Abstract
Mariout Lake is one of the Northern Nile-Delta Lakes in Egypt that receives agricultural, industrial and domestic effluents through several drains. The present study aims to evaluate the levels of some heavy metals (HMs) in water and edible parts of Oreochromis niloticus in Mariout Lake, in addition to studying several pollution indices and potential human health risks. The levels of the studied HMs in water were in the order of Fe > Zn > Mn > Pb > Cu > Ni > Cd. However, results of the pollution index, that concerns the effect of individual metal, concluded that Cd and Pb in water had serious pollution effects for aquatic life, while Cu, Fe, Mn, Ni, and Zn had not any pollution effects at different locations in the lake. The indices of the composite effects of all HMs (Metal Index and Heavy Metal Pollution Index) indicated the high pollution of Mariout Lake water, which may cause adverse effects on fish and different aquatic organisms. On the other side, the bioaccumulation factors of HMs in edible parts of O. niloticus were in the order of Zn > Cd > Cu > Ni > Pb > Mn > Fe. Although the target hazard quotient for all metals was less than the non-hazardous limit (THQ < 1), the non-carcinogenic hazard index (HI = 1.24) was classified in the moderate hazard risk level (1 < HI < 10) indicating low potential adverse effects on the exposed population due to consumption of O. niloticus caught from Mariout Lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potentially toxic element (PTE) pollution in the aquatic environment has become a global challenge in the twenty-first century. This is due to their abundance, persistence, inherent toxicity, non-degradability, and ubiquity in aquatic systems. Heavy metals are present in the environment in different phases: solid phase, liquid phase as free ions, or absorbed as solid colloidal particles. They may enter the aquatic ecosystem through natural and anthropogenic processes, including atmospheric deposition, erosion of the geological matrix, storm runoff, leaching from landfills, mining, shipping and harbor activities, and domestic, industrial, and agricultural runoff (Panico et al. 2018; Abd El-Aal et al. 2020; Bashir et al. 2020; Napoletano et al. 2021; Sánchez et al. 2022), causing worsening of the aquatic environment (Hamed et al. 2020). Intensive research on heavy metals in the aquatic and terrestrial environments has become important due to concerns regarding their overaccumulation and toxic effects on organisms and, eventually, on humans through the food chain (Volpe et al. 2015; Memoli et al. 2017; Kortei et al. 2020).
Heavy metal pollution endangers several aquatic species of fish, and continuous exposure to such toxic metals makes and forces fishes to take them directly from the environment through their blood and thus through contact with their organs and tissues (Goher et al. 2018). This causes physiological, morphological, and behavioral abnormalities alongside reproductive impairment in fish (Bristy et al. 2021). Consequently, there is a concomitant increase in human health risk through the consumption of fish contaminated with heavy metals. The long-term intake of heavy metals through foodstuffs, such as fish, may induce chronic accumulation, thereby damaging the human body (Talab et al. 2016; Adegbola et al. 2021).
The severity of toxic metals in humans has been known for ages, and exposure to these metals is continuously increasing in many areas, such as: Manzala Lake and Abou-Quir Bay. The toxicity of some heavy metals can be acute, while others can be chronic after long-term exposure. Heavy metals negatively affect humans, even at low concentrations, as they can cause abnormal development of a fetus; procreation failure; immune deficiency; carcinoma; organ dysfunction; physical, mental, and neurological disorders; renal tumor; nephritis; osteoporosis; nasopharyngeal congestion; increased blood pressure associated with cardiovascular diseases; reduced life expectancy; and, in some cases, death (Hamadani et al. 2020; Mohanta et al. 2020; Sánchez et al. 2022).
The most studied lagoons in the Mediterranean are located in Albania, Algeria, Egypt, France, Greece, Italy, Montenegro, Morocco, Spain, Tunisia, and Turkey. They provide ecosystem services that are essential for humans in many ecological, cultural, and medical uses. They also contribute to the overall productivity of coastal waters by supporting a variety of habitats and to the reduction in poverty by providing natural resources. Their stressors include overexploitation, pollution, climate changes, and biological invasions, often co-occurring in time and space and having cumulative effects. Such ecosystem changes can have large consequences on species abundance, bio-distributions, as well ecosystem functioning and services. Hence, it is of primary importance to study and protect lagoons (Parisi et al. 2022).
Mariout Lake is one of the five coastal northern lakes in Egypt. It is a brackish water body in the northern part of Egypt, between the Alexandria and El-Beheira governorates. It represents the southern boundary of Alexandria City and is located in the northwestern corner of the Egyptian Nile Delta. It is the smallest of the northern Delta lakes but perhaps the most threatened. Mariout Lake has been in existence for over 6000 years. The current lake is only a small remnant of its precursor, Lake Mareotis (the lake’s name in ancient times). During that period, the lake covered a large area south of Alexandria, extending over 40 km southeast and 70 km southwest along the Mediterranean coast. The maximum width of the lake was estimated to be 24.5 km, while its length was about 44.5 km. The lake had eight islands. Four of them disappeared, while the remaining four became peninsulas. There is no evidence in history that the lake had any connection with the sea, but it was always a freshwater lake, getting its water from River Nile through various branches stemming from the Canopy branch, which ends at the Mediterranean Sea at the old Canoup City (now known as Abou-Quir) (Shehata 2014). Fishing is a major activity in the lake that was characterized in the past by large fish harvest rates with reputed quality. Lake fishes are essential to the well-being and livelihood of about 7000 fishers and their families. Until the mid-1970s, Mariout Lake was highly productive, contributing no less than 75% of the national fish catch in the Alexandria area. In 1974, the fish catch attained its peak level of 17,000 t. Since the beginning of the 1980s, fish production has progressively decreased, reaching 5000 t in 2007. The simultaneous reclamation of great areas of Mariout Lake, excessive fishing pressure, and wastewater discharged into the lake have dramatically affected fish production (Omran and Negm 2017). However, there was a fluctuation in fish production of Mariout Lake during the last period, recording 12,301 t, 8058 t, and 15,510 t in 2015, 2017, and 2020, respectively (GAFRD 2020).
This study aims (a) to investigate the heavy metal (Fe, Mn, Zn, Cu, Ni, Pb, and Cd) distributions in water and muscles of the Tilapia species (Oreochromis niloticus) from Mariout Lake; (b) To track the long-term changes of the HMs levels over previous periods (c) to appraise the extent of direct and indirect pollution effects on the lake’s environmental situation due to the disposal of domestic, industrial, and agricultural effluents into the lake through the drains; (e) to determine the bioaccumulation levels of heavy metals in Oreochromis niloticus muscles; and (f) to assess the human health risks due to consumption of the edible parts of the Tilapia species (O. niloticus) caught from Mariout Lake.
Materials and methods
Study region
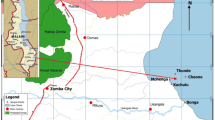
Mariout Lake lies between Latitude 31° 04′ 47′′ N and 31o 09′ 48′′ N, and Longitude 29° 51′ 40′′ E and 29° 55′ 14′′ E along the Mediterranean Sea coast of Egypt, as shown in Table 1 and Fig. 1. Its area secured 200km2 at the beginning of the twentieth century, but at the beginning of the twenty-first, it was only about 50km2. Mariout Lake has been decreased by more than 75% and is still shrinking. It has a very shallow bottom reaching 150 cm at its deepest location and a depth of 20 cm at shore locations around the lake periphery. It is a closed lake having no direct connection to the Mediterranean Sea. It is separated from the sea (by a 20 m) by the Desert Road, El-Nubaria Canal and El-Umoum Drain. Moreover, during February 2003, the remaining opened disposal sites to the beaches of Alexandria were locked and converted to Mariout Lake (Baraka 2012; Shehata 2014; Donia 2016; El-Kafrawy et al. 2017; Alnagaawy et al. 2018).
The lake is currently divided into four basins, as shown in Fig. 1, that are dissected by railroads, embankments and highways connecting Alexandria with the desert areas around it and with Cairo. The four basins are interconnected with each other by several breaches in the dykes of El-Umoum Drain and El-Nubaria Canal (EEAA 2011; Khalil et al. 2013; El-Naggar and Rifaat 2017). The four basins are:
-
1.
The Main (6000 Feddans) Basin: It is the northernmost water body and the largest basin in Mariout Lake as it covers an area of about 25km2 with an average water depth of 1.2 m (El-Shorbagi 2015; Afifi et al. 2016). It receives most of its water from the most heavily polluted drains (El-Qalaa and El-Umoum Drains) (Khalil et al. 2013).
