Abstract
Potentially hazardous elements (PHEs) are non-biodegradable and accumulate in places like water, soil, and plants where they endanger environmental health. There are a considerable number of wetlands having both national and worldwide importance in Türkiye. Regarding PHE accumulation, sediments and Ceratophyllum demersum were examined in the Miliç Wetland (MW), situated in a basin with intense hazelnut and rice farming, which is next to the international highway on the Central Black Sea Coast of Türkiye. The quantification of PHEs in the study subjects was undertaken using a validated inductively coupled plasma-mass spectrometry (ICP-MS) method, and mean concentrations (mg/kg) of PHEs in the sediments were in the order of Al (13,133) > Fe (10,790) > Mn (205.84) > Cu (17.95) > Cr (16.40) > Zn (15.55) > Ni (11.74) > Pb (9.17) > Co (6.30) > As (2.07) > Cd (0.19). The ecotoxicological risk was assessed using sediment quality guidelines (SQGs) and certain geological indices, indicating mostly low ecological risk, low pollution, and no hazardous risk. Based on the modified hazard quotient (mHQ) classification of values, Ni showed low contamination, while Cd, Pb, As, and Cu displayed very low contamination, and Zn presented minor contamination. The findings of total lifetime cancer risk (LCR), hazard quotient (HQ), and hazard index (HI) identified that exposure of adults or children to sediments containing PHEs would not represent a major health risk. As a recommendation, it is necessary to avoid the direct entrance of agricultural pesticides and fertilizers to enhance the sediment quality of the MW. Since the highway was constructed close to MW, this is considered a significant source of human-caused pollution. Consequently, all PHEs analyzed, except for Cd, displayed a bioconcentration factor (BCF) value of more than 1000, indicating that Ceratophyllum demersum is a promising plant for phytoremediation in PHE-polluted ecological systems involving wetlands, and it can efficiently be employed as an indicator species in biological screening investigations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands provide several ecological roles and are essential to the sustainability of an active ecosystem. In addition to being a huge carbon sink, they regulate rinse and flood water. Wetlands prevent soil erosion, act as a wind and storm barrier, minimize sea level rise, and preserve coastal areas. Also, numerous species nest and spawn in marsh habitats (Zedler & Kercher 2005; Xiao et al. 2019; Xia et al. 2020).
Sediment is generally reflected as the swamp of potentially hazardous elements (PHEs) in wetlands since sediment is a key repository for metals taken from the surrounding water. Once water chemistry changes in terms of redox or pH, sediment may become a reservoir for discharging metals into water columns (Cüce et al. 2022a, b; Aydin et al. 2021). On the other hand, PHEs have become an international concern for contamination owing to industrial expansions, which impact living things because of their high toxicity and bio-concentration capability (Radomirović et al. 2021a, b; Ustaoğlu 2021). An increase in PHE concentration in sediments harms the aquatic habitat regarding environmental health. Sediment stores PHEs in aquatic ecosystems because they can be easily circulated back into the water bodies (Töre et al. 2021; Sojka & Jaskuła 2022).
Sedimentary PHEs might have lithological or anthropological origins. Anthropogenic activities such as industrial expansion, farming, mining, the creation of council estates, transportation, garbage disposal, and wastewater runoff are the primary sources of pollution. Geological processes such as erosion and weathering cannot be overstated. In contrast to lithogenic metals, anthropogenic metals are highly bioavailable and transportable in the aquatic environment (Tokatli and Ustaoğlu 2020; Ustaoğlu 2021; Varol et al. 2022a, b). Due to the negative impact on aquatic biota, concerns about water and sediment quality have increased the focus on PHEs-polluted aquatic habitats over the last few decades. Hence, numerous investigations have been undertaken across the globe to determine the geochemical background (Proshad et al. 2021; Arisekar et al. 2022; Şimşek et al. 2022; Cüce et al. 2022c).
According to environmental monitoring studies, there are several non-essential components (Pb, Cd, Hg, and As) in PHEs that are harmful to plants, humans, aquatic species, and animals (Yüksel et al. 2015, 2018; Akinci et al. 2016; Bozalan et al. 2019). Furthermore, those toxic elements may impair critical soil microbial activities and limit plant growth and metabolism, even at low quantities. Also, it distresses the quality of water, leading to the creation of reactive oxygen species that can harm fish and other aquatic animals in the water (Böke Özkoç & Arıman 2022). Aside from these toxic metals, PHEs also include other elements that are essential to aquatic life, which are named macronutrients (phosphorus, sulfur, sodium, calcium, potassium, and magnesium) and micronutrients (copper, cobalt, iron, zinc, selenium, manganese, chromium, nickel, boron, and molybdenum). These elements are necessary for life, yet excessive quantities of these important metals may have harmful influences on the reproduction and metabolism of organisms (Yüksel et al. 2021a; Doğan et al. 2022).
The use of bio-accumulator organisms like plants or mussels to remove harmful waste from the environment is a popular bioremediation strategy. Integrative techniques, such as using plants and microbes, have been demonstrated to be very successful in reducing the impact of heavy metals on the environment (Dökmeci 2020). Underwater macrophytes are commonly used in phytoremediation to eliminate, convert, or stabilize some trace metals in water or sediments. Such plants have a crucial role in the intake, deposition, and reprocessing of trace elements in aquatic environments (Polechońska et al. 2018). Macrophytes like C. demersum grow quickly in muddy, still bodies of water with little light. In addition, C. demersum is considered a passive bioindicator with its distinctive characteristics that make it perfect for ecological biomonitoring studies in toxicity laboratory settings (Mahmoud et al. 2018; Hak et al. 2020). Likewise, bioconcentration is the phenomenon through which a contaminant is received by an organism from its ambient environment solely via its respiratory and dermal surfaces, excluding chemical exposure through food. A bioconcentration factor (BCF) is needed to determine target concentrations to investigate secondary poisoning hazards. As a result, the BCF is used as the primary screening criteria for potentially harmful items that are also persistent and bioaccumulative, such as PHEs (Arnot & Gobas 2006).
Sediment quality guidelines (SQGs) have been produced to describe the multi-dimensional effects of metal contamination on sediment and aquatic life. There are a variety of approaches for determining whether or not sediments are harmful to their ecology. Typically, the major purpose of a sediment ecotoxicity test is to determine whether polluted sediments are poisonous to benthic surrogates (Batley & Simpson 2013). In other words, SQG values are produced to increase the safety of benthic biodiversity, fisheries, and the quality of surface water. These values are an essential part of all methodologies for assessing sediment quality (Batley & Warne 2017; Zhang et al. 2021). Incorporating the deposition of PHEs into sediments, geochemical and statistical analyses are employed to interpret the ecological risk and pollution load in a systematic approach (Kumar et al. 2022).
Over the last decades, a series of pollution indices have been fully implemented, including the geo-accumulation index (Igeo), the enrichment factor (EF), the pollution load index (PLI), the contamination factor (CF), the toxic risk index (TRI), the prospective ecological risk factor (Eri), and the modified hazard quotient (mHQ). Likewise, the Pearson correlation coefficient (PCC), principal component analysis (PCA), cluster analysis (CA), and other multivariate statistical methods have been used to assess the PHEs level in environmental subjects, including water, sediments, and aquatic macrophytes. PCA is used to separate sensitive details from a set of multivariate data so that relationships between a smaller number of variables can effectively be examined. It can therefore be used to figure out the sources, such as anthropogenic and natural. These statistical analyses are applicable to achieve the associations between the analyzed data, identifying their sources, and categorizing them according to related features (Fural et al. 2020; Varol et al. 2020; Jaskula et al. 2021; Canpolat et al. 2022).
There are numerous wetland areas of national and international significance in Türkiye. The Miliç Wetland (MW), which is situated in Terme, Samsun (Central Black Sea area of Türkiye), is under pressure from the international motorway, high summer settlements, and hazelnut and rice farming activities. The objectives of this investigation included (i) quantification of potentially hazardous elements (PHEs) in sediments and C. demersum from the MW; (ii) assessing the ecotoxicological risk by undertaking sediment contamination indices (mHQ, Eri, TRI, CF, PLI, EF, and Igeo); (iii) using the USEPA risk model to assess potential risks to human health; (iv) interpreting the possible sources of contamination using multivariate statistical techniques; and (v) examination of the BCF for Ceratophyllum demersum species.
