–331. https://doi.org/10.1016/j.ecolind.2017.12.052
Chidiac S, El Najjar P, Ouaini N, El Rayess Y, El Azzi D (2023) A comprehensive review of water quality indices (WQIs): history, models, attempts and perspectives. Rev Environ Sci Biotechnol 22(2):349–395. https://doi.org/10.1007/s11157-023-09650-7
Chorol L, Gupta SK (2023) Evaluation of groundwater heavy metal pollution index through analytical hierarchy process and its health risk assessment via Monte Carlo simulation. Process Saf Environ Prot 170:855–864. https://doi.org/10.1016/j.psep.2022.12.063
Cüce H, Kalıpcı E, Ustaoğlu F, Kaynar İ, Baser V, Türkmen M (2022) Multivariate statistical methods and GIS based evaluation of the health risk potential and water quality due to arsenic pollution in the Kızılırmak River. Int J Sedim Res 37(6):754–765
Din IU, Muhammad S, Faisal S, Rehman IU, Ali W (2023) Heavy metal (loid) s contamination and potential risk assessment via groundwater consumption in the district of Hangu, Pakistan. Environ Sci Pollut Res 30(12):33808–33818
Doumbia T, Liousse C, Ouafo-Leumbe M-R, Ndiaye SA, Gardrat E, Galy-Lacaux C, Zouiten C, Yoboué V, Granier C (2023) Source Apportionment of Ambient Particulate Matter (PM) in Two Western African Urban Sites (Dakar in Senegal and Bamako in Mali). Atmosphere 14:684. https://doi.org/10.3390/atmos14040684
Drasovean R, Murariu G (2021) Water Quality Parameters and Monitoring Soft Surface Water Quality Using Statistical Approaches. IntechOpen.https://doi.org/10.5772/intechopen.97372
Du X, Shao F, Wu S, Zhang H, Xu S (2017) Water quality assessment with hierarchical cluster analysis based on Mahalanobis distance. Environ Monit Assess 189:335. https://doi.org/10.1007/s10661-017-6035-y
EC (1998) European council directive 98/83/ec of 3 november 1998 on the quality of water intended for human consumption. Off J Eur Communities 330:32. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31998L0083
Edet A, Offiong O (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 57:295–304. https://doi.org/10.1023/B:GEJO.0000007250.92458.de
El-Degwy AA, Negm NA, El-Tabl AS, Goher ME (2023) Assessment of heavy metal pollution in water and its effect on Nile tilapia (Oreochromis niloticus) in Mediterranean Lakes: a case study at Mariout Lake. Appl Water Sci 13:50. https://doi.org/10.1007/s13201-022-01858-2
Gao B, Gao L, Gao J, Xu D, Wang Q, Sun K (2019) Simultaneous evaluations of occurrence and probabilistic human health risk associated with trace elements in typical drinking water sources from Major River Basins in China. Sci Total Environ 666:139–146. https://doi.org/10.1016/j.scitotenv.2019.02.148
Gurung A, Adhikari S, Chauhan R, Thakuri S, Nakarmi S, Rijal D, Dongol BS (2019) Assessment of Spring Water Quality in the Rural Watersheds of Western Nepal. J Geosci Environ Prot 7:39–53. https://doi.org/10.4236/gep.2019.711004
Hamuna B, Wanimbo E (2021) Heavy Metal Contamination in Sediments and Its Potential Ecological Risks in Youtefa Bay, Papua Province. Indonesia. J Ecol Eng 22(8):209–222. https://doi.org/10.12911/22998993/139116
Hemachandra SCSM, Sewwandi BGN (2023) Application of water pollution and heavy metal pollution indices to evaluate the water quality in St. Sebastian Canal Colombo Sri Lanka. Environ Nanotechnol Monit Manag. https://doi.org/10.1016/j.enmm.2023.100790
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–306
Hristova E, Veleva B, Georgieva E, Branzov H (2020) Application of Positive Matrix Factorization Receptor Model for Source Identification of PM10 in the City of Sofia, Bulgaria. Atmosphere 11(9):890. https://doi.org/10.3390/atmos11090890
Jia C, Altaf AR, Li F, Ashraf I, Zafar Z, Nadeem AA (2023) Comprehensive assessment on groundwater quality, pollution characteristics, and ecological health risks under seasonal thaws: Spatial insights with Monte Carlo simulations. Groundw Sustain Dev 22:100952. https://doi.org/10.1016/j.gsd.2023.100952