-
2.
The Fisheries or Aquacultural (1000 Feddans) Basin: It lies south of the Main Basin. It is bordered by fish farms in the north and agricultural lands in the east. It covers an area of about 9.44km2 (849 Feddans) and its average water depth is 1.35 m. It also receives its water from El-Umoum and El-Qalaa Drains (Afifi et al. 2016).
-
3.
The North-West (3000 Feddans) Basin: It is a triangular lagoon located in the west of El-Umoum Drain. Its area is about 11.59km2 with an average water depth of 0.7 m. It receives its water through El-Nubaria Canal and El-Max pumping site that is located at the northern side of the basin (El-Naggar and Rifaat 2017).
-
4.
The South-West (5000 Feddans) Basin: It is bordered by agricultural lands in the east, and petroleum companies and human settlements at the west. It is partially divided by El‐Nubaria Canal. This basin is very shallow with an average water depth of 0.68 m and a surface area of 33.77km2. The main source of water is El-Omoum Drain at the northern region and El-Nubaria Canal at the middle region (EEAA 2011; Donia 2016).
Mariout Lake inflows and outflows
Mariout Lake receives daily a large amount of different pollutants from Alexandria City. The lake receives water from many drains and canals. (a) El-Qalaa Drain, which receives effluents from raw wastewater, irrigation drainage, agricultural runoff, and domestic and industrial waste from the eastern sector of Alexandria. Its water was thrown into the lake on the south-eastern side of the Main Basin. Now, it runs parallel to the southern border of the Aquacultural Basin and connects to El-Umoum Drain. (b) El-Umoum Drain, which represents the main water source for the Main Basin (Shehata 2014; Saad et al. 2017). (c) Petroleum Drain, which receives untreated industrial wastewater from the estimated 775 industries around the Mariout Lake area (Silim 2015; El-Naggar and Rifaat 2017). (d) El-Nubaria Canal, which lines the western side of the Main Basin and may be considered as fresh water source for the lake. (e) East Water Treatment Plant (EWTP), which discharges its treated effluents (90% domestic sewage, and only 10% industrial effluents) into El-Qalaa Drain. (f) West Water Treatment Plant (WWTP), which discharges its treated effluents (66% industrial effluents and 34% domestic sewage) directly into the Main Basin. Mariout Lake is not directly connected to the Mediterranean Sea. The only outflow from Mariout Lake is the El-Max pumping site. It comprises two buildings, each housing six pumps with nominal capacities of 12.5m3/s (Silim 2015). The fact that domestic, municipal, agricultural, and industrial wastes are continuously discharged into the lake makes El-Max pumping site essential to maintain the lake water level about 2.8–3.0 m below the mean sea level (Khalil et al. 2013; El-Khatib et al. 2020).
Sampling protocol
Subsurface water samples were collected seasonally from 13 selected sites in Mariout Lake in addition to El-Nubaria Canal and three drains (El-Qalaa Drain, El-Umoum Drain, and Petroleum Drain) during spring 2017–winter 2018, using a polyvinyl chloride Van-Dorn bottle with a capacity of 2L. Triplicate subsamples were collected from each site. The samples were kept in cleaned stopper plastic bottles of 1L capacity to analyze later in the laboratory. For heavy metals analyses, water samples were preserved with conc. HNO3 to reduce pH to be below 2 to stop bacterial growth, block oxidation reactions and prevent precipitation of the metals. The bottles were then stored in a refrigerator at 4°C to prevent change in volume due to evaporation.
Fish samples of Tilapia species (Oreochromis niloticus), a common and a famous fish species in Egypt; its native name is Bolti, were collected from the sites 2, 3, 6, 7, 10 and 11 during summer 2017 and winter 2018. The collected fish samples (10 from each site) nearly had the same size, length and weight. The samples were quickly transported into the laboratory for dissection and subsequent analyses. Fish caught from the selected sites were externally dried from water, then lateral white muscles were quickly and carefully removed, and kept frozen at −20°C for determination of heavy metals concentrations. Geographic positions of the samples were appointed using the GPS device (Garmin, ETrex model). The sampling locations along with their longitudes and latitudes are presented in Table 1.
Methodology
Physicochemical parameters in water
Temperature, pH and dissolved oxygen (DO) content of water samples collected from Mariout Lake were measured in-situ using Thermo Orion Star (A 329 multiparameter instrument). Water transparency was also measured in situ using a white/black Secchi disk (30 cm in diameter). However, total dissolved solids (TDS) and total suspended solids (TSS) in lake water were determined in the laboratory using the evaporation method where TDS were determined by filtering a volume of a sample with a glass microfiber filter (GF/C), and a known volume of the filtrate was evaporated at 180°C. TSS were calculated as the difference between TS (total solids) and TDS, where TS were measured by evaporating a known volume of a well-mixed sample (APHA 2005).
Heavy metals in water and fish
All glassware used were rinsed and washed with diluted HCl before usage. Merck analytical grades (USA) of all reagents were used to prepare standards for ICP instrument calibration under satisfying clean laboratory environment. A 1000 g/L multi-element certified standard solution was used as a stock solution for the instrument standardization. Standard reference materials, from National Institute of Standards and Technology (NIST), were used to validate analysis. Accuracy and precision of analysis was checked by replicate measurements of these reference materials, with recovery rates for metals between 97.1 and 103.8% which were within the acceptable recovery percentage range (80–110%) according to Huber (2007).
Water samples were digested using the Nitric Acid Digestion Method according to APHA (2005) as the following:
-
Put a well-mixed 250-ml water sample in a beaker on a hot plate and add 5 ml of conc. HNO3, then slow boil and evaporate till the lowest volume possible (50 ml) but before precipitation occurs. The digested solutions were cooled to room temperature.
-
Transfer to a 50-ml volumetric flask and dilute to the mark with deionized water if needed. Mix thoroughly and filter if necessary. The total concentrations of heavy metals in water were calculated according to the following equation.
$$ {\text{Metal}} \;{\text{Concentration}} \left( {{\mu g}/{\text{L}}} \right) = \frac{A \times B}{C} $$
where A is the total concentration of a metal in the digested solution (μg/l), B is the final volume of the digested solution (ml), and C is the initial water sample volume (ml).
Fish muscles were digested according to FAO/SIDA (1983) as in the following:
-
The fish flesh of Tilapia species (O. niloticus) collected from the same site were mixed together. A 10 ml portion of a freshly prepared mixture of nitric and perchloric acids (HNO3: HClO3 = 1: 1) was added to 5 g of muscles from each mixture in tightly closed Teflon vessels.
-
Closed vessels holder was placed at 160°C in a microwave oven (model Milestone, MLS-1200 mega, Germany) until the organic matter was broken down which was observed through the reduced volume and clarity of the solution. After the digestion process, the vessels were cooled and carefully opened.
-
Solutions were transferred to a 50-ml volumetric flask and diluted to the mark with deionized water, then filtered and transferred to plastic bottles. The concentrations of heavy metals in fish muscles were calculated according to the following equation:
$$ {\text{Metal}} \;{\text{Concentration}} \left( {{\mu g}/{\text{g }}\;{\text{wet}}\;{\text{wt}}.} \right) = \frac{A \times B}{{C \times 10^{3} }} $$
where A is the metal concentration in the digested solution (μg/l), B is the final volume of the digested solution (ml), C is the weight of fish muscle sample (g), and 103 is the unit conversion factor.
The total concentrations of the heavy metals (Fe, Mn, Zn, Cu, Ni, Pb, and Cd) in digested water and O. niloticus muscles samples collected from Mariout Lake were measured using Inductively Coupled Plasma Emission Spectrometry (ICP-ES) with Ultra Sonic Nebulizer (USN), model Perkin Elmer optima 7000, USA.
Blanks were prepared in each digestion procedure. Water and fish samples were analyzed in three replicates with relative standard deviations (RSDs) less than 10% of the trace elements. Concentrations below the detection limits were substituted with the reported detection limit for all non-detects, if concentrations in respective replicates of the sample were detected at least once above the detection limits. Wavelengths (nm) and detection limits (µg/L) of ICP-ES are 259.933, 257.604, 213.855, 324.747, 231.602, 220.35 and 226.499 nm, and 0.3, 0.04, 0.2, 0.3, 0.5, 1.5 and 0.1 µg/l for Fe, Mn, Zn, Cu, Ni, Pb and Cd, respectively.
