Abstract
Pen shells (PS), a type of shellfish, are abundantly consumed, and their inedible shell residues are often discarded near the coast without consideration of reutilization. This study sought to investigate the use of natural pen shells (NPS) and calcined pen shells (CPS) to stabilize Pb and As-contaminated soil. During the investigation, NPS and CPS were applied to the contaminated soil in amounts ranging from 1 to 10 wt% and cured for 28 days. After the curing process, the mineral phase was examined through X-ray powder diffraction (XRD) and scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM–EDX) analysis. The XRD and SEM–EDX results revealed the presence of riversideite and ettringite, which contribute to Pb and As stabilization in the CPS-treated soil. The leachability of Pb and As in the treated soil was further examined with three types of chemical extraction methods. Extraction results using 0.1 M HCl displayed a notable pH fluctuation in the extractant due to the residual amendments (NPS and CPS). The fluctuation resulted in a strong correlation of leached Pb and As with the pH of the extractant, which might hinder an accurate assessment of stabilization. In order to minimize the effect of pH, an EDTA-NH4OAc extraction was employed, suggesting its potential as a suitable assessment method. EDTA-NH4OAc extraction showed a higher effectiveness of CPS than NPS at 10 wt% of input amounts. In the SBET extraction, that uses a strongly acidic solution, a higher As leachability was observed by increasing the addition of CPS, which implied a CPS-related chemical fixation mechanism. The comparison of various extraction methods showed a higher CPS effectiveness as compared to NPS. However, it was recommended that CPS-treated soil required caution in strongly acidic conditions, especially for arsenic. This study explores the applicability of PS, which has not been investigated as an amendment for Pb and As-contaminated soil previously. Furthermore, this study revealed that utilization of various extraction methods is beneficial for gaining a comprehensive understanding of the role of CaCO3-based amendment in Pb and As-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past, the mining industry in the Republic of Korea thrived. However, there are 5100 abandoned mines, which result in large quantities of mining waste generation (KOMIR 2023). This abandoned waste in mining areas contains numerous toxic elements including heavy metals and metalloids (Moon et al. 2021). Among them, lead (Pb) and arsenic (As) are known as major pollutants that require significant consideration due to their severe toxicity (McBride et al. 2013; Raffa et al. 2021). Over time, the waste including a considerable amount of pollutants could be transported into the soil through wind and water (Karaca et al. 2018). Under these conditions, if pollutants are allowed to accumulate in the soil, they can cause long-term and continuous damage to animal and human health (Abreu et al. 2008; Bueno et al. 2009). Therefore, it is crucial to manage Pb and As contamination in the soil media to prevent risks to the ecosystem.
To remediate contaminated soils from mining activity, various methods have been reported including soil washing, phytoremediation, and electrokinetic techniques (Karaca et al. 2018; Liu et al. 2018; Moon et al. 2021). However, these methods are known to have issues associated with wastewater generation, high operational cost, or time consumption (Satyro et al. 2017; Liu et al. 2018). On the other hand, stabilization/solidification (S/S) is a technique aimed to minimize the negative impacts of pollutants on living organisms including humans. S/S of contaminated soil can be achieved by chemical amendments which reduce the mobility and toxicity of pollutants by encapsulation, precipitation, and adsorption processes (Yoon et al. 2010; Moon et al. 2018). Additionally, S/S has a cost advantage over other techniques (Lee et al. 2011), and its application is straightforward (Liu et al. 2018). As a matter of fact, S/S has been one of the most used techniques for contaminated soil at Superfund sites in the United States so far (USEPA 2023). Among many amendments, Portland cement and quicklime have been widely used for contaminated soil because of their cost-effectiveness (Correia et al. 2020). However, these materials are in high demand by numerous industries all over the world, raising environmental sustainability issues related to the depletion of natural resources such as limestone (Tun et al. 2020). Therefore, development of sustainable, eco-friendly, and renewable stabilizing amendments for the contaminated soil is an essential and sound proposition.