Materials and methods
A flowchart summarizes the sample collection, PHEs of interest, the analysis method, and statistical, ecological, and health risk indicators (Fig. 1).
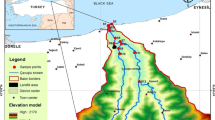
The Miliç coastal wetland, with its fauna and flora richness, draws great attention. However, there are agricultural, domestic, and industrial wastewater inputs reaching the wetland from the upper basin, where agricultural activities are quite intense. Rice and hazelnut farming are very common in the region. The wetland is also under pressure since the Miliç River flows parallel to both the Samsun-Ordu highway vicinity and the wetland. Therefore, a one-year study was planned between April 2019 and March 2020 to examine the sediment quality as well as bioaccumulation of C. demersum in the Miliç Wetland. The stations (M1, M2, M3, and M4) were selected from four different sites in the east–west direction over the approximately 10 km long stream. The mean annual rainfall in the Terme district is 964.8 mm, which is comparatively more than the entire rainfall average of Türkiye (623 mm). In the study area, the highest rainfall is observed in November, while the least is detected in May. In addition, the annual mean temperature of the study region is about 14.3 °C. The lowest temperature was measured in February (6.8 °C), and the highest mean temperature was measured in August (23.2 °C) (Coşkun & Dengiz 2016; Ustaoğlu et al. 2022a). The study region with the stations can be viewed in detail (Fig. 2).
Sixteen surface sediment samples were collected seasonally (winter, January 2020; autumn, October 2019; summer, July 2019; spring, April 2019) from four stations along the Miliç Wetland. A 20 × 20 × 20 cm Ekman Dredge grab was used to retrieve samples from a depth of 0–10 cm in the wetland bottom. To reduce cross-contamination between stations, the Ekman grab was cleaned with distilled water before each sampling. Each station was sampled three times, with each sample weighing roughly 250 g. Following that, the samples were put in polyethylene bags and transported using a cold chain to the laboratory. The sediments were dried 24 h at 103 °C. Each sample was ground in a mortar and sieved to a 63-micron before being stored at 4 °C until the day of analysis (Yüksel et al. 2022). C. demersum specimens were also sampled at the same stations. As was outlined by Haghnazar et al. (2021) and Ustaoğlu et al. (2022a), the leaves were rinsed with distilled water and dried for 24 h at an oven temperature of 80 °C. After using a mortar and pestle to grind the dried samples into a fine powder, 5 g of each sample was derived and kept at 4 °C until examined in polyethylene bags.
Analytical technique
Owing to the matrix elements (Cl, Mg, Na, K, and Ca) in environmental specimens, the determination of PHEs levels in sediments and macrophytes is challenging. As previously stated in detail (Yuksel & Arica 2018; Yüksel et al. 2021a), the digestion method not only liquefies the samples but also reduces the matrix effects due to the acidic content of the final solution. From this point of view, microwave digestion of research subjects was undertaken using a Milestone SK10 brand device. The samples were introduced to Teflon® tubes which were already filled with concentrated supra-pure grade HNO3 and H2O2 (9:3). The digestion procedure was conducted at 220 ºC for 20 min under 45 bar using maximum power. The digested solution was introduced into a 50.0-mL polypropylene falcon tube and diluted with ultra-pure water to a final volume of 50 mL. As a diluent, ultrapure water was employed exclusively throughout the sample preparation procedure. The multi-elemental profiles in samples were measured using an Agilent 7700X (USA) model Inductively Coupled Plasma-Mass Spectrophotometer (ICP-MS). Quality assurance and control were undertaken using triplicate analysis of the certified reference materials (UME EnvCRM 03 soil; Tubitak, Ankara, Türkiye) to validate the ICP-MS method through accuracy, precision, recovery, and the limit of detection. The standard stock solution (Agilent 8500–6940 2A, 27 element mix) was utilized to prepare the calibration curves. The outcomes of the validation report are illustrated in Table S1.
Examination of sediment pollution
Sediment samples may be utilized to monitor environmental pollution and determine ecological risk. Therefore, ecological indicators are broadly used to assess the pollution of surface sediments. Various indices were used in combination to provide a statement that was both dependable and consistent. Thus, we used the following tools (TRI, EF, Eri, CF, and Igeo) to evaluate pollution levels and figure out how the surface sediment from Miliç Wetland affects the environment. Furthermore, Table 1 provides a comprehensive description of the ecological risk assessment indices employed throughout this study.
Bio-concentration factor
The bio-concentration factor (BCF) measures how much an organism (macrophyte) has been enriched in PHEs compared to its natural environment. The term “water and sediments”” refers to the ratio between the concentration of heavy metals in an organism’s tissue and the concentration in the abiotic medium (Ali et al. 2021). Environmental risk assessment benefits the use of BCF since it provides quantitative information on the potential of organisms to absorb a pollutant from water. It is therefore employed as an initial screening criterion for hazardous, persistent, and bioaccumulative substances, including PHEs (McGeer et al. 2003). Metal accumulation in aquatic plants is influenced by a variety of factors, including metal concentrations in the aquatic medium, sediment’s physical and chemical characteristics, exposure period, growth dynamics of the organism, sampling time, and absorption methods (Bonanno 2011). A submerged aquatic plant, C. demersum, was used to assess BCF for the concentration of PHEs in its leaves. As was previously described by Ali and Khan (2017) and Ramachandra et al. (2018), BCF is then computed using the following Eq. (1):
where “C abiotic medium” represents the concentration of PHEs in the abiotic medium, “C organism” denotes the concentration of metals in the body of the macrophyte.
Potential human health risk assessment
Exposure to PHEs deposited in sediments by ingesting and dermatological contact was reflected as a risk factor for human health in this investigation. Health risk assessment indices are frequently employed to estimate cancer and non-cancer hazards. Therefore, numerous studies have been undertaken to determine the potential risk to human health from exposure to PHEs in sediment (Song et al. 2019; Saydam Eker 2020; Varol 2020; Kırıs and Baltas 2021; Ustaoğlu et al. 2022a,b; Yüksel et al. 2022). Individuals may be exposed to hazardous chemicals in three ways: via inhaling, ingesting food, or skin contact. Thus, the risk of injury to human health from skin contact and ingestion of hazardous components in sediment was studied in this research. To compute exposure degrees, the following formulas (2 and 3) were utilized (US EPA 2004, Song et al. 2019).
Inside Formula (2), AT specifies the number of days (non-cancerous; adult 7300 days, child 2190 days; carcinogenic adult 25,550 days); BW reflects the stated weight (adult 70 kg, child 15 kg); Exping denotes the ingestion exposure (mg/kg/day); EF represents the sediment exposure frequency (135 days per year); ED signifies the exposure duration (adult 20 years, child 6 years); IR symbolizes the daily ingestion quantity (adults 100 mg/day, children 200 mg/day); CF indicates the quantification unit conversion factor (106 kg/mg), and the abbreviation Csed refers to the concentration of PHEs in the sediment.
In Formula (3), Expderm reflects the dermal exposure (mg/kg/day); AF symbolizes the adhesion index of the trace metal on unit skin area (adult 0.07 mg/cm2, child 0.2 mg/cm2); SA represents the exposed skin area on the sediment (adult 6032 cm2, child 2373 cm2) and ABS designates the dermal absorption rate (As 0.03, other metals 0.001) (Iqbal et al. 2013; Varol 2020).
The hazard index (HI) and hazard quotient (HQ) were determined using Formulas (4) and (5).
HQing/derm and HI are the best hazard indicators to assess the level of risk that may be expected when exposure occurs through ingestion or dermal routes. Furthermore, reference values for unfavorable health consequences originating from metal contamination are symbolized as RfD. In particular, the reference value derived via swallowing is generally viewed as comparable to that derived via dermatological contact (Iqbal et al. 2013).