Statistics
The obtained results were analyzed using the Excel-Stat 2016 software for determination of the spatial and temporal variations by analysis of variance using the one-way ANOVA test (α = 0.05) which was also used for determination of the probability values (P-values); significance levels of tests were taken as significant when P ≤ 0.05 and highly significant when P ≤ 0.01. In addition, the relationships between the studied heavy metals were determined by calculating the Spearman correlation matrix using Minitab 20 software (confidence level = 95). Spearman correlation was specified after application of the normality test (Ryan-Joiner) on our results using the Minitab 20 software. Based on the correlation coefficient values (r) and number of samples (n), highly significant correlations were indicated when r ≥ 0.354 and significant correlations when 0.273 ≤ r < 0.354. Also, Microsoft Excel was used to design some of the figures in this study.
Metal quality indices (MQI) in water
Three different quality indices were used to determine the grades of metals contamination in Mariout Lake water for aquatic life utilizations. These indices are Pollution Index (PI), Metal Index (MI), and Heavy Metal Pollution Index (HPI).
Pollution index (PI)
IT is based on the individual metal concentration which means that each metal has its own PI value. It is categorized into 5 classes, as shown in Table S1. PI was calculated using the equation according to Caerio et al. (2005).
where Ci is the concentration of each heavy metal and Si is the metal level according to the national water quality criterion.
Metal index (MI)
IT is based on a total trend evaluation of the present status and by that summarizes the status of the study area by one value. The higher the concentration of a metal compared to its respective MAC value, the worse the quality of water. MI value > 1 is a threshold of warning (Bakan et al. 2010). MI was calculated by the equation according to Tamasi and Cini (2004).
where Ci is the concentration of each heavy metal, n is the number of measured heavy metals, and MAC is the maximum allowable concentration of the ith metal.
Heavy metal pollution index (HPI)
IT is a comprehensive tool or a rating model that assesses the overall water quality according to the composite effects of individual heavy metal. It is based on the weighted arithmetic quality mean method (Hassouna et al. 2019) and can be calculated from the following equation:
where Qi is the sub-index of the ith metal, n is the number of measured heavy metals, and Wi is the weight unit of the ith metal (between 0 and 1).
Wi and Qi were calculated according to the following equations:
where K is the proportionality constant, Ci is the concentration value of the ith metal, Si is the standard permissible value of the ith metal for aquatic life, the sign (−) indicates the numerical differences between the two values ignoring the algebraic sign, and Ii is the ideal value of the ith metal; in pure water, Ii = 0 and the previous equation converts to the following equation:
Finally, the critical heavy metal pollution index (HPI) score for the water suitability to aquatic life is 100 (Milivojević et al. 2016).
Bioaccumulation factor (BAF) estimation
Bioaccumulation is the process that causes an increase in chemical concentrations in aquatic organisms compared to those in water due to uptake by all the exposure routes, including dietary absorption, transport across the respiratory surfaces, and dermal absorption. Thus, it can be viewed as a combination of bio-concentration and food uptake. It is a number that describes the bioaccumulation as a ratio of the concentration of a chemical inside an organism to its concentration in the surrounding environment (Goher et al. 2015). The bioaccumulation factor (BAF) of a heavy metal in fish tissues was determined using the equation according to El-Khatib et al. (2020).
where Mtissue is the metal concentration in fish tissues (μg/g wet weight) and Mwater is the metal concentration in water (µg/l).
According to Olayinka-Olagunju et al. (2021), BAFs values of heavy metals are categorized as follows: BAF < 1000 means no probability of accumulation; 1000 < BAF < 5000 means bioaccumulative metal and BAF > 5000 means extremely bioaccumulative metal.
Human health risk assessment of fish consumption
The human health risks associated with the intake of Tilapia species (O. niloticus) from Mariout Lake by approximately one million people living in cities and villages around it were evaluated using Estimated Daily Intake (EDI), Target Hazard Quotient (THQ) and Hazard Index (HI) developed by the United States Environmental Protection Agency (USEPA 2013).
Estimated daily intake (EDI)
IT is a commonly used method that helps identify the number of pollutants consumed daily. The EDI of potentially toxic elements (PTEs) is directly proportional to the concentrations of PTEs in food and the amount of daily food consumption. Furthermore, human body weight significantly affects the tolerance of contaminants. EDI of heavy metals (mg/day/70 kg Egyptian person) was calculated by multiplying the overall average level of the heavy metals concentrations detected in the Tilapia species (O. niloticus) by the average daily consumable rate of the fish muscles for the general adult consumers (Ullah et al. 2017). It was determined using the following formula (Bristy et al. 2021):
Target hazard quotient (THQ)
IT is a ratio of the estimated exposure dose of a pollutant to the reference dose. It represents the risk of non-carcinogenic effects (Kortei et al. 2020). It must be noted that THQ is not a measure of risk, but indicates a level of concern. It was calculated for the individual heavy metal using the following equation:
where MC is the heavy metal concentration (mg/kg) in the fish flesh in wet weight basis, IR is the fish ingestion rate by a person per day, the per capita consumption of fish flesh in Egypt for human food is averaged to be 57 g/day for general population (FAO 2014), EF is the exposure frequency (365 days/year), ED is the life time exposure duration (70 years), RfD is the oral reference dose of individual heavy metal (mg/kg/day), as reported by USEPA (2013), BW is the average adult body weight of a consumer in Egypt (70 kg), as a default value suggested by USEPA (2013), ATn is the average exposure time for non-carcinogens (365 days/year × number of exposure years), and 10–3 is the unit conversion factor. It was considered that the fillet yield for Tilapia species (O. niloticus) is within 39.1% (Neira et al. 2016).
THQ < 1 means that adverse health effects are unlikely. THQ > 1 reveals probable adverse health effects. THQ > 10 indicates high chronic risks (Custodio et al. 2021). Although the THQ values are additive, they are not multiplicative; e.g., the level of concern at THQ of 20 is larger but not tenfold of that at THQ = 2 (Osakwe et al. 2014).
Hazard index (HI)
The overall potential for non-carcinogenic effects from all studied metals was assessed using the hazard index (HI). It was denoted as the sum of the target hazard quotient (THQ) values of all the tested heavy metals and was obtained using the equation according to USEPA (2011).
where i is the individual heavy metal.
If HI < 1, it is assumed that the non-carcinogenic adverse effects due to the fish consumption are negligible, while the potential for chronic effects may be a concern when HI > 1 (Custodio et al. 2021). Hence, HI < 1 means no hazard; 1 < HI < 10 means moderate hazard, while HI > 10 means high chronic hazard (Ukoha et al. 2014). Like THQ, HI does not measure the risk directly as it cannot define the dose–response relationship (Bristy et al. 2021).
Results and discussion
General physicochemical features of Mariout Lake water
The ranges and means of temperature, transparency, TDS, TSS, pH, and DO of Mariout Lake water during 2017–2018 are presented in Table 2. Temperature ranged between 15.6°C at site (1) during winter and 31.4°C at site (13) during summer season with highly temporal significant differences (p ≤ 0.01). Mariout Lake is characterized by water turbidity due to the deterioration effects of El-Qalaa and El-Umoum Drains. Transparency fluctuated between 15 cm at site (8) in spring and 65 cm in summer at site (11) with highly temporal significant differences (p ≤ 0.01) and significant differences (p ≤ 0.05) among sites. However, the maximum TDS value (9.68 g/L) was observed in winter at site (6), but the minimum value (2.78 g/L) was at site (8) during summer season with highly spatial significant differences (p ≤ 0.01). Conversely, the highest TSS value (148.62 mg/L) was recorded at site (8) during spring, but the lowest value (35.18 mg/L) was at site (6) in summer season with highly temporal significant differences (p ≤ 0.01) and spatial significant differences (p ≤ 0.05). TSS levels were higher than the permissible limits for aquatic organisms during all seasons and sites. On the other hand, Mariout Lake water is alkaline, whereas pH values fluctuated between 7.22 in summer at site (2) and 8.68 at site (12) in spring with highly significant differences (p ≤ 0.01) among seasons; the values were within the permissible levels for aquatic life (Elsayed et al. 2019). pH was positively correlated with DO (r = 0.3, p ≤ 0.05) which may be related to the photosynthetic action (Imam et al. 2020). Our results showed that DO ranged from 0.5 mg/L during spring to 9.14 mg/L during winter at sites (8) and (4), respectively, with significant differences (p ≤ 0.05) between seasons and sites.