In this context, waste-based amendments, such as red mud, steel slag, fly ash, acid mine drainage (AMD) sludge, and oyster shells, have been utilized to stabilize contaminated soils (Moon et al. 2013, 2015; Ko et al. 2015). Among these amendments, recycling oyster shells as a CaCO3-based material has gained a great deal of attention from several Korean research groups (Moon et al. 2010, 2015; Hong et al. 2010; Ok et al. 2011). This is due to the fact that oyster shells are the most produced shellfish in the Republic of Korea, with a production of 320,000 tons in 2021 (KOSIS 2023). Furthermore, upon consumption, oyster shells are often discarded along the seashore resulting in nuisance associated with repulsive odor and landscape degradation (Moon et al. 2013). The utilization of both natural and calcined oyster shells for the remediation of heavy metals and arsenic-contaminated soil, as a material reuse strategy, has been demonstrated to be highly effective (Ok et al. 2011; Moon et al. 2013). Consequently, other various CaCO3-based marine wastes such as mussel shells, cockle shells, and starfish have subsequently been investigated for their potential to stabilize contaminated soil (Ahmad et al. 2014; Islam et al. 2017; Moon et al. 2018; Park et al. 2023). Along with these CaCO3-based marine wastes, pen shells (PS, Atrina pectinata) are also abundantly produced shellfish in the Republic of Korea, with a production of 8300 tons in 2021 (KOSIS 2023), and they cause adverse effects in coastal areas similar to oyster shells (Jeon 2018). The government of Republic of Korea has recently enacted a regulation encouraging the reutilization of abandoned shellfish including PS (Park et al. 2023). However, little research has been reported in terms of the reuse of PS (Jeon 2018). Furthermore, to the best of our knowledge, there has been no research on the use of PS as a stabilizing amendment for contaminated soil to date.
Single extraction methods such as 0.1 M HCl, TCLP (toxic characteristic leaching procedure), EDTA-NH4OAc, and SBET (simplified bioaccessibility extraction test) have been broadly employed to assess the effectiveness of stabilizing amendments in contaminated soil (Kim et al. 2002; Ok et al. 2011; Moon et al. 2018; Aziz et al. 2019). In essence, these methods can indirectly predict mobile contaminants after the stabilization process (Han et al. 2020a). However, each extraction method may lead to contrasting results owed to differences in principal and chemical characteristics. For instance, Li et al. verified the reduction of As concentration in Portland cement stabilized soils by the DTPA extraction method, whereas As increase was observed by the TCLP method (Li et al. 2017). In this view, recent studies have utilized various extraction methods for the comprehensive evaluation of the stabilized soil (Aziz et al. 2019; Han et al. 2020b; Li et al. 2017).
Therefore, the purpose of this research was to evaluate the feasibility of using natural pen shells (NPS) and calcined pen shells (CPS) as stabilizing amendments for Pb and As-contaminated soil. Three extraction methods, 0.1 M HCl, EDTA-NH4OAc, and SBET, were applied to evaluate the stabilizing effect of PS in the contaminated soil. Additionally, the stabilization mechanism was investigated with scanning electron microscopy equipped with energy-dispersive X-ray (SEM–EDX) and X-ray diffraction analysis (XRD).
Materials and methods
Soil characterization
Pb- and As-contaminated soil was collected from the paddy fields around the Pungjeong mine in Bonghwa-gun, Gyeongsang province in the Republic of Korea. The collected soil was completely air-dried, mixed homogeneously, and then passed through a #10-mesh size sieve for the subsequent experiments. Soil pH and EC values were measured at a soil-to-water ratio of 1:5 (Lee et al. 2013; MOE 2018). Soil mechanical analysis was used to determine sand, silt, and clay portions in the soil (Miller and Miller 1987). The loss of ignition (LOI) method was employed to assess the organic content in solid materials (Schulte and Hopkins 1996). The organic content in the soil was calculated by the weight variation of soil samples before and after ignition. Exchangeable cations such as Ca, Mg, K, and Na were evaluated by 1 M-NH4OAc solution at pH 7 (RDA 2013). Total Pb and As concentrations were evaluated using the following procedures (MOE 2018); (1) the air-dried soil was pulverized to a size less than #100-mesh (0.149 mm); 0.25 g of soil was weighed and placed into a reaction bottle; (2) 3 mL of hydrochloric acid and 1 mL of nitric acid were added to the bottle; (3) the lid was then closed, and the mixture was heated in a heating block at 70 °C for 1 h; (4) after heating, 6 mL of distilled water was added to the reaction container, the lid was sealed, and the contents were thoroughly mixed using a vortex mixer; (5) the supernatant, obtained after centrifuging at 3000 rpm for 15 min, was filtered using a polyvinylidene fluoride (PVDF) syringe filter with a pore size of 0.45 μm. Subsequently, the filtered solution was analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES, iCAP7400DUO, Thermo Fisher Scientific, USA). The obtained soil physicochemical characteristics are presented in Table 1.