Karadeniz S, Ustaoğlu F, Aydın H, Yüksel B (2024) Toxicological risk assessment using spring water quality indices in plateaus of Giresun Province/Türkiye: a holistic hydrogeochemical data analysis. Environ Geochem Health. https://doi.org/10.1007/s10653-024-02054-8
Krishnamoorthy N, Thirumalai R, Lenin SM, Anusuya M, Manoj KP, Hemalatha E, Prasad MM, Munjal N (2023) Assessment of underground water quality and water quality index across the Noyyal River basin of Tirupur District in South India. Urban Clim 49:101436. https://doi.org/10.1016/j.uclim.2023.101436
Kubicz J, Lochyński P, Pawełczyk A, Karczewski M (2021) Effects of drought on environmental health risk posed by groundwater contamination. Chemosphere 263:128145. https://doi.org/10.1016/j.chemosphere.2020.128145
Kumar P, Singh RP (2023) Assessment of Edaphic pollution indices and bioaccumulation of trace metals in Solanum lycopersicum, Spinacia oleracea and Triticum aestivum: an associated health risk evaluation. Environ Monit Assess 195:588. https://doi.org/10.1007/s10661-023-11170-4
Laonamsai J, Pawana V, Chipthamlong P, Chomcheawchan P, Kamdee K, Kimmany B, Julphunthong P (2023) Groundwater Quality Variations in Multiple Aquifers: A Comprehensive Evaluation for Public Health and Agricultural Use. Geosciences 13(7):195. https://doi.org/10.3390/geosciences13070195
Long X, Liu F, Zhou X, Pi J, Yin W, Li F, Huang S, Ma F (2021) Estimation of spatial distribution and health risk by arsenic and heavy metals in shallow groundwater around Dongting Lake plain using GIS mapping. Chemosphere 269:128698. https://doi.org/10.1016/j.chemosphere.2020.128698
Lukhabi DK, Mensah PK, Asare NK, Pulumuka-Kamanga T, Ouma KO (2023) Adapted Water Quality Indices: Limitations and Potential for Water Quality Monitoring in Africa. Water 15(9):1736. https://doi.org/10.3390/w15091736
Mahipal SS, Rajeev K (2019) Contaminant of Heavy Metals in Groundwater & its Toxic Effects on Human Health & Environment. Int J Environ Sci Nat Res 18(5):555996. https://doi.org/10.19080/IJESNR.2019.18.555996
Malakar A, Snow DD, Ray C (2019) Irrigation Water Quality—A Contemporary Perspective. Water 11(7):1482. https://doi.org/10.3390/w11071482
Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E (2020) Environmental and Health Impacts of Air Pollution: A Review. Front Public Health 8:14. https://doi.org/10.3389/fpubh.2020.00014
Mitra S, Chakraborty AJ, Tareq AM, Emran TB, Nainu F, Khusro A, Idris AM, Khandaker MU, Osman H, Alhumaydhi FA, Simal-Gandara J (2022) Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J King Saud Univ-Sci 34:101865. https://doi.org/10.1016/j.jksus.2022.101865
Mohammadi AA, Zarei A, Majidi S, Ghaderpoury A, Hashempour Y, Saghi MH, Alinejad A, Yousefi M, Hosseingholizadeh N, Ghaderpoori M (2019) Carcinogenic and non-carcinogenic health risk assessment of heavy metals in drinking water of Khorramabad, Iran. MethodsX 6:1642–1651. https://doi.org/10.1016/j.mex.2019.07.017
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metals in drinking water and develop-ment of heavy metal pollution index. J Environ Sci Health Part A 31:283–289
Mukherjee I, Singh UK, Chakma S (2022) Evaluation of groundwater quality for irrigation water supply using multi-criteria decision-making techniques and GIS in an agroeconomic tract of Lower Ganga basin, India. J Environ Manag 309:114691. https://doi.org/10.1016/j.jenvman.2022.114691
Myers RA, Gyimah E, Gbemadu K, Osei B, Akoto O (2023) Appraising groundwater quality and the associated health risks of heavy metal contamination at Suame magazine. Sci Afr 21:e01794. https://doi.org/10.1016/j.sciaf.2023.e01794
Nafi AW, Taseidifar M (2022) Removal of hazardous ions from aqueous solutions: Current methods, with a focus on green ion flotation. J Environ Manag 19:115666. https://doi.org/10.1016/j.jenvman.2022.115666
Nihalani S, Meeruty A (2021) Water quality index evaluation for major rivers in Gujarat. Environ Sci Pollut Res 28:63523–63531. https://doi.org/10.1007/s11356-020-10509-5