Heavy metals in Mariout Lake water
The occurrence of metal contaminants in Mariout Lake water has become an increasing concern. This situation has arisen due to the rapid growth of the population and, thus, the increase in human activity (Silim 2015). The concentrations of the studied heavy metals in Mariout Lake water were found in the ranges of 266.50–1145.40, 18.60–103.20, 18.00–130.40, 5.00–23.23, 5.60–19.62, 10.55–59.51, and 3.30–9.90 µg/l for Fe, Mn, Zn, Cu, Ni, Pb, and Cd, respectively, over the study year. Generally, the annual average contents were in the order of Fe > Zn > Mn > Pb > Cu > Ni > Cd with respective values of 682.70 ± 248.87, 60.42 ± 31.55, 58.91 ± 22.42, 33.55 ± 11.47, 12.40 ± 4.37, 12.09 ± 3.6, and 6.35 ± 1.39 µg/l, respectively, as shown in Table 3. ANOVA results indicated that there were highly significant spatial differences (p ≤ 0.01) for Fe and Mn concentrations in Mariout Lake water. However, Pb and Cd concentrations showed highly temporal significant differences (p ≤ 0.01), while Cu concentrations showed significant differences between seasons (p ≤ 0.05). Generally, the seasonal variations in heavy metal content may be attributed to the fluctuation of the amounts of agricultural drainage water, industrial wastes, and illegal untreated domestic sewage discharged into the lake. Domestic effluents can contain fairly high concentrations of metals, such as Fe, Zn, Cu, Ni, and Pb, which are derived from various household products such as cleaning materials, toothpaste, and cosmetics (Goher et al. 2019a).
The maximum concentrations of all heavy metals in Mariout Lake water were observed in the Main Basin (sites 8 and 9) due to the effects of domestic, agricultural, and industrial effluents from El-Qalaa Drain, El-Umoum Drain, and the WWTP located in front of the site (8). These data agreed with those obtained by El-Rayis and Saad (1990) and Ali et al. (2020); they concluded that heavy metals increased in the Main Basin more than in the other three basins. However, the minimum concentration values of Fe, Mn, Cu, and Pb were recorded in the North-West Basin (sites 12 and 13). The lowest value of Ni concentrations (5.60 μg/l) was found at site (1) in the South-West Basin, and that of Zn concentrations (18.00 μg/l) was determined at site (5) in the Aquacultural Basin.
The distribution patterns of iron and manganese concentrations in Mariout Lake water increased during the cold rainy seasons (autumn and winter). Generally, Fe concentrations in Mariout Lake water increase in the rainy seasons due to water flowing from nearby shores carrying soil along with it, as the earth’s crust contains abundant amounts of metals (Vasistha and Ganguly 2020). However, increasing Mn values in lake water are mainly attributed to the decomposition of organic debris by microbial activity (Ali et al. 2020). Conversely, the highest concentrations of Zn, Ni, Pb, and Cd were observed in the dry hot seasons, which may be attributed to metal liberation from bottom sediment into the overlying water column under the effects of high temperatures and the fermentation process (Tafa and Assefa 2014; Abd El-Aal et al. 2020).
Table 3 illustrates that the concentrations of Cu, Pb, and Cd in Mariout Lake water exceeded the maximum permissible limits of the USEPA (2021) and CCME (2021) guidelines for aquatic life, but Mn and Ni concentrations were below the standard permissible limits of the USEPA (2021) and CCME (2021) guidelines. However, following the USEPA (2021) guidelines, the concentrations of Fe and Zn in lake water were below the permissible levels (1000 and 120) µg/l, respectively, but exceeded the permissible standards for aquatic life of CCME (2021) guidelines, which are (300 and 7) µg/l, respectively. This indicates the poor water quality of Mariout Lake, especially in the sites facing the outlets of the drains, and this may cause negative human health effects (Ghannam 2021).
The statistical analysis of the Spearman correlation coefficient matrix in Table 4 showed that the positive significant correlations among the metals Fe/Mn (r = 0.396, p ≤ 0.01) and Fe/Cu (r = 0.333, p ≤ 0.05) suggest that there are associations between these metals as they have similar distribution dynamics and mutual dependence as well as they originate from a common source in the aquatic ecosystem during transportation and/or the deposition reactions (Masoud et al. 2005; Ali et al. 2020). The chemical behavior of nickel as a strong chelating transition element is clear from its strong correlation with other metals, such as iron [Ni/Fe (r = 0.329, p ≤ 0.05)], due to its tendency to form more stable organic complexes (Wangersky 1986).
Generally, heavy metals have high ecological significance because they persist in the environment and can remain for a very long time; they can potentially accumulate in water reservoirs and thus enter the food chain. Under certain environmental conditions, heavy metals may accumulate and concentrate in living organisms in highly toxic concentrations and cause serious harm and ecological damage (Bashir et al. 2020; Sánchez et al. 2022).
Some PTEs, such as Cu, Zn, Fe, Ni, and Mn, benefit humans at low concentrations and play a biochemical role in the life processes of all aquatic organisms but become toxic in unregulated quantities. However, the intake of some others, such as Cd and Pb, is highly poisonous to humans, even at quite low concentrations (Ghannam 2021; Sánchez et al. 2022).
Heavy metals in Mariout Lake inflows
The seasonal variations of heavy metal concentrations in Mariout Lake inflows are graphically represented in Fig. 2. The concentrations of the studied metals in the lake drains were found in the ranges of 271.00–1970.60, 23.00–97.40, 46.12–97.20, 5.40–32.00, 4.80–25.60, ND–56.93, and 9.40–17.60 µg/l for Fe, Mn, Zn, Cu, Ni, Pb, and Cd, respectively, over the study year. The annual average contents were in the order of Fe > Zn > Mn > Pb > Cu > Ni > Cd. The highest concentration values of Zn, Ni, and Cd were observed in the petroleum drain, but the maximum concentrations of Mn, Cu, and Pb were observed in the El-Umoum Drain. However, the highest iron concentration value was recorded in the El-Qalaa Drain. The discharged heavy elements into the lake either precipitate to the bottom sediment, are uptaken by living organisms, or increase the levels of these metals in the lake water (Goher et al. 2019b).
Long-term changes of heavy metals in Mariout Lake water
The long-term analyses of the measured heavy metals (Fe, Mn, Zn, Cu, Ni, Pb, and Cd) in Mariout Lake water showed significantly irregular vibrations from 1981 to 2020, as indicated in the supplementary file (Table S2). The high fluctuation in the concentrations of all the measured metals may be attributed to the quality and quantity of the waste discharged into the lake via drains, especially El-Qalaa and El-Umoum Drain. In addition, some studies focused only on the Lake Main Basin, like El-Bestauy (1993), Amr et al. (2005), Abaza et al. (2009), and Afifi et al. (2016), but others studied the fishery basin, like Khalil (1998) and El-Khatib et al. (2020). However, some researchers collected water samples monthly from the lake, for example, Saad et al. (1981) and El-Bestauy (1993), while others studied seasonal variations, like Khalil (1998), El-Shorbagi (2015), Ali et al. (2020), and El-Khatib et al. (2020), but Afifi et al. (2016) explored heavy metal concentrations of water samples collected only in the winter season. In addition, some studies determined only the concentrations of dissolved heavy metals, as in Saad et al. (1981), El-Bestauy (1993), and El-Shorbagi (2015), while others measured the total heavy metal concentrations in Mariout Lake water, like Ashmawy et al. (2018), Abd El-alkhoris et al. (2020), and Morsy et al. (2020). On the other hand, Table S2 (in the supplementary file) shows the levels of the studied metals in Mariout Lake water in comparison to the other Northern Nile-Delta Lakes.
Metal quality indices
Pollution index (PI)
According to the PI values, Mariout Lake water suffers from obviously different contamination grades with the measured metals for aquatic life utilization. Mn, Fe, Zn, Cu, and Ni recorded no pollution effects at any sites along the lake, but Cd and Pb exhibited serious pollution effects at all the studied locations according to the aquatic life criteria, as indicated in Table 5.
Metal index (MI)
According to the MI values, all the selected sites along Mariout Lake were seriously threatened by metal pollution for aquatic life usage (MI > 1). The MI values ranged from 24.48 to 35.34 along the studied sites in Mariout Lake. As indicated in Table 6, the most polluted site was site (8) in the Main Basin.