Preparation of stabilizing amendments
Pen shells (PS) to be used as amendments were purchased from a market. After removing the edible core, the surface of PS was thoroughly washed with tap water and brushed to eliminate salt residues. It was then rinsed three times with deionized water and left in a hood to dry completely. All types of dried PS were ground with a mixer and sorted into two groups: those passing through #10-mesh (2 mm) and a 20-mesh (0.85 mm) sieve. The samples sorted through #20-mesh were labeled as NPS (natural pen shells). A portion of PS passed through a #10-mesh size sieve was subsequently placed into an alumina crucible and calcined at 900 °C for 2 h in a furnace; the calcined samples were labeled as calcined pen shells (CPS). All produced amendments were stored in polypropylene (PP) containers and placed in a desiccator to protect all samples from moisture. The samples were only taken out and used when necessary for the experiments. The major chemical compositions of NPS and CPS were determined by X-ray fluorescence (XRF).
Soil stabilization
Contaminated soil aliquots of 50 g were placed in a PP container. Subsequently, NPS and CPS were added in proportions of 1 to 10 wt% relative to the mass of the contaminated soil. After achieving a uniform mixture of soil and amendments, 20 wt% of distilled water equivalent to the total mixture weight was added to obtain a fully hydrated mixture. All hydrated samples were tightly covered and cured for 4 weeks at ambient conditions. Following the curing process, the samples were thoroughly dried in a hood. Subsequently, the dried samples were ground according to each extraction method.
Extraction of Pb and As
The efficiency of the stabilized soil was assessed using each single extraction method: 0.1 M HCl, EDTA-NH4OAc, and SBET, as detailed in Table 2. All reagents, namely, hydrochloric acid (HCl), sodium salt of ethylene-diamine-tetra acetic acid (Na-EDTA), ammonium acetate (NH4OAc), and glycine used for this single extraction method, were purchased from Daejung Chemical Co. (Republic of Korea) with guaranteed reagents (GR) grade. Following extraction, all solutions were centrifuged at 3000 rpm to separate the solid and liquid phases. The supernatant was then filtered using a 0.45-μm syringe filter. Subsequently, the filtered solution was analyzed using ICP-OES to determine the concentrations of Pb and As. The multi-element standard solution (Agilent Technologies, USA), containing both Pb and As, was diluted with 2% nitric acid to obtain standards of specific concentrations for a calibration curve. The conditions used were RF power of 1150 watts, plasma flow rate of 12 L/min, nebulizer flow rate of 0.6 L/min, and plasma view in axial mode for all elements. All experiments were conducted in triplicate, and two qualified standard solutions were applied for each of the 10 samples for quality assurance and quality control (QA/QC). All experimental procedures for the single extraction method are illustrated in Fig. 1.
XRD and SEM–EDX analysis
XRD analysis was employed to verify the mineralogical phase of both amendments and stabilized soils. Each thoroughly dried sample was ground with a mortar and pestle until the particle size passed through the #200-mesh size (0.075 mm). The diffraction pattern of the samples was measured using a powder X-ray diffractometer (X’Pert3, Malvern Panalytica, UK) with Cu Kα radiation in the range of 5 to 65° at a scanning rate of 0.4° min−1 and a scanning step of 0.02°. JADE software version 7.1 (MDI 2005) with PDF-2 reference database (ICDD 2002) was utilized to identify each mineralogical phase. Field emission scanning electron microscopy (FE-SEM, S-4800, Hitachi, Japan) equipped with EDX (EMAX, Horiba, Japan) was used to examine the surface morphology of the stabilized soil. For the analysis, the thoroughly dried samples were set on non-porous carbon tape and coated with platinum under vacuum conditions prior to the analysis.