Arsenic, cadmium, copper, chromium, and lead are examples of metals that pose a risk of cancer (IARC 2012). Employing Formulas (6) and (7), the total lifetime cancer risk (LCR) associated with each exposure pathway to these PHEs was computed.
Arsenic, chromium, cadmium, and lead are characterized by the slope factors of 1.5, 0.5, 6.3, and 8.50E − 3 mg/kg/day, correspondingly (USEPA 2004). According to the USEPA, the authorized threshold for carcinogenic risk is between 10−4 and 10−6. Carcinogenic risk less than 10–6 is minimal, whereas carcinogenic risk greater than 10−4 exceeds tolerable limit levels.
Statistical analysis
Before statistical analysis, variables were checked for normality using the Kolmogorov–Smirnov test. The test results showed that all PHEs were normally distributed (p > 0.05), except for Mn and Co (p < 0.05). The Krustal-Wallis test was used for Mn and Co, which did not show a normal distribution, and a one-way analysis of variance (ANOVA) was used for the others to evaluate the differences in PHE levels between sampling stations and seasons. SPSS 22.0, minitap 18, and OriginPro 2021 are all examples of statistical software which was utilized for principal component analysis (PCA), cluster analysis (CA), and correlation matrix analysis, respectively. These statistical methods enabled us to assess the likely origins and interrelationships of PHEs in the surface sediments of the research region. Kaiser–Meyer–Olkin (KMO) and Bartlett’s sphericity tests were used to determine whether or not the data set was appropriate for PCA (Dević et al. 2020). A KMO score greater than 0.5 indicates that the data set is acceptable for principal component analysis. Additionally, using the same version of SPSS software, descriptive statistical analyses, Kolmogorov–Smirnov (K–S) tests, and one-way ANOVA analyses were conducted. PAST 4.06 software was used to create box plots, while Origin pro 2021 was employed to obtain the heatmap graphs illustrating Spatio-temporal distributions.
Results and discussions
Assessing the spatiotemporal distribution of PHEs in specimens using a validated ICP-MS method
Since the sediment has a great ability to store PHEs, this buffer is a particularly effective medium for tracking these elements. Sediment specimens can therefore be used to assess the potential risk to microflora and chronic contamination (Sojka et al. 2022). PHEs are naturally occurring ecological components. However, their biochemical stability and geochemical activity have been modified as a result of their widespread use for human benefits. PHEs are eminent ecological pollutants because of their continued toxicity in the bio cumulative landscape and environment. Environmental assets such as marine ecosystems and soil are polluted by great amounts of PHEs, including chromium, cadmium, lead, nickel, and zinc. Volcanic explosions and corrosion of metal-bearing rocks are examples of natural sources, whereas anthropogenic sources can be derived from industrial operations, including mining and agricultural production. Chronic exposure to these PHEs may result in negative health consequences for aquatic life and human life. Irreversibly, contamination of aquatic and coastal habitats with PHEs is a significant ecological problem for public health. Therefore, PHE pollution is a serious issue that must be taken into consideration (Yüksel et al. 2010, 2017, 2021b; Kahal et al. 2020; Ali et al. 2021; Rani et al. 2021).
Numerous instrumental approaches may be conducted to assess PHE concentrations in environmental specimens. Among those methods, the microwave-assisted ICP-MS technique remains a viable option as it has high selectivity and an aptitude for multi-element analysis (Arica et al. 2018; Yüksel et al. 2021a). The concentrations of PHEs in sediment and C. demersum specimens were measured right after the ICP-MS method was proven to be accurate and reliable, based on the method validation report.
Table 2 provides datasets and descriptive statistics for the PHEs analyzed. Thus, the mean concentrations of PHEs in the sediments (in the unit of mg/kg) were as follows: Al (13,133 ± 2514) > Fe (10,790 ± 2444) > Mn (205.84 ± 112.35) > Cu (17.95 ± 7.07) > Cr (16.40 ± 4.06) > Zn (15.55 ± 2.24) > Ni (11.74 ± 3.37) > Pb (9.17 ± 2.31) > Co (6.30 ± 4.00) > As (2.07 ± 1.43) > Cd (0.19 ± 0.02). Our results stress that most of the PHEs in MW are higher than in previously reported values for the sediments of the Terme River (Ustaoğlu et al. 2022a), which is located in the same district. However, the levels of most of the PHEs in MW are lower than in the Çavuşlu stream sediments, which have been impacted by the garbage disposal facility in the vicinity. Based on this comparison, preliminary ideas for PHEs resources in MW are that the resource is more likely lithogenic. Yet, multivariate statistical tests and ecological indicator assessments were then undertaken for more accurate results.
Table 3 shows an overview of our findings in comparison with earlier publications involving the earth’s crust, PEL, and TEL values. Background metal levels were calculated using the global average shale value (Turekian & Wedepohl 1961). Herewith, no PHEs exceeded their corresponding TEL or PEL values in our investigation. Table 2 also provides seasonal fluctuations. As it has been pointed out in Fig. 3, the box and whisker graphs of the PHEs examined demonstrated that Al, Cd, and Zn levels were significantly different in various seasons (p < 0.05). However, this circumstance for cobalt was only observed between stations.
In natural waterways, aluminum ions tend to stay on the sediments, which act as a sink for the organisms that eat them. Several reports have shown that aluminum can have a major effect on the growth of plants and other organisms in acidic environments (Leleyter et al. 2018). In our study, the winter-Al level was significantly higher than in the fall and summer (p < 0.05). This may be explained by the fact that weathering of rocks in the winter season can release more minerals than other seasons in the study region. Therefore, the aluminum content in the surface sediments of MW has a high probability of lithogenic origin.
In 52 different locations throughout the globe, more than 70% of the sediments showed varying degrees of cadmium pollution, which is a priority contaminant that must be addressed globally. Cadmium in sediments impacts both the environment and human health (Jin et al. 2021, 2022). In MW, surface sediment cadmium levels in the fall are lower than in the spring and winter. Since Cd levels were very low and ranged between 0.15 and 0.23 mg/kg, it does not point to any toxic responses.
The anthropogenic sources of zinc contamination in sediments are mostly a result of human activities such as atmospheric dispersion, coal-burning power plants, municipal wastewater discharges, and metal industrial operations, as well as commercial items such as fertilizers and wood preservatives that contain zinc (ATSDR, 2005; Dong et al. 2012). In our study, Zn levels in the winter were significantly higher than in the fall season. Likewise, in aluminum, Zn content in the surface sediment of MW also showed a strong likelihood of being lithogenic in origin. To limit the impacts of PHEs, preventative and corrective steps must be taken. Priority efforts include legal controls, monitoring studies, and the use of soluble and harmless chemicals in environmental compartments. Toxic metals-free pesticides, wastewater treatment plants, and renewable energy sources are other essential measures (Dökmeci 2020).
Estimating the degree of metal enrichment
The most often used techniques for estimating whether an element is of natural or anthropogenic origin are listed as Igeo, CF, and EF. Figure 4 illustrates the Igeo values obtained for PHEs in sediments visually using a heat map. The metals are itemized in the following order based on their average: As (− 3.80), Zn (− 3.34), Al (− 3.33), Ni (− 3.24), Cr (-3.06), Mn (− 2.95), Fe (− 2.91), Co (− -2.87), Cu (− 2.42), Pb (− 1.88), Cd (− 1.88) (-1.35). Since Igeo < 0 means that the sediment is unpolluted, PHEs suggested no contamination in the sediments of MW.
Figure 5 explains the computed CF values for the PHEs using a hot map. For all sites, the mean CF values of the metals were as follows: As (0.6) = Zn (0.16) < Ni (0.17) < Cr (0.18) < Cu (0.40) < Pb (0.46) < Cd (0.63). Given that CF 1 indicates low contamination, it is clear that PHEs exhibited only a little contamination in MW sediments based on the CF results.