Heavy metal pollution index (HPI)
Table 7 shows that the HPI values in Mariout Lake water ranged from 772.24 to 1151.33. The most polluted site was site (8) in the Main Basin. These results demonstrate that all the studied metals have significant polluting effects on aquatic life usage. Following a critical HPI value of 100, the data indicate that aquatic organisms living in Mariout Lake may be exposed to greater risks. Nadmitov et al. (2015) reported that at an HPI > 100, the overall pollution levels must be assessed as undesirable for an aquatic ecosystem.
Heavy metals in fish
In this study, Tilapia species (O. niloticus) caught from Mariout Lake was selected to assess the metals’ accumulation pattern because Egypt is the second-largest producer of Tilapia after China (FAO 2014). Besides its ecological value, Tilapia is economically important as a food source for low-income families. It is one of the most common fish species used in toxicological studies (Osman et al. 2010) because it presents some characteristics that may make it a good biomonitor for describing the quality of aquatic systems and testing levels of metal pollution (Ghannam 2021).
The seasonal variations of heavy metal concentrations in edible tissues of the Tilapia species (O. niloticus) caught from Mariout Lake are graphically represented in Fig. 3. The concentrations of the studied metals were found in the ranges of 14.79–30.54 and 10.25–13.96, 1.33–3.08 and 0.9–2.24, 3.44–19.4 and 2.93–4.7, 1.15–1.45 and 0.84–1.43, 0.58–2.49 and 0.11–2.47, 1.27–2.06 and 1.04–1.57, and 0.73–0.96 and 0.56–0.87 µg/g for Fe, Mn, Zn, Cu, Ni, Pb, and Cd during the summer and winter seasons, respectively. In general, the annual average contents of O. niloticus muscles in the lake were in the order of Fe > Zn > Mn > Pb > Ni > Cu > Cd.
The maximum concentration values of all the measured heavy metals in O. niloticus muscles from Mariout Lake were observed in the summer season at site (2) in the South-West Basin for Fe, Mn, and Pb; at site (11) in the North–West Basin for Zn and Cd; and in the Main Basin at sites (7 and 10) for Cu and Ni, respectively. In general, higher concentrations of heavy metals in fish are mainly attributed to industrial effluents, municipal sewage runoff, untreated waste dumping, and urbanization (Ali and Chidambaram 2021). On the other hand, the minimum concentrations of all metals were recorded in the winter season at site (10) in the Main Basin for Fe, Mn, and Cu; at site (6) in the Aquacultural Basin for Zn and Cd; at site (2) in the South–West Basin for Ni; and at site (7) in the Main Basin for Pb. ANOVA results indicated that there were temporal significant differences (p ≤ 0.05) for Fe, Mn, and Cu concentrations in muscles of O. niloticus from Mariout Lake. However, Pb concentrations showed high temporal significant differences (p ≤ 0.01).
Table 8 illustrates the minimum, maximum, and annual means of the measured heavy metal concentrations in edible tissues of the Tilapia species (O. niloticus) caught from Mariout Lake compared to the international standard levels. It is clear that the concentrations of Fe, Zn, and Cu were below the international permissible limits, but that of Cd exceeded the corresponding standard guidelines. However, Pb concentrations were below the guideline value of 2.0 μg/g (ANZECC 2000; MAFF 2000) but exceeded the other international permissible levels. Furthermore, Ni concentrations were found to be higher than the estimated guideline of (0.5–0.6) µg/g (FAO 1983) but were below the standard level of (70–80) µg/g (USFDA 1993). The concentrations of Mn also exceeded the guideline of 1.0 μg/g (WHO 1989) but were below the permissible limit of 20 µg/g stipulated by Dural et al. (2007). On the other hand, Table S3 (in the supplementary file) shows the levels of the studied metals in O. niloticus muscles from Mariout Lake and in other Northern Nile-Delta Lakes.
Bioaccumulation factor (BAF)
Kucuksegin et al. (2006) have identified the bioaccumulation of heavy metals in the tissues of marine organisms as an indirect measure of the abundance and availability of metals in the marine environment. It is always important to determine the bioaccumulation capacity of heavy metals by fish to assess the potential risks to human health and to take appropriate actions to protect public health and the environment (Ambedkar and Muniyan 2011). Generally, many factors may affect metals’ uptake and accumulation in aquatic organisms, including the organism characteristics, metal concentrations, exposure time, mode of metals’ uptake, and physiochemical conditions of water (Goher et al. 2019b; Ghannam 2021).
The BAFs of the measured heavy metals in the edible tissues of the Tilapia species (O. niloticus) caught from Mariout Lake are represented in Fig. 4. Results showed that the BAF values were 12.48–112.26, 10.3–124.22, 35.46–1010.66, 76.4–200, 11.96–210.45, 20.18–118.79, and 78.4–160.78 for Fe, Mn, Zn, Cu, Ni, Pb, and Cd, respectively. The highest BAF (1010.66) was observed for Zn in the summer season at site (11) in the North-West Basin, but the lowest BAF (10.3) was recorded for Mn in the winter season at site (7) in the Main Basin. Generally, the accumulation order of metals in O. niloticus muscles from the lake was Zn > Cd > Cu > Ni > Pb > Mn > Fe. Cadmium bioaccumulation is generally hazardous, as it is a nonessential trace element. According to the categories of BAFs obtained by Olayinka–Olagunju et al. (2021), all the studied heavy metal BAF values were less than 1000 (BAFs < 1000), indicating no probability of accumulation, except the highest BAF for Zn in the summer season (1010.66) at site (11), which indicates that Zn may be a bioaccumulative metal because it was identified that phytoplanktons bloom during summer, and subsequently, its consumption results in bioaccumulation (Ali and Chidambaram 2021).
Human health risk assessment
Risk assessment is a methodology that identifies, characterizes, and analyzes toxic elements to qualify their adverse effects within a specified timeframe and to estimate the potential risk levels for humans. This approach was applied to identify the exposure and tendency of the contaminated fish in Mariout Lake to the human body (Sánchez et al. 2022).
Estimated daily intake (EDI)
As fish consumption is a possible source of heavy metal accumulation in humans, it is important to consider the daily intake of metals through fish consumption. The following assumptions were made in this study to estimate the risks of heavy metals from Tilapia species (O. niloticus) consumption at the extreme: the ingested dose was equal to the absorbed pollutant dose, and cooking did not affect the pollutants, as assumed by Kortei et al. (2020) and Yacoub et al. (2021). Table 9 shows that the EDI values of all heavy metals through consumption of 0.057 kg of fish flesh by a 70 kg Egyptian person per day are lower than their provisional tolerable daily intake (PTDI70) values for each metal. The results revealed that Mn, Ni, Cu, Pb, and Cd constituted the lowest daily intake, while Fe and Zn constituted the highest daily intake, as shown in Fig. 5. These results are supported by Yacoub et al. (2021) for El-Manzala Lake.
Target hazard quotient (THQ) and hazard index (HI)
Table 9 and Figs. 6, 7 show the non-carcinogenic human hazard risks of heavy metals through edible tissue exposure routes using the seven measured metals to calculate the THQ and HI values. The present results indicate that the obtained THQ values due to the exposure route for all investigated heavy metals do not demonstrate the risks (THQ < 1) associated with the consumption of 57 g/day of O. niloticus from Mariout Lake. The THQ values were in the order of Cd (0.651) > Pb (0.338) > Mn (0.112) > Ni (0.054) > Cu (0.025) > Fe (0.018) > Zn (0.017). Results showed that the THQ values of the most studied metals were ten times less than the non-hazardous limit (THQ < 1). Therefore, there are no non-carcinogenic health risks from ingestion of these metals individually through Tilapia species (O. niloticus) consumption, according to Custodio et al. (2021).
The IRFb value is the amount of edible fish tissues that must be consumed daily for the health risks of each element to become evident (THQ ≥ 1). IRFb-calculated values in Table 9 revealed that only a small amount of edible tissues of the Tilapia species (O. niloticus) caught from Mariout Lake is required to produce adverse health effects for Cd and Pb, whereas larger amounts of edible fish tissues are required to produce health risks for Fe, Mn, Zn, Cu, and Ni.
The health risk assessment of metal exposure from the consumption of Tilapia fish species from Mariout Lake should allow for the combined effects of the various heavy metals studied. Therefore, the HI value is necessary to assess the health risk associated with fish consumption (Zhu et al. 2016). The total non-carcinogenic HI value for the analyzed heavy metals was 1.241, as shown in Table 9 and Fig. 7.