Results and discussion
Characterization of amendments
Figure 2 and Table 3 present XRD and XRF results showing the mineralogical phases and major chemical composition for the NPS and CPS, respectively. XRD analysis confirmed that NPS is composed of calcium carbonate (CaCO3) in the phases of both calcite (PDF 00–005-0586) and aragonite (PDF 00–041-1475), while CPS primarily consisted of calcium oxide (CaO, PDF 00–048-1467). XRF showed that NPS and CPS consisted of 95.90% and 95.05% CaO. These results aligned with previous studies that investigated CaCO3-based waste such as oyster shells and eggshells (Ok et al. 2011; Ahmad et al. 2012).
Analysis of stabilized soils
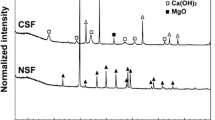
Figure 3 shows the mineralogical phases for the untreated soil and soil treated with 10 wt% NPS and CPS content. The untreated soil primarily consisted of quartz, albite, and muscovite. Following the NPS treatment, new peaks for calcite were observed in the treated soil. According to published research, Pb and As in soil can be stabilized with non-calcined CaCO3-based materials through the formation of PbCO3 and Pb(OH)2 (Ahmad et al. 2012; Lee et al. 2013), as well as Ca–As precipitates (Pérez-Sirvent et al. 2019). However, due to the detection limit of XRD (i.e., less than 1 wt% concentration of Pb and As in soil), the stabilizing products (i.e., precipitates) related to Pb and As may not be observed (Yoon et al. 2010).
XRD patterns for the contaminated soil and 10 wt% of natural pen shell (NPS)- and calcined pen shell (CPS)-treated soil. The respective PDF references are 00–005-0586 (calcite), 00–046-1045 (quartz), 00–009-0466 (albite), 00–007-0025 (muscovite), 00–004-0733 (portlandite), and 00–029-0329 (riversideite-9A)
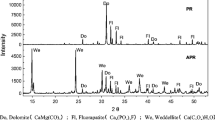
Following the CPS treatment, additional peaks associated with portlandite (Ca(OH)2, PDF 00–044-0733) and riversideite (Ca5Si6O16(OH)2•2(H2O), PDF 00–029-0329) were also observed. The presence of portlandite is ascribed to the hydration reaction of calcium oxide in CPS during the stabilization process. It is widely recognized that quicklime (CaO) treatment can lead to the formation of portlandite as well as Pb(OH)2 and As precipitates with calcium ions in lime (Moon et al. 2004; Ahmad et al. 2012). However, the absence of those precipitates in our study might be attributed to the aforementioned reason. Meanwhile, portlandite significantly increases soil pH and the solubility of silicon (Si) and aluminum (Al) in clay minerals, which could potentially result in the formation of calcium silicate hydrate (CSH) (Dermatas and Meng 2003). Riversideite is a mineral related to CSH and is one of the pozzolanic reaction products (Taylor 1953). It has been reported that Pb could be substituted into the Pb–O-Si silicate tetrahedral structure of CSH (Rose et al. 2000), while As could be fixed into the CSH structure (Guo et al. 2017). Furthermore, SEM–EDX analysis of CPS-treated soil (Fig. 4) revealed the presence of ettringite (Ca6Al2(SO4)3(OH)1226H2O), a pozzolanic reaction product demonstrating a needle-like morphology (Moon et al. 2011). Ettringite is capable of substituting calcium for lead, while sulfate ions in ettringite can replace oxyanions that have similar structures and radius, such as those of arsenic (As) chromium (Cr), selenium (Se), and vanadium (V) (Guo et al. 2017). Likewise, EDX analysis further confirmed the presence of Pb and As within the ettringite structure, aligning with previous research findings. Based on these XRD and SEM–EDX analyses, it was postulated that the NPS treatment could contribute to the formation of stabilizing precipitates, while the CPS treatment could result in both precipitation and chemical fixation processes by the formation of pozzolanic reaction products to stabilize Pb and As. The stabilization mechanisms of Pb and As by NPS and CMP is further illustrated in Fig. 5.