OECD (2018) Considerations for assessing the risks of combined exposure to multiple chemicals, series on testing and assessment no. 296, environment, health and safety division, environment directorate. https://www.oecd.org/content/dam/oecd/en/publications/reports/2018/12/considerations-for-assessing-the-risks-of-combined-exposure-to-multiple-chemicals_dc88379f/ceca15a9-en.pdf
Öncü T, Yüksel B, Binay E, Şen N (2024) LC-MS/MS Investigation of nitrosamine impurities in certain Sartan group medicinal products available in Istanbul, Türkiye. Ann Pharm Fr 82(1):72–83. https://doi.org/10.1016/j.pharma.2023.08.002
Radfard M, Hashemi H, Baghapour MA et al (2023) Prediction of human health risk and disability-adjusted life years induced by heavy metals exposure through drinking water in Fars Province, Iran. Sci Rep 13:19080. https://doi.org/10.1038/s41598-023-46262-1
Rahman MS, Parvez S, Begum BA, Quraishi SB, Choudhury TR, Fatema KJ, Hosen MM, Bodrud-Doza M, Rahman LS, Sattar MA (2022) Chemometric appraisal of water quality for domestic and agricultural purposes: a case study from establishing Rooppur Nuclear Power Plant (NPP) area, Pabna District, Bangladesh. Environ Sci Pollut Res Int 29(37):56620–56641. https://doi.org/10.1007/s11356-022-19308-6
Rajkumar H, Naik PK, Rishi MS (2020) A new indexing approach for evaluating heavy metal contamination in groundwater. Chemosphere 245:125598. https://doi.org/10.1016/j.chemosphere.2019.125598
Ramsay L, Petersen MM, Hansen B, Schullehner J, van der Wens P, Voutchkova D, Kristiansen SM (2021) Drinking Water Criteria for Arsenic in High-Income, Low-Dose Countries: The Effect of Legislation on Public Health. Environ Sci Technol 55(6):3483–3493. https://doi.org/10.1021/acs.est.0c03974
Rashid A, Schutte BJ, Ulery A, Deyholos MK, Sanogo S, Lehnhoff EA, Beck L (2023) Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 13(6):1521. https://doi.org/10.3390/agronomy13061521
Ravikumar P, Aneesul Mehmood M, Somashekar RK (2013) Water quality index to determine the surface water quality of Sankey tank and Mallathahalli lake, Bangalore urban district, Karnataka, India. Appl Water Sci 3:247–261. https://doi.org/10.1007/s13201-013-0077-2
Rezaei A, Hassani H, Hassani S, Jabbari N, Mousavi SBF, Rezaei S (2019) Evaluation of groundwater quality and heavy metal pollution indices in Bazman basin, southeastern Iran. Groundw Sustain Dev 9:100245. https://doi.org/10.1016/j.gsd.2019.100245
Saleem M, Iqbal J, Shah MH (2019) Seasonal variations, risk assessment and multivariate analysis of trace metals in the freshwater reservoirs of Pakistan. Chemosphere 216:715–724. https://doi.org/10.1016/j.chemosphere.2018.10.173
Saleh HN, Panahande M, Yousefi M, Asghari FB, Oliveri CG, Talaee MAA (2019) Carcinogenic and noncarcinogenic risk assessment of heavy metals in groundwater wells in Neyshabur Plain, Iran. Biol Trace Elem Res 190(1):251–261
Şener Ş (2023) Groundwater quality, heavy metal pollution, and health risk assessment using geospatial techniques and index methods in Eber wetland and surroundings (Afyonkarahisar/Turkey). Environ Sci Pollut Res Int 30(18):51387–51411. https://doi.org/10.1007/s11356-023-25857-1
Şener E, Şener Ş, Varol S (2023) Appraisal of groundwater quality with WQI and human health risk assessment in Karamık wetland and surroundings (Afyonkarahisar/Turkey). Environ Geochem Health 45(5):1499–1523
Shi H, Zeng M, Peng H, Huang C, Sun H, Hou Q, Pi P (2022) Health Risk Assessment of Heavy Metals in Groundwater of Hainan Island Using the Monte Carlo Simulation Coupled with the APCS/MLR Model. Int J Environ Res Public Health 19(13):7827. https://doi.org/10.3390/ijerph19137827
Siddiqua A, Hahladakis JN, Al-Attiya WAKA (2022) An overview of the environmental pollution and health effects associated with waste landfilling and open dumping. Environ Sci Pollut Res Int 29(39):58514–58536. https://doi.org/10.1007/s11356-022-21578-z