This result revealed that the risks of consuming edible tissues of Tilapia species (O. niloticus) caught from Mariout Lake slightly exceed the threshold value of 1. The human health risk assessment for heavy metal contamination delineated low-hazard risks in edible tissues. According to Ukoha et al. (2014), the HI value (HI = 1.241) is classified in the moderate hazard risk level (1 < HI < 10). This moderate value is mainly based on the THQ values of Cd (0.651) and Pb (0.338). In this study, the major health risk-contributing metal was Cd (53.6%), followed by Pb (27.82%), Mn (9.19%), Ni (4.43%), Cu (2.06%), and Fe (1.5%), and Zn (1.37%).
Conclusion
Mariout Lake is one of the five Egyptian Mediterranean lagoons. Although it is the smallest one of them, it is the most polluted. It is a closed lake that has no direct connection with the Mediterranean Sea. It receives a huge amount of wastes via different drains. The results indicated that the highest concentrations of the measured heavy metals are observed in the Main Basin due to the effects of domestic, agricultural, and industrial effluents from El-Qalaa and El-Umoum Drains. The long-term changes of the studied metals in Mariout Lake water showed significantly irregular vibrations from 1981. The pollution index detected that Cd and Pb have serious pollution effects on aquatic life, while the metal index and heavy metal pollution index indicated the high-water pollution of Mariout Lake and it is inappropriate for fish and different aquatic organisms. In spite of that, the levels of most metals in O. niloticus muscles were within the international permissible limits. Generally, the bioaccumulation order of heavy metals in O. niloticus muscles was Zn > Cd > Cu > Ni > Pb > Mn > Fe. The obtained results showed that the target hazard quotient due to the ingestion of HMs through O. niloticus was less than the hazard limit (THQ < 1), while the hazard index recorded a slight increase (HI = 1.241), indicating moderate potential non-carcinogenic health risks from the collective ingestion of these metals through consumption of muscles of O. niloticus from Mariout Lake. Our study recommends concerted efforts of all the concerned and responsible authorities to enforce law 48/1982 that prevents the dumping of pollutants into the water bodies, in addition to the application of biological and chemical treatment for wastewater discharged into Mariout Lake to rapidly rehabilitate it and improve its water quality.
Data availability
All data will be available from the corresponding author upon request.
Code availability
Non applicable.
References
Abd El-Aal RF, El-Sayed SM, Attia MS, Donia NS, Goher ME (2020) Pollution indices and distribution pattern of heavy metals in Qarun Lake water, Egypt. Egypt J Aquat Biol Fish 24(1):593–607. https://doi.org/10.21608/EJABF.2020.75893
Abd El-alkhoris YS, Abbas MS, Sharaky AM, Fahmy MA (2020) Assessment of water and sediments pollution in Maruit Lake, Egypt. Egypt J Aquat Biol Fish 24(1):145–160. https://doi.org/10.21608/EJABF.2020.70681
Adegbola IP, Aborisade BA, Adetutu A (2021) Health risk assessment and heavy metal accumulation in fish species (Clarias gariepinus and Sarotherodon melanotheron) from industrially polluted Ogun and Eleyele Rivers, Nigeria. Toxicol Rep 8:1445–1460. https://doi.org/10.1016/j.toxrep.2021.07.007
Afifi AA, Shalaby AA, Issa YM, Rostom NG (2016) Geo-spatial variability assessment of water pollutants concentration in Mariut Lake, Egypt. Egypt J Soil Sci 56(2):231–248. https://doi.org/10.21608/EJSS.2016.2351
Ali A, Chidambaram S (2021) Assessment of trace inorganic contaminates in water and sediment to address its impact on common fish varieties along Kuwait Bay. Environ Geochem Health 43(2):855–883. https://doi.org/10.1007/s10653-020-00559-6
Ali MM, Ali ML, Proshad R, Islam S, Rahman Z, Tusher TR, Kormoker T, Abdullah-Al M (2020) Heavy metal concentrations in commercially valuable fishes with health hazard inference from Karnaphuli river Bangladesh. Hum Ecol Risk Assess 26(10):2646–2662. https://doi.org/10.1080/10807039.2019.1676635
Alnagaawy AM, Sherif MH, Assy MG, Shehata AS (2018) Impact of industrial pollutants on some water quality parameters of Edku, Mariout Lakes and the Nile River. Int J Environ 7(1):1–15
Ambedkar G, Muniyan M (2011) Bioaccumulation of some heavy metals in the selected five freshwater fish from Kollidam River, Tamilnadu India. Adv Appl Sci Res 2(5):221–225
Amr HM, El-Tawila MM, Ramadan MH (2005) Assessment of pollution levels in fish and water of Main Basin, Lake Mariut. J Egypt Public Health Assoc 80(1–2):51–76
ANZECC/ARMCANZ (2000) Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environmental Conservation Council & Agriculture and Resource Management Council of Australian and New Zealand, Canberra, 1–123
APHA-American Public Health Association (2005) Standard methods for the examination of water and wastewater. 21th ed., 1015 pp, AWWA, WCPF, Washington DC
Arafa MM, Ali AT (2008) Effect of some heavy metals pollution in Lake Mariout on Oreochromis niloticus fish. Egypt J Comp Pathol Clin Pathol 21(3):191–201
Ashmawy KI, Hiekai FA, Abo-Akadda SS, Laban NE (2018) The inter-relationship of water quality parameters and fish parasite occurrence. Alex J Vet Sci 59(1):97–106. https://doi.org/10.5455/ajvs.299584
Bakan G, Őzkoç HB, Tülek S, Cüce H (2010) Integrated environmental quality assessment of Kızılırmak River and its coastal environment. Turk J Fish Aquat Sci 10(4):453–462. https://doi.org/10.4194/trjfas.2010.0403
Baraka H (2012) Egypt’s lakes: a truly tragic environmental tale. Egyptian Independent
Bashir I, Lone FA, Bhat RA, Mir SA, Dar ZA, Dar SA (2020) Concerns and threats of contamination on aquatic ecosystems. In: Hakeem KR, Bhat RA, Qadri H (eds) Bioremediation and biotechnology, 1–26. https://doi.org/10.1007/978-3-030-35691-0_1
Bristy MS, Sarker KK, Baki MA, Quraishi SB, Hossain MM, Islam A, Khan MF (2021) Health risk estimation of metals bioaccumulated in commercial fish from coastal areas and rivers in Bangladesh. Environ Toxicol Pharmacol 86:103666. https://doi.org/10.1016/j.etap.2021.103666
Caerio S, Costa MH, Ramos TB, Fernandes F, Silveira N, Coimbra A, Mederios G, Painho M (2005) Assessing heavy metal contamination in Sado Estuary sediment: an index analysis approach. Ecol Indic 5(2):151–169. https://doi.org/10.1016/j.ecolind.2005.02.001
CCME-Canadian Council of Ministers of the Environment (2021) Canadian water quality guidelines for the protection of aquatic life. Summary Table: https://ccme.ca/en/summary-table
Custodio M, Peñaloza R, Ochoa S, Cuadrado W (2021) Human risk associated with the ingestion of artichokes grown in soils irrigated with water contaminated by potentially toxic elements, Junin Peru. Saudi J Biol Sci 28(10):5952–5962. https://doi.org/10.1016/j.sjbs.2021.06.054
Donia N (2016) Water quality modelling of Northern Lakes case study (Egyptian Northern Lakes). In: Rashed MN (ed) Lake sciences and climate change, 175–190. https://doi.org/10.5772/63526
Dural M, Göksu MZ, Őzak AA (2007) Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chem 102(1):415–421. https://doi.org/10.1016/j.foodchem.2006.03.001
EC-European Community (2005) Official Journal of the European Union, 78:L16/43-L16/45
EEAA-Egyptian Environmental Affairs Agency (2011) Abstract of Annual Report (2010–2011) for the environmental program of the Egyptian lakes (Lake Maruit), 1:1–14
El-Bestauy F (1993) Studies on the occurrence and distribution of pollution metals in fresh water phytoplankton and bacteria in Lake Mariut, Alexandria, Egypt. Ph.D. Thesis, university of Manchester, 324p