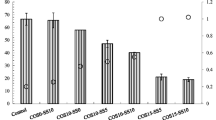
0.1 M HCl extraction
Unlike aqua regia, which is capable of extracting nearly all contaminants in soil media, the 0.1 M HCl extraction method can target highly mobile pollutants, making it applicable for assessing stabilized soil (Jeong et al. 2011). Figure 7a, b displays the leached Pb and As concentrations from the soil samples treated with NPS and CPS using the 0.1 M HCl extraction method. For the NPS treatment, Pb and As concentrations decreased up to 99.1 wt% and 96.8 wt%, respectively. This demonstrates that Pb and As concentrations can be decreased in proportion to the NPS content. CPS showed maximum efficiency at a content of 3 wt%, indicating higher efficiency than NPS. This finding agrees with prior reports that use both natural and calcined CaCO3-based wastes for heavy metal and arsenic-contaminated soils (Moon et al. 2015; Park et al. 2023).
At the same time, the pH of the extractant after the extraction procedure presented an upward trend with increasing contents of NPS and CPS (Fig. 7a, b). At 10 wt% NPS and CPS content, the soil pH was 5.83 and 7.29, respectively, as compared to 1.31 in the untreated soil. This finding suggests a significant correlation between leached Pb and As with the pH of the extractant (Table 4) and is in agreement with published research. As a matter of fact, in a study by Ahmad et al. (2012), Pb-contaminated soil stabilized by natural and calcined eggshells was assessed through the TCLP method using a strong organic acid solution similar to the 0.1 M HCl method. Their research showed a reduction trend in Pb concentration and an elevation in the pH of the extractant with increasing inputs of amendments, demonstrating a strong negative correlation between Pb concentration and the extractant pH. They asserted that the elevated pH of the extractant may contribute to a decrease in Pb solubility resulting in high stabilization efficiency (Ahmad et al. 2012). In that sense, the results of our study (Table 4) clearly demonstrate a significant correlation between leached Pb, As, and the pH of the extractant and reaffirm published research.
Nevertheless, unlike the pH of the extractant, soil pH did not increase past a certain point despite increased amendment addition (Fig. 6). This phenomenon might be attributed to the presence of unreacted NPS (Fig. 2), resulting from the nature of calcium carbonate which has low solubility (0.014 g/L (Hong et al. 2010)) and is not reactive with acids at a pH above 8.3. Consequently, it was assumed that unreacted NPS which can buffer hydrogen ions in the stabilized soils, neutralized the acidic extractant (0.1 M HCl solution) during extraction and elevated the pH, thereby affecting stabilization efficiency. Likewise, CPS composed of calcium oxide is more reactive than calcium carbonate but still has a lower solubility (1.15 g/L (Hong et al. 2010)), leading to an increased extractant pH due to the presence of residual CPS. This suggests that the residual CaCO3-based amendments can influence extractant pH during acid-based extraction and present a challenge in the precise assessment of stabilization efficiency. In this context, Li et al. reported that the increased pH of the extractant could lead to a decrease in As concentration (Li et al. 2018). In addition, Moon et al. (2010) stated that an assessment for the stabilized soil should focus on precipitation, adsorption, and chemical fixation effects, rather than solely alleviating the leachability of pollutants through pH adjustments (Moon et al. 2010). Therefore, these extraction results suggest that the utilization of various extraction methods is necessary to better comprehend the effect of CaCO3-based amendments on the contaminated soil.