The pollution load index (PLI) examines the scale at which surface sediment is related to PHEs that may have an effect on the sedimental microflora and fauna (Yadav & Yadav 2018). In this study, the pollution load index (PLI) for all PHEs were listed in ascending order of Al = As = Zn = 0.16 < Ni (0.17) < Cr (0.18) < Fe (0.23) < Mn (0.24) < Co (0.33) < Cu (0.40) < Pb (0.46) < Cd (0.63). The PLI values for all PHEs ranged between 0.16 and 0.63. Thus, the mean value of PLI was characterized as 0.25 ± 0.15, indicating low pollution in sediments of MW (PLI < 1).
The EF values for the metals in sediment samples were calculated. As seen in Fig. 6, the mean EF values for the metals for all stations were in increasing order of As (0.97), Al (1.00) = Zn (1.00), Ni (1.05), Cr (1.11), Fe (1.39), Mn (1.48), Co (2.02), Cu (2.43), Pb (2.78), Cd (3.86). The mean EF value obtained for arsenic was < 1, which is associated with no enrichment, but the mean EF value calculated for Mn, Fe, Cr, Ni, Zn, and Al was < 2, which is compatible with minor enrichment. On the other hand, Cd, Pb, Cu, and Co had EF values within 2 ≤ EF < 5, suggesting considerable enrichment. Additionally, EF values provide insight into the cause of metal deposition in sediments. When EF exceeds 1.5, the enrichment is most likely due to human factors. However, metals with an EF between 0.05 and 1.50 are considered to have a lithogenic origin (Ravisankar et al. 2015; Tepe et al. 2022). In this research, sources of PHEs (Mn, Fe, Cr, Ni, Zn, Al, and As) with an EF value < 1.5 were identified as lithogenic, while sources of Co, Cu, Pb, and Cd with an EF value > 1.5 were identified as anthropogenic.
Ecotoxicological Investigation of PHEs
The integrated potential ecological risk factor (Eri) is used to quantify the ecological risk associated with a specific aquatic system (Ma and Han 2019). In our study, certain indices (Eri and TRI) were employed to investigate the ecological risks of PHEs. Thus, the mean Eri values of the PHEs for all stations were in ascending order of Zn (0.16) < Cr (0.36) < Ni (0.86) < As (1.59) < Cu (1.99) < Pb (2.28) < Cd (19.99). As can be seen in Fig. 7, the heatmap of Eri for PHEs is illustrated. It was therefore decided that no PHEs posed a significant risk due to the predicted Eri values being lower than 40.
The objective of TRI is to present information concerning the possible ecological harm of all quantified metals combined in the study regions. Reporting the outcome of the TRI examination may facilitate effective decision-making throughout all stages, including academia, industry, government, and non-governmental agencies, as well as the general public. Therefore, all sample stations were assigned TRI values. According to the categorization of TRI values, no stations present a toxicity concern throughout any season (TRI ≤ 5). In contrast, the mean TRI value derived for all stations for the four seasons is 2.00, suggesting that there is no hazardous risk. Figure 8 illustrates the graphical representation of the TRI research.
The modified hazard quotient (mHQ) is a technique for regulating the severity and danger of any PHEs to aquatic and living species. This marker is derived from the assessment of the PHE content in sediments based on the distribution of unfavorable ecological synoptic impacts for the quantitative thresholds such as TEL, PEL, and SEL (Mortazavi 2018). As can be seen in Fig. 9, mHQ values were as follows Zn (0.44) < Cd (0.62) < Pb (0.63) < As (0.73) < Cu (0.87) < Cr (0.88) < Ni (1.07). According to the classification of values, Ni showed low contamination, while Cd, Pb, As, and Cu displayed very low contamination. Lastly, Zn presented minor contamination as its corresponding mHQ level was lower than 0.5.
Contaminant source identification using multivariate statistical analysis
Numerous multivariate data analyses, particularly PCA, HCA, and PCC, were used to determine the source and amount of PHEs accumulated in the sediments of MW. The sample data collected in this investigation was sufficient for PCA, as determined by the KMO test (0.5 ≤ KMO = 0.538) and Bartlett’s sphericity test (p < 0.001). PCA showed three main components with eigenvalues greater than 1, accounting for 81.49% of the total variance (Table S2). Therefore, the component plots in rotated space revealed that the PHEs in the sediment specimens were linked to three distinct sources (Fig. 10).
In PC1, metals (Fe, Cu, Al, and Zn) demonstrated significant positive loading values of more than 0.8, accounting for 40.71 percent of the total variance. Conversely, PC2 explained 22.62 percent of the total variation in terms of hazardous metals (Ni, As, Cr, and Co) with positive loading values greater than 0.6. Regarding PC3, which accounts for 18.16 percent of the total variation, elements (Pb, Cd, and Mn) possess positive loading values greater than 0.6. The PCA indicated that metals in PC1 (Fe, Cu, Al, and Zn) could have originated from lithological inputs, whereas metals in PC2 (Ni, As, Cr, and Co) could be interpreted using both anthropogenic and lithological inputs. However, the metals in PC3 (Cd, Mn, and Pb) were probably related to anthropogenic sources. These findings were also consistent with the outcome of EF and preliminary hypotheses based on ICP-MS results. Given that the roadway was built near MW, it is certainly a substantial contributor to anthropogenic contamination. In addition, agricultural activities such as using fertilizers and pesticides are significant potential sources of anthropogenic pollution.
Next, further verification of PCA was conducted using HCA. According to the results shown in Fig. 11, the outputs of the PCA were matched in the HCA dendrogram, revealing three distinct clusters. The first cluster included the elements iron, copper, zinc, and aluminum, whereas the second cluster had the elements cobalt, chromium, nickel, and arsenic. The elements that made up the third cluster were manganese, cadmium, and lead.
The strength of a linear link between two variables may be measured using PCC as a statistical tool. In other words, high degrees of positive correlation between PHEs imply that the respective metals in sediment are derived from the same inputs (Islam et al. 2018; Yüksel et al. 2022). As a result, PCC was tasked with determining whether or not the various metal concentrations in the sediments are connected. Figure 12 shows the results of the PCC test, which emphasized the compelling correlations between certain PHEs: Zn-Al (r = 0.81), Zn-Fe (r = 0.79), Al-Cu (r = 0.78), Fe-Cu (0.74), Ni-As (0.54), Pb–Cd (0.68), Pb-Mn (r = 0.60), and Mn-Cd (0.52). In this investigation, strong correlations were discovered between PHEs within the same cluster and principal component, suggesting that the outputs of PCA, HCA, and PCC were mutually reinforcing.
Analysis of prospective adverse health effects
The health risk assessments for carcinogenic and non-carcinogenic illnesses that may have been caused by PHEs in sediments were studied both for adults and children who underwent ingestion and skin contact. In other words, the research included the risk of inadvertent silt consumption by children and adults participating in recreational activities in MW water. The results of this health risk assessment are shown in Table 4, which provides essential information on HQ, HI, THI, and LCR values.
HI values were listed in ascending order of Cd < Zn < Mn < Ni < Cu < Fe < Al < As < Cr < Pb < Co for children. Cobalt had the strongest HI value among adults (1.11E-02), whereas zinc had the lowest HI value (2.75E − 05). Typically, HQ and HI values are less than 1.0, indicating that neither children nor adults are at risk of non-carcinogenic risks. In addition, the THI values for children (3.37E − 01) and adults (3.64E − 02) demonstrate that the cumulative effects of 11 PTEs do not provide a non-carcinogenic health risk. The reason why children have greater THI values than adults can be explained by the fact that children are more susceptible to non-cancer health risks that may occur in the form of exposure to PHEs (Varol 2020).