Sirajudeen J, Pravinkumar J (2021) Heavy metal analysis of groundwater of Thirukoviloor, Villuppuram, Tamil Nadu, India. Mater Today: Proc 36(4):828–831. https://doi.org/10.1016/j.matpr.2020.07.012
Taşdelen S (2021) Hydrogeochemical Characteristics of Spring Waters for Irrigation, Gökpınar Basin Case, Denizli, Turkey. J Agric Sci 27(4):516–525. https://doi.org/10.15832/ankutbd.744844
Tekin-Özan S, Tunç M, Bakioğlu-Acar B (2024) Evaluation of some heavy metals and selenium pollution in Karataş Lake (Burdur/Türkiye) using various pollution indices and statistical analysis. Mar Pollut Bull 199:115927
Tokatli C (2019) Sediment quality of Ergene River Basin: Bio-ecological risk assessment of toxic metals. Environ Monit Assess 191(11):706. https://doi.org/10.1007/s10661-019-7885-2
Tokatli C, Ustaoğlu F (2020) Health risk assessment of toxicants in Meriç River Delta Wetland, Thrace Region, Turkey. Environ Earth Sci 79:426. https://doi.org/10.1007/s12665-020-09171-4
Tokatli C, Titiz AM, Uğurluoğlu A, Islam MS, Ustaoğlu F, Islam ARMT (2022) Assessment of the effects of COVID-19 lockdown period on groundwater quality of a significant rice land in an urban area of Türkiye. Environ Sci Pollut Res Int 29(47):71752–71765. https://doi.org/10.1007/s11356-022-20959-8
Tokatlı C, Varol M, Ustaoğlu F (2023) Ecological and health risk assessment and quantitative source apportionment of dissolved metals in ponds used for drinking and irrigation purposes. Environ Sci Pollut Res 30(18):52818–52829
Topaldemir H, Taş B, Yüksel B, Ustaoğlu F (2023) Potentially hazardous elements in sediments and Ceratophyllum demersum: an ecotoxicological risk assessment in Miliç Wetland, Samsun, Türkiye. Environ Sci Pollut Res Int 30:26397–26416. https://doi.org/10.1007/s11356-022-23937-2
TS 266 (2005) Water intended for human consumption. Turkish standards institute, Report No: 13.060.20, Ankara, Türkiye
Turdi M, Yang L (2016) Trace Elements Contamination and Human Health Risk Assessment in Drinking Water from the Agricultural and Pastoral Areas of Bay County, Xinjiang, China. Int J Environ Res Public Health 13(10):938. https://doi.org/10.3390/ijerph13100938
Tudi M, Daniel Ruan H, Wang L, Lyu J, Sadler R, Connell D, Chu C, Phung DT (2021) Agriculture development, pesticide application and its impact on the environment. Int J Environ Res Public Health 18(3):1112. https://doi.org/10.3390/ijerph18031112
Ullah A, Ali W, Muhammad S, Ijaz J, Amir F, Iqbal S (2023) Comprehensive evaluation of hydro-chemical processes, suitability, health risks, and sources of groundwater contamination using compositional data analysis, Nizampur basin, Pakistan. Groundw Sustain Dev 23:101021
USEPA (2004) Risk Assessment Guidance for Superfund, Vol. 1, Human Health Evaluation Manual. Part E (supplemental guidance for dermal risk assessment), EPA/540/R/99/005. Office of Superfund Remediation and Technology Innovation, Washington, DC, USA. https://www.epa.gov/sites/default/files/2015-09/documents/part_e_final_revision_10-03-07.pdf
Ustaoğlu F, Aydın H (2020) Health risk assessment of dissolved heavy metals in surface water in a subtropical rivers basin system of Giresun (North-Eastern Turkey). Desalin Water Treat 194:222–234. https://doi.org/10.5004/dwt.2020.25900
Ustaoğlu F, Tepe Y, Aydin H, Akbas A (2020a) Evaluation of surface water quality by multivariate statistical analyses and WQI: case of comlekci stream,(Giresun-Turkey). Fresenius Environ Bull 29(01):167–177
Ustaoğlu T, Tepe Y, Taş B (2020b) Assessment of stream quality and health risk in a subtropical Turkey river system: A combined approach using statistical analysis and water quality index. Ecol Ind 113:105815. https://doi.org/10.1016/j.ecolind.2019.105815
Ustaoğlu F, Taş B, Tepe Y, Topaldemir H (2021) Comprehensive assessment of water quality and associated health risk by using physicochemical quality indices and multivariate analysis in Terme River, Turkey. Environ Sci Pollut Res Int 28(44):62736–62754. https://doi.org/10.1007/s11356-021-15135-3