El-Kafrawy SB, Donia NS, Mohamed AM (2017) Water quality assessment based on CWQI and NDWI indices in Mariout Lake Egypt. MOJ Ecol Environ Sci 2(5):224–232. https://doi.org/10.15406/mojes.2017.02.00039
El-Khatib ZI, Azab AM, Abo-Taleb HA, Al-Absawy AN, Toto MM (2020) Effect of heavy metals in irrigation water of different fish farms on the quality of cultured fish. Egypt J Aquat Biol Fish 24(5):261–277. https://doi.org/10.21608/ejabf.2020.104648
El-Naggar NA, Rifaat AE (2017) Hydrodynamic and water quality modeling of Lake Mariout (Nile Delta, Northern Egypt). In: Negm AM, Bek MA, Abdel-Fatah S (eds) Egyptian Coastal Lakes and Wetlands: Part I-characteristics and hydrodynamics, The Handbook of Environmental Chemistry (2019), 71:241–264
El-Rayis OA, Saad M (1990) Heavy metal pollution in Lagoon Maryout on the southeastern coast of the eastern Mediterranean Sea. J King Abdulaziz Univ Mar Sci 1:17–26
Elsayed FA, Okbah MA, El-Syed SM, Eissa MA, Goher ME (2019) Nutrient salts and eutrophication assessment in Northern Delta Lakes: case study Burullus Lake Egypt. Egypt J Aquat Biol Fish 23(2):145–163. https://doi.org/10.21608/EJABF.2019.30239
El-Sharkawy MH (2010) Quantitative assessment and treatment of some industrial pollutants along the Nile River at Giza region. Ph.D. Thesis, Faculty of Science, Benha University, Egypt
El-Shorbagi EK (2015) Physico-chemical and environmental studies on Lake Mariut, Egypt. M.Sc. Thesis, Faculty of Science, Alexandria University, Egypt, 188p
EU-European Union (2001) Commission Regulation as regards heavy metals. Directive 22:466
FAO/SIDA (1983) Manual of methods in aquatic environment research [Part 9. Analyses of metals and organochorines in fish]. FAO Fish Technical Paper, 212:33p
FAO/WHO-Food and Agriculture Organization/World Health Organization (1989) Evaluation of certain food additives and the contaminants mercury, lead and cadmium, WHO technical report series No. 505. In: Aquatic environment monitoring report No. 52, Center for Environment, Fisheries and Aquaculture Science, Lowestoft, UK
FAO/WHO-Food and Agriculture Organization/World Health Organization (2010) Joint FAO/WHO expert committee on food additives (JECFA). Summary report of the seventy-third meeting of JECFA in the WHO technical report series: 12–13, Geneva, Switzerland
FAO-Food and Agriculture Organization (1983) Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fishery Circular 464:5–100
FAO-Food and Agriculture Organization (2014) Report on fisheries and aquaculture markets in the Middle East. http://www.thefishsite.com/articles/1870/fao-report-fisheries-and-aquaculture-marketsin-the-middle-east
GAFRD (2020) Fish statistics year book, 30th Edition. The General Authority for Fishery Resources Development, Egypt, 102p.
Ghannam HE (2021) Risk assessment of pollution with heavy metals in water and fish from River Nile Egypt. Appl Water Sci 11(7):125. https://doi.org/10.1007/s13201-021-01449-7
Goher ME, Abdo MH, Mangood AH, Hussein MM (2015) Water quality and potential health risk assessment for consumption of Oreochromis niloticus from El-Bahr El-Pharaony Drain Egypt. Fresenius Environ Bull 24(11):3590–3602
Goher ME, Ali MH, El-Sayed SM (2019a) Heavy metals contents in Nasser Lake and the Nile River, Egypt: an overview. Egypt J Aquat Res 45(4):301–312. https://doi.org/10.1016/j.ejar.2019.12.002
Goher ME, El-Rouby WM, El-Dek SI, El-Sayed SM, Noaemy SG (2018) Water quality assessment of Qarun Lake and heavy metals decontamination from its drains using nanocomposites. IOP Conf Ser: Mater Sci Eng 464(1):012003. https://doi.org/10.1088/1757-899X/464/1/012003
Goher ME, Mahdy EM, Abdo MH, El Dars FM, Korium MA, Elsherif AA (2019b) Water quality status and pollution indices of Wadi El-Rayan Lakes, El-Fayoum, Egypt. Sustain Water Resour Manag 5(2):387–400. https://doi.org/10.1007/s40899-017-0162-z
Hamadani H, Rashid SM, Rehman MU, Ali R, Rashid M, ur-Rahman M, Hussain I, Gul G, ul-Haq Z (2020) Global scenario of remediation techniques to combat environmental pollution. In: Hakeem KR, Bhat RA, Qadri H (eds) Bioremediation and biotechnology, 93–106. https://doi.org/10.1007/978-3-030-35691-0_5
Hamed EAE, Khaled A, Ahdy H, Ahmed HO, Abdel Razek FA (2020) Health risk assessment of heavy metals in three invertebrate species collected along Alexandria Coast, Egypt. Egypt J Aquat Res 46(4):389–395. https://doi.org/10.1016/j.ejar.2020.11.001
Hassouna ME, Goher ME, El-Sayed SM, Hassan RA (2019) Integrated approach to quality indices and health risk assessment of water in the Bahr Yusuf Canal, Fayoum, Egypt. Oceanol Hydrobiol Stud 48(4):337–354. https://doi.org/10.2478/ohs-2019-0031
Huber L (2007) Validation and qualification in analytical laboratories, 2nd edn. CRC Press, Boca Raton, p 288p
Imam N, El-Sayed SM, Goher ME (2020) Risk assessments and spatial distributions of natural radioactivity and heavy metals in Nasser Lake, Egypt. Environ Sci Pollut Res 27(2):25475–25493. https://doi.org/10.1007/s11356-020-08918-7
Khalil MK, El Zokm GM, Fahmy MA, Said TO, Shreadah MA (2013) Geochemistry of some major and trace elements in sediments of Edku and Mariut Lakes, North Egypt. World Appl Sci J 24(3):282–294. https://doi.org/10.5829/idosi.wasj.2013.24.03.75115
Khalil MT (1998) Impact of pollution on productivity and fisheries of Lake Mariut, Egypt. J Aquat Biol Fish 2(2):1–17. https://doi.org/10.21608/EJABF.1998.1621
Kortei NK, Heymann ME, Essuman EK, Kpodo FM, Akonor PT, Lokpo SY, Boadi NO, Ayim-Akonor M, Tettey C (2020) Health risk assessment and levels of toxic metals in fishes (Oreochromis noliticus and Clarias anguillaris) from Ankobrah and Pra Basins: impact of illegal mining activities on food safety. Toxicol Rep 7:360–369. https://doi.org/10.1016/j.toxrep.2020.02.011
Kucuksezgin F, Kontas A, Altay O, Uluturhan E, Darılmaz E (2006) Assessment of marine pollution in Izmir Bay; Nutrient, heavy metal and total hydrocarbon concentrations. Environ Int 32(1):41–51. https://doi.org/10.1016/j.envint.2005.04.007
MAFF-Ministry of Agriculture, Fisheries and Food (2000) Monitoring and surveillance of non-radioactive contaminants in the aquatic environment and activities regulating the disposal of wastes at sea, 1997. In: Aquatic environment monitoring report No. 52. Center for Environment, Fisheries and Aquaculture Science, Lowestoft, UK
Masoud MS, Elewa AA, Ali AE, Mohamed EA (2005) Distribution of some metal concentrations in water and sediment of Lake Edku, Egypt. Bull Chem Technol Macedonia 24(1):21–34
Memoli V, Esposito F, De Marco A, Arena C, Vitale L, Tedeschi A, Magliulo V, Maisto G (2017) Metal compartmentalization in different biomass portions of Helianthus annuus L. and Sorghum bicolor L. grown in an agricultural field inside an urban fabric. Appl Soil Ecol 121:118–126. https://doi.org/10.1016/j.apsoil.2017.09.035
Milivojević J, Krstić D, Šmit B, Djekić V (2016) Assessment of heavy metal contamination and calculation of its pollution index for Uglješnica River, Serbia. Bull Environ Contam Toxicol 97(5):737–742. https://doi.org/10.1007/s00128-016-1918-0
Mohanta VL, Naz A, Mishra BK (2020) Distribution of heavy metals in the water, sediments, and fishes from Damodar River Basin at steel city, India: a probabilistic risk assessment. Hum Ecol Risk Assess 26(2):406–429. https://doi.org/10.1080/10807039.2018.1511968
Morsy KM, Mishra AK, Galal MM (2020) Water quality assessment of the Nile Delta Lagoons. Air Soil Water Res 13(1):1–11. https://doi.org/10.1177/1178622120963072