Particular PHEs (Pb, Cd, As, and Cr) were taken into account to estimate LCR solely for adults concerning the cancerogenic health risk associated with ingestion and cutaneous administration. The sequence of decreasing LCR values was as follows: Cr (1.24E − 06) > As (4.71E − 07) > Cd (1.81E − 07) > Pb (1.18E − 08). Consequently, these LCR values calculated in this study are below the target risk limit (1.00E-04), indicating that there is no cancerogenic risk for adults regardless of whether a piece of sediment is unintentionally ingested or contacted dermally (Kırıs & Baltas 2021; Ustaoğlu 2021). Therefore, accidental consumption of MW sediment or skin contact was not anticipated to pose major carcinogenic hazards, highlighting that MW is not contaminated by industrial waste. This point is consistent with previous studies conducted in Terme River (Ustaoğlu et al., 2022a), Sera Lake Nature Park in Trabzon (Ustaoğlu et al. 2022b), Çömlekçi Stream in Giresun (Ustaoğlu, 2021), Çayeli, Rize (Kırıs & Baltas 2021). Çavuşlu stream sediments, in the vicinity of a garbage disposal facility, showed the collective effect of PHEs based on THI and stressed possible non-carcinogenic health risks for children (Yüksel et al. 2022).
Bioconcentration factor of Ceratophyllum demersum
Toxic metals may be accumulated and tolerated by plants growing in aquatic environments with a high content of trace metals. Biomonitoring may therefore be conducted with some of these accumulating plant species, which indicate the mineral composition of their habitats (Favas et al. 2012; Polechońska et al. 2018). Ceratophyllum demersum has no roots and is a rapid-growing subaquatic plant in polluted water. Therefore, it receives alimentary supplements from the surrounding aquatic column without the complex aspect of differential root or shoot absorption of nutrients (Hak et al. 2020). As evident from Table S3, PHEs contents in the C. demersum specimen were as follows: Mn (14,908 ± 8758) > Al (3255 ± 3043) > Fe (3014 ± 2371.06) > Zn (59.25 ± 25.70) > Ni (16.73 ± 3.88) > Co (16.24 ± 15.62) > Cu (14.80 ± 6.21) > As (8.41 ± 6.21) > Cr (3.20 ± 2.38) > Pb (1.53 ± 1.07) > Cd (0.8 ± 0.31).
Researchers have used bio-concentration factor (BCF) to evaluate the amount of PHEs absorbed by plants in their environment since BCF is a ratio of PHE levels in plant tissues to the concentration of elements in water (Zayed et al. 1998). Positive phytoremediation species are those with a BCF level larger than 1000 (Ahmad et al. 2016; Matache et al. 2013). In our research, BCF levels were predicted as in the following order: Cd (716) < Pb (1381) < Ni (1542) < Cr (1930) < As (2104) < Cu (2401) < Zn (2463) < Al (3304) < Fe (4943) < Co (7395) < Mn (33,814). Consequently, all PHEs analyzed, except for Cd, displayed a BCF value of more than 1000, indicating that C. demersum is a promising plant for phytoremediation.
The use of hyperaccumulator plants for the phytoremediation of polluted ecological systems like wetlands is regarded as a cost-effective and environment-friendly strategy. Aquatic plants such as C. demersum can effectively be considered for the phytoremediation of aquatic environments polluted with PHEs (Aasim et al. 2020). Furthermore, Qadri et al. (2022) investigated the capability potential of C. demersum on PHEs removal. They concluded that the tolerance of the macrophytes to contaminated conditions identifies them as a potent and long-lasting phytoremediation technique for restoring damaged ecosystems without disrupting the environmental mechanism. Consistent with the previous literature, our findings demonstrate that it is a potential phytoremediation agent for restoring environmental quality in wetlands.
Conclusion
This research can be considered an inaugural study to examine the accumulation of PHEs in the sediments of MW in the vicinity of the international highway, which is situated in a basin with intense hazelnut and rice farming. Consequently, this study highlights the following themes:
-
The mean concentrations of PHEs in the sediments (in the unit of mg/kg) were as follows: Al (13,133) > Fe (10,790) > Mn (205.84) > Cu (17.95) > Cr (16.40) > Zn (15.55) > Ni (11.74) > Pb (9.17) > Co (6.30) > As (2.07) > Cd (0.19).
-
No PHEs in the sediments exceeded their corresponding TEL, PEL, and average shale values in our investigation.
-
The outcome of the CF and Igeo examinations suggested low or no contamination in the surface sediments of MW.
-
Ecotoxicological risk assessment based on Eri, PLI, and TRI offered low ecological risk, low pollution, and no hazardous risk, respectively.
-
Based on the mHQ classification of values, Ni showed low contamination, while Cd, Pb, As, and Cu displayed very low contamination. Lastly, Zn presented minor contamination.
-
The findings of LCR, HQ, and THI indicated that exposure of adults or children to sediments containing PHEs would not represent a major health risk.
-
Data analysis based on multivariate statistics (PCC, HCA, and PCA) revealed that the PHEs deposits in the sediments of MW were associated with lithological/geological origin and human influences.
-
Certain PHEs in PC3 (Cd, Mn, and Pb) were related to anthropogenic inputs, agreeing with the findings of EF and the initial hypothesis based on ICP-MS data.
-
Seeing that the highway was constructed close to MW, it is undoubtedly a significant source of human-caused pollution.
-
Agricultural operations such as the use of fertilizers and pesticides are also substantial potential sources of human-made pollution.
-
To enhance the sediment quality of the MW, it is necessary to avoid the direct entrance of agricultural pesticides and fertilizers.
-
C. demersum is a promising plant for phytoremediation in PHE-polluted ecological systems involving wetlands, and it can efficiently be used as an indicator species in biological monitoring studies.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
References
Aasim M, Aydın S, Karakaş M, Aydın ME, Soğukpınar C, Sevinc C (2020) Bioaccumulation potential of In vitro regenerated plants of Ceratophyllum demersum against Chromium - a lab study. Asian J Agric Biol 8(3):233–239. https://doi.org/10.35495/ajab.2019.11.516
Agency for Toxic Substances and Disease Registry (ATSDR) (2005) Toxicological profile for Zinc. U.S. Department of Health and Human Services, Public Health Service, Atlanta
Ahmad SS, Reshi ZA, Shah MA (2016) Heavy metal accumulation in the leaves of Potamogeton natans and Ceratophyllum demersum in a Himalayan RAMSAR site: management implications. Wetl Ecol Manag 24:469–475. https://doi.org/10.1007/s11273-015-9472-9
Akinci I, Tutkun E, Turksoy VA, Yilmaz H, Yuksel B, Kayaalti Z, Soylemezoglu T, Yılmaz H, Abusoglu S (2016) Toxic metal and essential trace element levels of blood donors. J Clin Anal Med 7(6):816–819. https://doi.org/10.4328/jcam.4630
Ali MM, Hossain D, Imran A, Khan S, Begum M, Osman HM (2021) Environmental pollution with heavy metals: a public health risk concern. In Nazal MK, Zhao H (Eds), Heavy Metals-Their Environmental Impacts and Mitigation. IntechOpen. https://doi.org/10.5772/intechopen.96805
Ali H, Khan E (2017) Environmental chemistry in the twenty-first century. Environ Chem Lett 15:329–346. https://doi.org/10.1007/s10311-016-0601-3