Ustaoğlu F, Yüksel B, Tepe Y, Aydın H, Topaldemir H (2024) Metal pollution assessment in the surface sediments of a river system in Türkiye: Integrating toxicological risk assessment and source identification. Mar Pollut Bull 203:116514. https://doi.org/10.1016/j.marpolbul.2024.116514
Varol S, Davraz A (2015) Evaluation of the groundwater quality with WQI (Water Quality Index) and multivariate analysis: a case study of the Tefenni plain (Burdur/Turkey). Environ Earth Sci 73:1725–1744. https://doi.org/10.1007/s12665-014-3531-z
Varol M, Tokatli C (2021) Impact of paddy fields on water quality of Gala Lake (Turkey): An important migratory bird stopover habitat. Environ Pollut 287:117640. https://doi.org/10.1016/j.envpol.2021.117640
Varol M, Tokatlı C (2022) Seasonal variations of toxic metal (loid) s in groundwater collected from an intensive agricultural area in northwestern Turkey and associated health risk assessment. Environ Res 204:111922
Varol M, Tokatli C (2023) Evaluation of the water quality of a highly polluted stream with water quality indices and health risk assessment methods. Chemosphere 311:137096. https://doi.org/10.1016/j.chemosphere.2022.137096
Wang J, Liu G, Liu H, Lamc P (2017) Multivariate statistical evaluation of dissolved trace elements and a water quality assessment in the middle reaches of Huaihe River, Anhui, China. Sci Total Environ 583:421–431. https://doi.org/10.1016/j.scitotenv.2017.01.088
Wang J, Zhou W, Zhao M, Guo X (2023) Water quality assessment and pollution evaluation of surface water sources: The case of Weishan and Luoma Lakes, Xuzhou, Jiangsu Province, China. Environ Technol Innov 32:103397. https://doi.org/10.1016/j.eti.2023.103397
Wang Z, Zhang Z, Adimalla N, Guo H, Pei J, Liu H (2024) Spatial distribution and enrichment mechanisms of high fluoride groundwater in geotherm-affected Pliocene aquifers of the Guide basin, China. J Geochem Explor 256:107356
WHO (2011) Guidelines for Drinking-Water Quality, 4th ed., Vol. 38, World Health Organization, pp 1–564, Geneva. https://doi.org/10.1590/S1413-41522011000400002
World Water Quality Alliance (2021) Assessing groundwater quality: a global perspective: importance, methods and potential data sources. a report by the friends of groundwater in the world water quality alliance. information document annex for display at the 5th session of the united nations environment assembly, Nairobi 2021. https://www.unep.org/explore-topics/water/what-we-do/improving-and-assessing-world-water-quality-partnership-effort?gad_source=1&gclid=Cj0KCQjwq_G1BhCSARIsACc7Nxr1kN4QdxMle7lULD623k6tzWW9gWFOmN88vPASEpFzAml_DIo8WKkaAvr8EALw_wcB
Xiao J, Wang L, Deng L, Jin Z (2019) Characteristics, sources, water quality and health risk assessment of trace elements in river water and well water in the Chinese loess plateau. Sci Total Environ Part 2(650):2004–2012. https://doi.org/10.1016/j.scitotenv.2018.09.322
Yang C, Jiang X, Ma L, Xiao D, Liu X, Ying Z, Li Y, Lin Y (2023) Transcriptomic and metabolomic profiles provide insights into the red-stipe symptom of morel fruiting bodies. J Fungi 9(3):373. https://doi.org/10.3390/jof9030373
Yüksel B, Arica E (2018) Assessment of toxic, essential, and other metal levels by icp-ms in Lake Eymir and Mogan in Ankara, Turkey: an environmental application. At Spectrosc 39(5):179–184. https://doi.org/10.46770/AS.2018.05.001
Yüksel B, Mergen G, Söylemezoglu T (2010) Assessment of arsenic levels in human hair by hydride generation atomic absorption spectrometry: a toxicological application. Spectroscopy 31:1–5
Yüksel B, Kayaalti Z, Söylemezoglu T, Türksoy VA, Tutkun E (2015) GAAS determination of arsenic levels in biological samples of workers occuputionally exposed to metals: an application in analytical toxicology. Spectrosc 36:171–176. https://doi.org/10.46770/AS.2015.04.004
Yüksel B, Şen N, Ali Türksoy V, Tutkun E, Söylemezoğlu T (2018) Effect of exposure time and smoking habit on arsenic levels in biological samples of metal workers in comparison with controls. Marmara Pharm J 22(2):218–226. https://doi.org/10.12991/mpj.2018.59