Nadmitov B, Hong S, In Kang S, Chu JM, Gomboev B, Janchivdorj L, Lee C, Khim JS (2015) Large-scale monitoring and assessment of metal contamination in surface water of the Selenga River Basin (2007–2009). Environ Sci Pollut Res 22(4):2856–2867. https://doi.org/10.1007/s11356-014-3564-6
Napoletano P, Colombo C, Di Iorio E, Memoli V, Panico SC, Ruggiero AG, Santorufo L, Maisto G, De Marco A (2021) Integrated approach for quality assessment of technosols in experimental mesocosms. Sustainability 13(16):9101. https://doi.org/10.3390/su13169101
Neira R, García X, Lhorente JP, Filp M, Yáñez JM, Cascante AM (2016) Evaluation of the growth and carcass quality of diallel crosses of four strains of Nile tilapia (Oerochromis niloticus). Aquaculture 451:213–222. https://doi.org/10.1016/j.aquaculture.2015.08.033
Olayinka-Olagunju JO, Dosumu AA, Olatunji-Ojo AM (2021) Bioaccumulation of heavy metals in Pelagic and Benthic fishes of Ogbese River, Ondo State, South-Western Nigeria. Water Air Soil Pollut 232(2):44. https://doi.org/10.1007/s11270-021-04987-7
Omran EE, Negm AM (2017) Adaptive management zones of Egyptian coastal lakes. In: Negm AM, Bek MA, Abdel-Fatah S (eds) Egyptian Coastal Lakes and Wetlands: Part I—Characteristics and Hydrodynamics, The Handbook of Environmental Chemistry (2019), 71:37–60
Osakwe JO, Adowei P, Horsfall M (2014) Heavy metals body burden and evaluation of human health risks in African Catfish (Clarias Gariepinus) from Imo River Nigeria. J Dev Drugs 4(2):78–89. https://doi.org/10.4172/2329-6631.S1.009
Osman GM, Ali E, Hashem M, Mostafa M, Mekkawy I (2010) Genotoxicity of two pathogenic strains of zoosporic fungi (Achlya klebsiana and Aphanomyces laevis) on erythrocytes of Nile Tilapia Oreochromis niloticus. Ecotoxicol Environ Saf 73(1):24–31. https://doi.org/10.1016/j.ecoenv.2009.08.021
Panico SC, Memoli V, Esposito F, Maisto G, De Marco A (2018) Plant cover and management practices as drivers of soil quality. Appl Soil Ecol 129:128–135. https://doi.org/10.1016/j.apsoil.2018.05.001
Parisi C, De Marco G, Labar S, Hasnaoui M, Grieco G, Caserta L, Inglese S, Vangone R, Madonna A, Alwany M, Hentati O, Guerriero G (2022) Biodiversity studies for sustainable Lagoon: thermophilic and tropical fish species vs. endemic commercial species at Mellah Lagoon (Mediterranean, Algeria). Water 14(4):635. https://doi.org/10.3390/w14040635
Saad AS, Massoud MA, Amer RA, Ghorab MA (2017) Assessment of the physico-chemical characteristics and water quality analysis of Mariout Lake, southern of Alexandria Egypt. J Environ Anal Toxicol 7(1):421–440. https://doi.org/10.4172/2161-0525.1000421
Saad MA, Ezzat AA, El-Rayis OA, Hafez H (1981) Occurrence and distribution of chemical pollutants in Lake Mariut, Egypt. II. Heavy metals. Water Air Soil Pollut 16(4):401–407. https://doi.org/10.1007/BF01048131
Sánchez ER, Martínez JM, Morales MM, Mendoza OT, Alberich MV (2022) Ecological and health risk assessment of potential toxic elements from a mining area (water and sediments): the San Juan-Taxco River system, Guerrero Mexico. Water 14(4):518. https://doi.org/10.3390/w14040518
Shehata DH (2014) Management of water quality in Lake Maryout using engineered wetland. M.Sc. Thesis, Faculty of Engineering, Ain Shams University, Egypt
Silim AR (2015) A water quality numerical model for Lake Maryout. Ph.D. Thesis, Faculty of Engineering, Ain Shams University, Egypt
Tafa B, Assefa E (2014) Detection of copper and zinc (heavy metals) in water of Lake Chamo Arbaminch Ethiopia. World J Chem Educ 2(3):42–47. https://doi.org/10.12691/wjce-2-3-3
Talab AS, Goher ME, Ghannam HE, Abdo MH (2016) Chemical compositions and heavy metal contents of Oreochromis niloticus from the main irrigated canals (Rayahs) of Nile Delta. Egypt J Aquat Res 42(1):23–31. https://doi.org/10.1016/j.ejar.2016.01.003
Tamasi G, Cini R (2004) Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the Province of Siena. Sci Total Environ 327(1–3):41–51. https://doi.org/10.1016/j.scitotenv.2003.10.011
Türkmen M, Türkmen A, Tepe Y, Töre Y, Ateş A (2009) Determination of metals in fish species from Aegean and Mediterranean seas. Food Chem 113(1):233–237. https://doi.org/10.1016/j.foodchem.2008.06.071
Ukoha PO, Ekere NR, Udeogu UV, Agbazue VE (2014) Potential health risk assessment of heavy metals (Cd, Cu and Fe) concentrations in some imported frozen fish species consumed in Nigeria. Int J Chem Sci 12(2):366–374
Ullah AK, Maksud MA, Khan SR, Lutfa LN, Quraishi SB (2017) Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol Rep 4:574–579. https://doi.org/10.1016/j.toxrep.2017.10.002
USEPA-United States Environmental Protection Agency (2011) Mid-Atlantic risk assessment, Washington. http://www.epa.gov/regshwmd/risk/human/Index.htm
USEPA-United States Environmental Protection Agency (2013) Regional screening level (RSL) fish ingestion table. http://www.epa.gov/reg3hwmd/risk/human/rbconcentration_table/usersguide.htm
USEPA-United States Environmental Protection Agency (2021) National recommended water quality criteria-Aquatic life criteria table. https://www.epa.gov/wqc/nationalrecommended-water-quality-criteria-aquatic-life-criteria-table
USFDA-United States Food and Drug Administration (1993) Guidance document for nickel in shell fish. DHHS/PHS/ FDA/CFSAN/office of seafood, Washington
Vasistha P, Ganguly R (2020) Assessment of spatio-temporal variations in lake water body using indexing method. Environ Sci Pollut Res 27(33):41856–41875. https://doi.org/10.1007/s11356-020-10109-3
Volpe MG, Nazzaro M, Di Stasio M, Siano F, Coppola R, De Marco A (2015) Content of micronutrients, mineral and trace elements in some Mediterranean spontaneous edible herbs. Chem Cent J 9:57. https://doi.org/10.1186/s13065-015-0137-9
Wangersky PJ (1986) Biological control of trace metal residence time and speciation: a review and synthesis. Mar Chem 18(2–4):269–297. https://doi.org/10.1016/0304-4203(86)90013-7
WHO-World Health Organization (1989) Environment health criteria No. 85. Heavy metals environmental aspects, Geneva, Switzerland
Yacoub AM, Mahmoud SA, Abdel-Satar AM (2021) Accumulation of heavy metals in Tilapia fish species and related histopathological changes in muscles, gills and liver of Oreochromis niloticus occurring in the area of Qahr El-Bahr, Lake Al-Manzalah Egypt. Oceanol Hydrobiol Stud 50(1):1–15. https://doi.org/10.2478/oandhs-2021-0001
Zhu H, Xu Y, Yan B, Guan J, Zhou Q, Liang Y (2016) Risk assessment of heavy metals contamination in sediment and aquatic animals in downstream waters affected by historical gold extraction in Northeast China. Hum Ecol Risk Assess 22(3):693–705. https://doi.org/10.1080/10807039.2015.1104626
Acknowledgements
This study was supported with facilities from Chemistry Lab in the National Institute of Oceanography and Fisheries (Fresh Water and Lakes Division). We also thank Dr. Yasser Hagag for his help in collection of the samples.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Field study and sampling, A A. El-D, M E. G; Sample processing and analysis, Amira A. El-D, M E. G; Writing of the first draft and final text, Amira A. El-D, M E. G; Review, editing and approving of the final text, all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
All authors voluntarily agree to participate in this research study.
Consent to publish
All authors voluntarily approved the publication of this research study.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Degwy, A.A., Negm, N.A., El-Tabl, A.S. et al. Assessment of heavy metal pollution in water and its effect on Nile tilapia (Oreochromis niloticus) in Mediterranean Lakes: a case study at Mariout Lake. Appl Water Sci 13, 50 (2023). https://doi.org/10.1007/s13201-022-01858-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01858-2