Alkan N, Alkan A, Erüz C (2018) Determination of metals in water and sediment samples of the Sürmene River, Turkey. In Aloui F, Dincer I (Eds), Exergy for A Better Environment and Improved Sustainability 2: Applications (pp. 175–181). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-319-62575-1_13
Arica E, Yuksel B, Yener I, Dolak I, Gok E, Yilmaz E (2018) ICP-MS determination of lead levels in autopsy liver samples: an application in forensic medicine. Atmos Spectrosc 39(2):62–66. https://doi.org/10.46770/AS.2018.02.002
Arisekar U, Shakila RJ, Shalini R, Jeyasekaran G, Keerthana M, Arumugam N, Almansour AI, Perumal K (2022) Distribution and ecological risk assessment of heavy metals using geochemical normalization factors in the aquatic sediments. Chemosphere 294:133708. https://doi.org/10.1016/j.chemosphere.2022.133708
Arnot JA, Gobas FA (2006) A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev 14(4):257–297. https://doi.org/10.1139/a06-005
Aydin H, Ustaoğlu F, Tepe Y, Soylu EN (2021) Assessment of water quality of streams in northeast Turkey by water quality index and multiple statistical methods. Environ Forensics 22(1–2):270–287. https://doi.org/10.1080/15275922.2020.1836074
Batley GE, Simpson S (2013) Sediment quality guidelines. In: Férard JF, Blaise C (eds) Encyclopedia of Aquatic Ecotoxicology. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5704-2_92
Batley GE, Warne MSJ (2017) Harmonization of water and sediment quality guideline derivation. Integr Environ Assess Manag 13(3):458–459. https://doi.org/10.1002/ieam.1903
Benson NU, Adedapo AE, Fred-Ahmadu OH, Williams AB, Udosen ED, Ayejuyo OO, Olajire AA (2018) New ecological risk indices for evaluating heavy metals contamination in aquatic sediment: a case study of the Gulf of Guinea. Reg Stud Mar Sci 18:44–56. https://doi.org/10.1016/j.rsma.2018.01.004
Böke Özkoç H, Arıman S (2022) Contamination and risk assessment of heavy metals in coastal sediments from the Mid-Black Sea, Turkey. Stoch Environ Res Risk Assess. https://doi.org/10.1007/s00477-022-02300-4
Bonanno G (2011) Trace element accumulation and distribution in the organs of Phragmites australis (common reed) and biomonitoring applications. Ecotoxicol Environ Saf 74(4):1057–1064. https://doi.org/10.1016/j.ecoenv.2011.01.018
Bozalan M, Turksoy VA, Yüksel B, Güvendik G, Soylemezoglu T (2019) Preliminary assessment of lead levels in soft plastic toys by flame atomic absorption spectroscopy. Turk Hij Den Biyol Derg 76(3):243–254. https://doi.org/10.5505/TurkHijyen.2019.58234
Canpolat Ö, Varol M, Okan ÖÖ, Eriş KK (2022) Sediment contamination by trace elements and the associated ecological and health risk assessment: a case study from a large reservoir (Turkey). Environ Res 204:112145. https://doi.org/10.1016/j.envres.2021.112145
Coşkun A, Dengiz O (2016) Determination of some basic physiographic characteristics and soil mapping for flood lands. Turk J Agric Res 3(1):1–13. https://doi.org/10.19159/tutad.55780
Cüce H, Kalipci E, Ustaoğlu F, Dereli MA, Türkmen A (2022b) Integrated spatial distribution and multivariate statistical analysis for assessment of ecotoxicological and health risks of sediment metal contamination, Ömerli Dam (Istanbul, Turkey). Wat Air Soil Poll 233:199. https://doi.org/10.1007/s11270-022-05670-1
Cüce H, Kalipci E, Ustaoglu F, Baser V, Türkmen M (2022a) Ecotoxicological health risk analysis of potential toxic elements accumulation in the sediments of Kızılırmak River. Int J Environ Sci Techno.https://doi.org/10.1007/s13762-021-03869-z
Cüce H, Kalipci E, Ustaoglu F, Kaynar İ, Baser V, Türkmen M (2022c) Multivariate statistical methods and GIS based evaluation of the health risk potential and water quality due to arsenic pollution in the Kızılırmak River. Int J Sediment Reshttps://doi.org/10.1016/j.ijsrc.2022c.06.004
Dević GJ, Ilić MV, Zildzović SN, Avdalović JS, Miletić SB, Bulatović SS, Vrvić MM (2020) Investigation of potentially toxic elements in urban sediments in Belgrade, Serbia. Environ. Sci. Health Part A Toxic Hazard. Subst Environ Eng 55:765–775. https://doi.org/10.1080/10934529.2020.1741999
Doğan M, Çavuşoğlu K, Yalçin E, Acar A (2022) Comprehensive toxicity screening of Pazarsuyu stream water containing heavy metals and protective role of lycopene. Scientific Reports, Sci Rep 12:16615. https://doi.org/10.1038/s41598-022-21081-y
Dökmeci AH (2020) Environmental impacts of heavy metals and their bioremediation. In Nazal MK, Zhao H (Eds), Heavy Metals - Their Environmental Impacts and Mitigation, IntechOpen, London. https://doi.org/10.5772/intechopen.95103
Dong C, Chen C, Chen C (2012) Contamination of zinc in sediments at river mouths and channel in Northern Kaohsiung Harbor. Taiwan Int J Environ Sci Dev 3:517–521. https://doi.org/10.7763/IJESD.2012.V3.278
Dundar MS, Altundag H, Eyupoglu V, Keskin SC, Tutunoglu C (2012) Determination of heavy metals in lower Sakarya River sediments using a BCR-sequential extraction procedure. Environ Monit Assess 84(1):33–41. https://doi.org/10.1007/s10661-011-1944-7
Favas PJ, Pratas J, Prasad MN (2012) Accumulation of arsenic by aquatic plants in large-scale field conditions: opportunities for phytoremediation and bioindication. Sci Total Environ 433:390–397. https://doi.org/10.1016/j.scitotenv.2012.06.091
Fural Ş, Kükrer S, Cürebal İ (2020) Geographical information systems based ecological risk analysis of metal accumulation in sediments of İkizcetepeler Dam Lake (Turkey). Ecol Ind 119:106784. https://doi.org/10.1016/j.ecolind.2020.106784
Haghnazar H, Hudson-Edwards KA, Kumar V, Pourakbar M, Mahdavianpour M, Aghayani E (2021) Potentially toxic elements contamination in surface sediment and indigenous aquatic macrophytes of the Bahmanshir River, Iran: Appraisal of phytoremediation capability. Chemosphere 285:131446. https://doi.org/10.1016/j.chemosphere.2021.131446
Hak K, Ritchie RJ, Dummee V (2020) Bioaccumulation and physiological responses of the Coontail, Ceratophyllum demersum exposed to copper, zinc and in combination. Ecotoxicol Environ Saf 189:110049. https://doi.org/10.1016/j.ecoenv.2019.110049
Hakanson L (1980) An ecological risk index for aquatic pollution control a sedimentological approach. Water Res 14(8):975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
IARC (International Agency for Research on Cancer) (2012) Personal habits and indoor combustions. IARC Monogr Eval Carcinog Risks Hum 100E:373–501
Iqbal J, Tirmizi SA, Shah MH (2013) Statistical apportionment and risk assessment of selected metals in sediments from Rawal Lake (Pakistan). Environ Monit Assess 185(1):729–743. https://doi.org/10.1007/s10661-012-2588-y
Islam MS, Hossain MB, Matin A, Sarker MSI (2018) Chemosphere assessment of heavy metal pollution, distribution and source apportionment in the sediment from Feni River Estuary, Bangladesh. Chemosphere 202:25–32. https://doi.org/10.1016/j.chemosphere.2018.03.077
Jaskuła J, Sojka M (2022) Assessment of spatial distribution of sediment contamination with heavy metals in the two biggest rivers in Poland. Catena 211:105959. https://doi.org/10.1016/j.catena.2021.105959
Jaskuła J, Sojka M, Fiedler M, Wrożyński R (2021) Analysis of spatial variability of river bottom sediment pollution with heavy metals and assessment of potential ecological hazard for the Warta River, Poland. Minerals 11(3):327. https://doi.org/10.3390/min11030327