EDTA-NH4OAc extraction
Figure 7c, d presents the concentrations of Pb and As after applying NPS/CPS and EDTA-NH4OAc extraction. This extraction method was developed to assess adverse effects of metals on nematode biocommunities around the soil rhizosphere (Lakanen and Erviö 1971). It uses both ammonium ions and EDTA solution to exchange trace elements and to form stable chelates, potentially allowing the extraction of bioavailable toxic elements (Hammer and Keller 2002). All extractant pH values including untreated soil and the soils subjected to NPS and CPS were measured in the range of 4.65 to 5.18 (Fig. 7c, d). The relatively lower pH variation compared to the 0.1 M HCl extraction method might be attributed to the buffer effect of the ammonium solution, suggesting that EDTA-NH4OAc could be more effective for the assessment of stabilization effects.
With 10 wt% inputs of NPS or CPS, the Pb concentration decreased by as much as 22.5 wt% and 38.4 wt%, respectively (Fig. 7c). This indicates that both NPS and CPS applications could mitigate the bioavailability of Pb in soil, though CPS was more effective. Similarly, As concentrations after NPS addition decreased proportionally to input amounts of the amendment. On the other hand, a 15 wt% increase in the leached As concentration was recorded with 1 wt% input of CPS which followed a decreasing trend with higher input amounts. Unlike Pb, As (Fig. 7d) was present in the soil in the form of oxidized anions that render the surface charge less positive and potentially desorbed at elevated soil pH (Marin et al. 1993). Thus, the desorption of As might be attributed to the elevated pH achieved by a 1 wt% CPS input (Fig. 7d). However, a subsequent decrease in As concentration was observed with increased input of CPS. This might result from the formation of Ca–As precipitates and pozzolanic reaction products such as ettringite and CSH, as previously reported since these stabilizing products can be formed under relatively high calcium concentrations and elevated pH conditions (Chrysochoou and Dermatas 2006; Moon et al. 2011). Considering the leaching characteristics, the addition of 10 wt% CPS appeared to be appropriate to effectively stabilize Pb and As-contaminated soil.
The EDTA-NH4OAc extraction method showed different leaching results compared to 0.1 M HCl extraction. As aforementioned, this might be attributed to minimizing the effects of increased pH. Thus, the EDTA-NH4OAc method, by reducing the effect of pH, can presumably reflect better the geochemical properties of the target elements in stabilized soil, especially in the case of As. In line with this research, Aziz et al. (2019) applied limestone and steel slag to heavy metals and arsenic-contaminated soil. They revealed the highest significance and correlation for leached Pb and As by the EDTA-NH4OAc extraction with the concentration of Pb and As in earthworms exposed to the stabilized soil. Hence, they insisted on the high applicability of the EDTA-NH4OAc extraction method to assess the bioavailability of Pb and As for the stabilized soil.
Simplified bioaccessibility extraction test (SBET)
SBET, as a simplified version of the physiologically based extraction test (PBET), aims to simulate gastrointestinal conditions to evaluate the bioavailability of the contaminant for humans (Ruby et al. 1996; Kim et al. 2002). Figure 7e, f shows Pb and As concentrations from the soils treated with NPS and CPS extracted by the SBET method. The soil treated with NPS presented a marginal reduction in leached concentrations of Pb and As as the input amount increased, though the results lacked significance. Similarly, the soil stabilized with CPS exhibited a trend consistent with that of NPS in the case of Pb. This result contradicted the results from the 0.1 M HCl and EDTA-NH4OAc extraction methods. This might be ascribed to the dissolution of stabilizing substances under the relatively strong acidic condition of the SBET method.
Notably, As concentrations in the CPS-treated soil showed a pronounced increasing trend with higher input amounts. Compared to the As concentration of CPS-treated soil leached by the EDTA-NH4OAc method, this result could provide further support for the stabilization mechanisms in line with existing reports. In other words, an increase in CPS input leads to an increase in calcium concentration and soil pH levels. The elevated pH results in higher arsenic mobility. Subsequently, mobile As can be stabilized by the formation of Ca–As precipitates and pozzolanic reaction products through precipitation and chemical fixation. As a result, the As leaching trend for CPS-treated soils using the EDTA-NH4OAc method could be associated with those stabilization mechanisms and the relatively higher pH conditions (i.e., pH 4.6 ~ 5.1 (Fig. 7e, f). However, in the case of the SBET method, higher As leachability with an increase in CPS input amounts might result from the dissolution of stabilized mobile arsenic under highly acidic conditions.