Yüksel B, Ustaoglu F, Arica E (2021) Impacts of a garbage disposal facility on the water quality of Çavuşlu stream in Giresun, Turkey: A health risk assessment study by a validated ICP-MS assay. Aquat Sci Eng 36(4):181–192
Yüksel B, Ustaoğlu F, Yazman MM, Şeker ME, Öncü T (2023) Exposure to potentially toxic elements through ingestion of canned non-alcoholic drinks sold in Istanbul, Türkiye: A health risk assessment study. J Food Compos Anal 121:105361. https://doi.org/10.1016/j.jfca.2023.105361
Yüksel B, Ustaoğlu F, Aydın H, Tokatlı C, Topaldemir H, Islam MS, Muhammad S (2024) Appraisal of metallic accumulation in the surface sediment of a fish breeding dam in Türkiye: A stochastical approach to ecotoxicological risk assessment. Mar Pollut Bull 203:116488. https://doi.org/10.1016/j.marpolbul.2024.116488
Zaman M, Shahid SA, Heng L (2018) Irrigation Water Quality. In: Zaman M, Shahid SA, Heng L (eds) Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques. Springer, Cham. https://doi.org/10.1007/978-3-319-96190-3_5
Zeng X, Liu Y, You S, Zeng G, Tan X, Hu X, Huang L, Li F (2015) Spatial distribution, health risk assessment and statistical source identification of the trace elements in surface water from the Xiangjiang River, China. Environ Sci Pollut Res 22:9400–9412. https://doi.org/10.1007/s11356-014-4064-4
Acknowledgements
The corresponding author, Dr. Bayram Yüksel, dedicates this work to his late father, Süleyman Yüksel (1960–2023). Throughout his life, Süleyman Yüksel provided unwavering support, guidance, and encouragement, playing an instrumental role in shaping Dr. Yüksel's academic journey and personal development. His father’s dedication and belief in the value of education were a constant source of inspiration, motivating Dr. Yüksel to pursue his goals with determination and perseverance. Without his father’s support and influence, Dr. Yüksel's achievements and the completion of this work would not have been possible. This dedication is a heartfelt tribute to a man whose legacy continues to inspire and drive excellence.
Funding
This research project has been financially supported by Giresun University Scientific Research Office under award number FEN-BAP-A-290224–43.
Author information
Authors and Affiliations
Contributions
F.U. and H.T. conceived of the presented idea. B.Y. and M.M.Y. collected the samples. F.U. and M.M.Y. developed the theory and performed the computations. M.M.Y. and N.S. verified the analytical methods. Y.T. encouraged F.U. and C.T- to investigate the ecological indicators and supervised the findings of this work. B.Y. prepared the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Declarations
The authors of this document attest that its contents have been thoroughly reviewed and approved. Furthermore, they assert that the evaluation and endorsement process did not feature any additional individuals who meet the criteria for authorship but are not explicitly identified. Furthermore, this confirms that the arrangement of authors in the document has been approved unanimously by all relevant parties.
Ethical approval
The research does not require ethical committee approval, participant assent, or publication consent.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Completing of interests
The authors declare that no actual, concealed, or perceived conflicts of interest exist in relation to the present manuscript.
Additional information
Responsible Editor: Xianliang Yi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yazman, M.M., Yüksel, B., Ustaoğlu, F. et al. Investigation of groundwater quality in the Southern Coast of the Black Sea: application of computational health risk assessment in Giresun, Türkiye. Environ Sci Pollut Res 31, 52306–52325 (2024). https://doi.org/10.1007/s11356-024-34712-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34712-w