Jin C, Li Z, Huang M, Wen J, Ding X, Zhou M, Cai C (2021) Laboratory and simulation study on the Cd(II) adsorption by lake sediment: Mechanism and influencing factors. Environ Res 197:111138. https://doi.org/10.1016/j.envres.2021.111138
Jin C, Li Z, Huang M (2022) Cadmium immobilization in lake sediment using different crystallographic manganese oxides: Performance and mechanism. J Environ Manage 313:114995. https://doi.org/10.1016/j.jenvman.2022.114995
Kahal A, El-Sorogy AS, Qaysi S, Almadani S, Kassem OM, Al-Dossari A (2020) Contamination and ecological risk assessment of the Red Sea coastal sediments, southwest Saudi Arabia. Mar Pollut Bull 154:111125. https://doi.org/10.1016/j.marpolbul.2020.111125
Kırıs E, Baltas H (2021) Assessing pollution levels and health effects of heavy metals in sediments around Cayeli copper mine area, Rize. Turkey Environ Forensics 22(3–4):372–384. https://doi.org/10.1080/15275922.2020.1850572
Kukrer S, Tunc IO, Erginal AE, Bay Ö, Kılıç Ş (2021) Distribution, sources and ecological risk assessment of metals in Kura river sediments along a human disturbance gradient. Environ Forensicshttps://doi.org/10.1080/15275922.2021.1940378
Kumar S, Islam ARMT, Hasanuzzaman M, Salam R, Islam MS, Khan R, Rahman MS, Pal SC, Ali MM, Idris AM, Gustave W, Elbeltagi A (2022) Potentially toxic elemental contamination in Wainivesi River, Fiji impacted by gold-mining activities using chemometric tools and SOM analysis. Environ Sci Pollut Res 29(28):42742–42767. https://doi.org/10.21203/rs.3.rs-941620/v1
Leleyter L, Baraud F, Reinert T, Gouali S, Lemoine M, Gil O (2018) Fate of aluminium released by sacrificial anodes – contamination of marine sediments by environmentally available compounds. C R Geosci 350(5):195–201. https://doi.org/10.1016/j.crte.2018.05.003
Ma L, Han C (2019) Water quality ecological risk assessment with sedimentological approach. In: Summers K (ed) Water Quality-Science, Assessments and Policy. IntechOpen, London, pp 1–6. https://doi.org/10.5772/intechopen.88594
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39(1):20–31. https://doi.org/10.1007/s002440010075
Mahmoud KM, Mahmoud HA, Sayed SS (2018) Potential role of Ceratophyllum demersum in bioaccumulation and tolerance of some heavy metals. Egypt J Aquat Biol Fish 22(4):1–12. https://doi.org/10.21608/EJABF.2018.9738
Matache ML, Marin C, Rozylowicz TA (2013) Plants accumulating heavy metals in the Danube River wetlands. J Environ Health Sci Engineer 11:39. https://doi.org/10.1186/2052-336X-11-39
McGeer JC, Brix KV, Skeaf JM, DeForest DK, Brigham SI, Adams WJ, Green A (2003) Inverse relationship between bioconcentration factor and exposure concentration for metals: implications for hazard assessment of metals in the aquatic environment. Environ Toxicol Chem 22:1017e1037. https://doi.org/10.1002/etc.5620220509
Mortazavi S (2018) Survey of modified hazard quotient, potential ecological risk factor and toxicity units of heavy metals in surface sediments of some wetlands of Iran. Arch Hyg Sci 7(4):251–263
Müller G (1981) Die Schwermetallbelastung der sedimente des Neckars und seiner Nebenflüsse: eine Bestandsaufnahme. Chem Ztg 105:157–164
Polechońska L, Klink A, Dambiec M, Rudecki A (2018) Evaluation of Ceratophyllum demersum as the accumulative bioindicator for trace metals. Ecol Indic 93:274–281. https://doi.org/10.1016/j.ecolind.2018.05.020
Proshad R, Al-Mamaun A, Islam MS, Khadka S, Kormoker T, Uddin MM, Modeo L (2021) Investigation of trace metals in riverine waterways of Bangladesh using multivariate analyses: spatial toxicity variation and potential health risk assessment. Environ Sci Pollut Res 28:31872–31884. https://doi.org/10.1007/s11356-021-13077-4
Qadri H, Uqab B, Javeed O, Dar GH, Bhat RA (2022) Ceratophyllum demersum-An accretion biotool for heavy metal remediation. Sci Total Environ 806(2):150548. https://doi.org/10.1016/j.scitotenv.2021.150548
Radomirović M, Stanković S, Mandić M, Jović M, Mandić LJ, Dragović S, Onjia A (2021a) Spatial distribution, radiological risk assessment and positive matrix factorization of gamma-emitting radionuclides in the sediment of the Boka Kotorska Bay. Mar Pollut Bull 169:112491. https://doi.org/10.1016/j.marpolbul.2021.112491
Radomirović M, Mijatović N, Vasić M, Tanaskovski B, Mandić M, Pezo L, Onjia A (2021b) The characterization and pollution status of the surface sediment in the Boka Kotorska Bay, Montenegro. Environ Sci Pollut Res 28:53629–53652. https://doi.org/10.1007/s11356-021-14382-8
Ramachandra TV, Sudarshan PB, Mahesh MK, Vinay S (2018) Spatial patterns of heavy metal accumulation in sediments and macrophytes of Bellandur wetland, Bangalore. J Environ Manage 206:1204–1210. https://doi.org/10.1016/j.jenvman.2017.10.014
Rani S, Ahmed K, Xiongzhi X, Keliang C, Islam S, Habibullah-Al-Mamun, (2021) Occurrence, spatial distribution and ecological risk assessment of trace elements in surface sediments of rivers and coastal areas of the East Coast of Bangladesh, North-East Bay of Bengal. Sci Total Environ 801:149782. https://doi.org/10.1016/j.scitotenv.2021.149782
Ravisankar R, Sivakumar S, Chandrasekaran A, Kanagasabapathy KV, Prasad MVR, Satapathy KK (2015) Statistical assessment of heavy metal pollution in sediments of the east coast of Tamilnadu using Energy Dispersive X-ray Fluorescence Spectroscopy (EDXRF). Appl Radiat Isot 102:42–47. https://doi.org/10.1016/j.apradiso.2015.03.018
Saydam Eker Ç (2020) Distinct contamination indices for evaluating potentially toxic element levels in stream sediments: a case study of the Harşit Stream (NE Turkey). Arab J Geosci 13:1175. https://doi.org/10.1007/s12517-020-06178-w
Şimşek A, Özkoç HB, Bakan G (2022) Environmental, ecological and human health risk assessment of heavy metals in sediments at Samsun-Tekkeköy, North of Turkey. Environ Sci Pollut Res 29:2009–2023. https://doi.org/10.1007/s11356-021-15746-w
Sojka M, Jaskuła J (2022) Heavy metals in river sediments: contamination, toxicity, and source identification–a case study from Poland. Int J Environ Res Public Health 19(17):10502. https://doi.org/10.3390/ijerph191710502
Sojka M, Jaskuła J, Barabach J, Ptak M, Zhu S (2022) Heavy metals in lake surface sediments in protected areas in Poland: concentration, pollution, ecological risk, sources and spatial distribution. Sci Rep 12:15006. https://doi.org/10.1038/s41598-022-19298-y
Song J, Liu Q, Sheng Y (2019) Distribution and risk assessment of trace metals in riverine surface sediments in gold mining area. Environ Monit Assess 191(3):191. https://doi.org/10.1007/s10661-019-7311-9
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu. Hawaii Environ Geol 39(6):611–627. https://doi.org/10.1007/s002540050473
Tepe Y, Şimşek A, Ustaoğlu F, Taş B (2022) Spatial–temporal distribution and pollution indices of heavy metals in the Turnasuyu Stream sediment. Turkey Environ Monit Assess 194(11):818. https://doi.org/10.1007/s10661-022-10490-1
Tokatli C (2019) Sediment quality of Ergene River Basin: bio-ecological risk assessment of toxic metals. Environ Monit Assess 191(11):706. https://doi.org/10.1007/s10661-019-7885-2
Tokatli C, Ustaoğlu F (2020) Health risk assessment of toxicants in Meriç river delta wetland, Thrace region. Turkey Environearth Sci 79:426. https://doi.org/10.1007/s12665-020-09171-4
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol Meeresunters 33(1):566. https://doi.org/10.1007/BF02414780