As described, the stabilization of Pb and As-contaminated soil with NPS and CPS addition appears insignificant in reducing risks to the human body according to the SBET extraction results. Nonetheless, it is worth noting that the SBET extraction method assumes direct ingestion of the contaminated soil, which is a relatively rare circumstance. Considering the cost-effectiveness of PS, it is believed that this concern could be mitigated. In addition, significant results could be achieved by comparing the conflicting test data between the three extraction methods, especially for As leachability. Hence, for future research, utilization of various extraction methods may be essential for a detailed assessment of CaCO3-based amendments (i.e., quicklime, eggshells, and oyster shells) for the stabilization of contaminated soils.
Conclusions
In this study, the reutilization of two types of pen shell-based amendments, NPS and CPS, was investigated for the stabilization of Pb and As-contaminated soil. After 28 days of curing, mineral phases of riversideite and ettringite were identified through XRD and SEM–EDX analyses. These mineral phases were engaged in the stabilization of Pb and As in the soil. The results of the 0.1 M HCl extraction tests for the stabilized soil revealed that the leached Pb and As were highly dependent on the pH of the extractant, which hindered the accurate assessment of the stabilization efficiency. This hindrance is derived from the significant pH increase of the extractant by the residual NPS and CPS. The EDTA-NH4OAc extraction method, with reduced pH fluctuation of the extractant, showed potential for reflecting the geochemical properties of the contaminants in soil. The extraction results of EDTA-NH4OAc method showed that CPS was more effective than NPS, and input amounts of 10 wt% of CPS were recommended. The results of the SBET extraction, which reflected strongly acidic conditions, showed elevated As concentrations with increased addition of CPS, indicating Pb and As stabilization via the chemical fixation mechanism. Overall, this study demonstrated the effectiveness of CPS for stabilizing Pb and As in soil, while caution was recommended in the case of strongly acidic conditions. Moreover, it appeared that applying various extraction methods is beneficial for gaining a better understanding of the effects and mechanisms of action of CaCO3-based amendments (i.e., pen shells) on Pb- and As-contaminated soil.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abreu MM, Matias MJ, Magalhães MCF, Basto MJ (2008) Impacts on water, soil and plants from the abandoned Miguel Vacas copper mine, Portugal. J Geochem Explor 96:161–170. https://doi.org/10.1016/j.gexplo.2007.04.012
Ahmad M, Hashimoto Y, Moon DH et al (2012) Immobilization of lead in a Korean military shooting range soil using eggshell waste: an integrated mechanistic approach. J Hazard Mater 209–210:392–401. https://doi.org/10.1016/j.jhazmat.2012.01.047
Ahmad M, Lee SS, Lim JE et al (2014) Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere 95:433–441. https://doi.org/10.1016/j.chemosphere.2013.09.077
Aziz AA, Lee BT, Han HJ, Kim KW (2019) Assessment of the stabilization of heavy metal contaminants in soils using chemical leaching and an earthworm bioassay. Environ Geochem Health 41:447–460. https://doi.org/10.1007/s10653-018-0173-1
Bueno PC, Bellido E, Rubí JAM, Ballesta RJ (2009) Concentration and spatial variability of mercury and other heavy metals in surface soil samples of periurban waste mine tailing along a transect in the Almadén mining district (Spain). Environ Geol 56:815–824. https://doi.org/10.1007/s00254-007-1182-z
Chrysochoou M, Dermatas D (2006) Evaluation of ettringite and hydrocalumite formation for heavy metal immobilization: literature review and experimental study. J Hazard Mater 136:20–33. https://doi.org/10.1016/j.jhazmat.2005.11.008