Töre Y, Ustaoğlu F, Tepe Y, Kalipci E (2021) Levels of toxic metals in edible fish species of the Tigris River (Turkey); threat to public health. Ecol Indic 123:107361. https://doi.org/10.1016/j.ecolind.2021.107361
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Bull Geol Soc Am 72(2):175–192. https://doi.org/10.1130/0016-7606(1961)72[175:DOTEIS]2.0.CO;2
USEPA (2004) Risk assessment guidance for superfund, Vol. 1, Human health evaluation manual. Part E (supplemental guidance for dermal risk assessment), EPA/540/R/99/005.Office of Superfund Remediation and Technology Innovation, Washington, DC
Ustaoğlu F (2021) Ecotoxicological risk assessment and source identification of heavy metals in the surface sediments of Comlekci stream, Giresun, Turkey. Environ Forensics 22:130–142. https://doi.org/10.1080/15275922.2020.1806148
Ustaoğlu F, Tepe Y (2018) Determination of the Sediment Quality of Pazarsuyu Stream (Giresun, Turkey) by multivariate statistical methods. Turk J Food Agric Sci 6(3):304–312. https://doi.org/10.24925/turjaf.v6i3.304-312.1696
Ustaoğlu F, Tepe Y, Aydin H (2020) Heavy metals in sediments of two nearby streams from Southeastern Black Sea coast: Contamination and ecological risk assessment. Environ Forensics 21(2):145–156. https://doi.org/10.1080/15275922.2020.1728433
Ustaoğlu F, Kükrer S, Taş B, Topaldemir H (2022a) Evaluation of metal accumulation in Terme River sediments using ecological indices and a bioindicator species. Environ Sci Pollut Res 29:47399–47415. https://doi.org/10.1007/s11356-022-19224-9
Ustaoğlu F, Islam MS, Tokatli C (2022b) Ecological and probabilistic human health hazard assessment of heavy metals in Sera Lake Nature Park sediments (Trabzon, Turkey). Arab J Geosci 15:597. https://doi.org/10.1007/s12517-022-09838-1
Varol M (2020) Environmental, ecological and health risks of trace metals in sediments of a large reservoir on the Euphrates River (Turkey). Environ Res 187:109664. https://doi.org/10.1016/j.envres.2020.109664
Varol M, Şen B (2012) Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris River, Turkey. CATENA 92:1–10. https://doi.org/10.1016/j.catena.2011.11.011
Varol M, Canpolat Ö, Eriş KK, Çağlar M (2020) Trace metals in core sediments from a deep lake in eastern Turkey: vertical concentration profiles, eco-environmental risks and possible sources. Ecotoxicol Environ Saf 189:110060. https://doi.org/10.1016/j.ecoenv.2019.110060
Varol M, Ustaoğlu F, Tokatlı C (2022a) Ecological risks and controlling factors of trace elements in sediments of dam lakes in the Black Sea Region (Turkey). Environ Res 205:112478. https://doi.org/10.1016/j.envres.2021.112478
Varol M, Ustaoğlu F, Tokatlı C (2022b) Ecological risk assessment of metals in sediments from three stagnant water bodies in Northern Turkey. Curr Pollution Rephttps://doi.org/10.1007/s40726-022-00239-2
Xia P, Ma L, Sun R, Yang Y, Tang X, Yan D, Lin T, Zhang Y, Yi Y (2020) Evaluation of potential ecological risk, possible sources and controlling factors of heavy metals in surface sediment of Caohai Wetland. China Sci Total Environ 740:14023. https://doi.org/10.1016/j.scitotenv.2020.140231
Xiao H, Shahab A, Li J, Xi B, Sun X, He H, Yu G (2019) Distribution, ecological risk assessment and source identification of heavy metals in surface sediments of Huixian karst wetland. China Ecotoxicol Environ Saf 185:109700. https://doi.org/10.1016/j.ecoenv.2019.109700
Yadav A, Yadav PK (2018) Pollution load index (PLI) of field irrigated with wastewater of Mawaiya drain in Naini suburbs of Allahabad district. Curr World Environ 13(1):159–164. https://doi.org/10.12944/CWE.13.1.15
Yüksel B, Mergen G, Söylemezoğlu T (2010) Assessment of arsenic levels in human hair by hydride generation atomic absorption spectrometry: a toxicological application. At Spectrosc 31(1):1–5
Yüksel B, Kayaalti Z, Söylemezoglu T, Türksoy VA, Tutkun E (2015) GAAS determination of arsenic levels in biological samples of workers occuputionally exposed to metals: an application in analytical toxicology. At Spectrosc 36(4):171–176. https://doi.org/10.46770/as.2015.04.004
Yüksel B, Kaya S, Kaya-Akyüzlü D, Kayaaltı Z, Söylemezoglu T (2017) Validation and optimization of an analytical method based on cold vapor atomic absorption spectrometry for the determination of mercury in maternal blood, cord blood, and placenta samples. At Spectrosc 38(4):112–116
Yüksel B, Şen N, Türksoy VA, Tutkun E, Söylemezoğlu T (2018) Effect of exposure time and smoking habit on arsenic levels in biological samples of metal workers in comparison with controls. Marmara Pharm J 22(2):218–226. https://doi.org/10.12991/mpj.2018.59
Yüksel B, Ustaoğlu F, Arıca E (2021) Impacts of a garbage disposal facility on the water quality of Çavuşlu Stream in Giresun, Turkey: a health risk assessment study by a validated ICP-MS assay. Aquat Sci Eng 36(4):181–192. https://doi.org/10.26650/ASE2020845246
Yüksel B, Arıca E, Söylemezoğlu T (2021b) Assessing reference levels of nickel and chromium in cord blood, maternal blood and placenta specimens from Ankara, Turkey. J Turk Ger Gynecol Assoc 22(3):187–195. https://doi.org/10.4274/jtgga.galenos.2021.2020.0202
Yüksel B, Ustaoğlu F, Tokatli C, Islam MS (2022) Ecotoxicological risk assessment for sediments of Çavuşlu stream in Giresun, Turkey: association between garbage disposal facility and metallic accumulation. Environ Sci Pollut Res 29:17223–17240. https://doi.org/10.1007/s11356-021-17023-2
Yuksel B, Arica E (2018) Assessment of toxic, essential, and other metal levels by ICP-MS in lake Eymir and Mogan in Ankara, Turkey: an environmental application. At Spectrosc 39:179–184. https://doi.org/10.46770/AS.2018.05.001
Zayed A, Gowthaman S, Terry N (1998) Phytoaccumulation of traces elements by wetland plants: I. Duckweed J Environ Qual 27:715–721
Zedler JB, Kercher S (2005) Wetland Resources: Status, Trends, Ecosystem Services, and Restorability. Annu Rev Environ Resour 30:39–74. https://doi.org/10.1146/annurev.energy.30.050504.144248
Zhang G, Bai J, Zhao Q, Lu Q, Jia J, Wen X (2016) Heavy metals in wetland soils along a wetland-forming chronosequence in the Yellow River Delta of China: levels, sources and toxic risks. Ecol Indic 69:331–339. https://doi.org/10.1016/j.ecolind.2016.04.042
Zhang Y, Li H, Yin J, Zhu L (2021) Risk assessment for sediment associated heavy metals using sediment quality guidelines modified by sediment properties. Environ Pollut 275:115844. https://doi.org/10.1016/j.envpol.2020.115844
Funding
This research study was financially supported by the Ordu University Scientific Research Projects Coordination Unit (BAP). Project Number: B-1912.
Author information
Authors and Affiliations
Contributions
HT and BT established the idea of the paper and participated in its project and organization. BY prepared the manuscript. FU contributed to the acquisition and explanation of data. HT, BT, BY and FU provided a critical review and significantly revised the manuscript text. All authors read and agreed with the ultimate manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Alexandros Stefanakis
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Topaldemir, H., Taş, B., Yüksel, B. et al. Potentially hazardous elements in sediments and Ceratophyllum demersum: an ecotoxicological risk assessment in Miliç Wetland, Samsun, Türkiye. Environ Sci Pollut Res 30, 26397–26416 (2023). https://doi.org/10.1007/s11356-022-23937-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23937-2