Correia AAS, Matos MPSR, Gomes AR, Rasteiro MG (2020) Immobilization of heavy metals in contaminated soils—performance assessment in conditions similar to a real scenario. Appl Sci 10:1–18. https://doi.org/10.3390/app10227950
Dermatas D, Meng X (2003) Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils. Eng Geol 70:377–394. https://doi.org/10.1016/S0013-7952(03)00105-4
Guo B, Liu B, Yang J, Zhang S (2017) The mechanisms of heavy metal immobilization by cementitious material treatments and thermal treatments: a review. J Environ Manage 193:410–422
Hammer D, Keller C (2002) Changes in the rhizosphere of metal-accumulating plants evidenced by chemical extractants. J Environ Qual 31:1561–1569
Han H-J, Ko M-S, Ko JI, Lee J-U (2020a) Study on soil extraction methods for contamination assessment of heavy metals in soil. J Korean Soc Miner Energy Resour Eng 57:471–482
Han H-J, Lee JU, Ko MS, Kim KW (2020b) Comparison of five extraction methods for evaluating cadmium and zinc immobilization in soil. Environ Geochem Health 42:4203–4212. https://doi.org/10.1007/s10653-020-00650-y
Hong CO, Kim SY, Gutierrez J et al (2010) Comparison of oyster shell and calcium hydroxide as liming materials for immobilizing cadmium in upland soil. Biol Fertil Soils 46:491–498. https://doi.org/10.1007/s00374-010-0458-8
ICDD (2002) Powder diffraction file. PDF-2 Database Release, USA
Islam MN, Taki G, Nguyen XP et al (2017) Heavy metal stabilization in contaminated soil by treatment with calcined cockle shell. Environ Sci Pollut Res 24:7177–7183. https://doi.org/10.1007/s11356-016-8330-5
Jeon C (2018) Adsorption behavior of cadmium ions from aqueous solution using pen shells. J Ind Eng Chem 58:57–63. https://doi.org/10.1016/j.jiec.2017.09.007
Jeong S-K, An J-S, Kim Y-J et al (2011) Study on heavy metal contamination characteristics and plant bioavailability for soils in the Janghang smelter area. J Soil Groundw Environ 16:42–50
Karaca O, Cameselle C, Reddy KR (2018) Mine tailing disposal sites: contamination problems, remedial options and phytocaps for sustainable remediation. Rev Environ Sci Biotechnol 17:205–228
Kim J-Y, Kim K-W, Lee J-U et al (2002) Assessment of As and heavy metal contamination in the vicinity of Duckum Au-Ag mine, Korea. Environ Geochem Health 24:213–225
Ko MS, Kim JY, Park HS, Kim KW (2015) Field assessment of arsenic immobilization in soil amended with iron rich acid mine drainage sludge. J Clean Prod 108:1073–1080. https://doi.org/10.1016/j.jclepro.2015.06.076
KOMIR (2023) Statistics of national mine (by ore type). Korea Mine Rehabilitation and Mineral Resources Corporation, Weonju (in Korean), Republic of Korea
KOSIS (2023) Statistics of fisheries and species. Republic of Korea
Lakanen E, Erviö R (1971) A comparison of eight extractants for the determination of plant available micronutrients in soil. Acta Agralia Fennica 123:223–232
Lee SH, Kim EY, Park H et al (2011) In situ stabilization of arsenic and metal-contaminated agricultural soil using industrial by-products. Geoderma 161:1–7. https://doi.org/10.1016/j.geoderma.2010.11.008
Lee SS, Lim JE, El-Azeem SAMA et al (2013) Heavy metal immobilization in soil near abandoned mines using eggshell waste and rapeseed residue. Environ Sci Pollut Res 20:1719–1726. https://doi.org/10.1007/s11356-012-1104-9
Li JS, Beiyuan J, Tsang DCW et al (2017) Arsenic-containing soil from geogenic source in Hong Kong: leaching characteristics and stabilization/solidification. Chemosphere 182:31–39. https://doi.org/10.1016/j.chemosphere.2017.05.019
Li JS, Wang L, Cui JL et al (2018) Effects of low-alkalinity binders on stabilization/solidification of geogenic As-containing soils: spectroscopic investigation and leaching tests. Sci Total Environ 631–632:1486–1494. https://doi.org/10.1016/j.scitotenv.2018.02.247
Liu L, Li W, Song W, Guo M (2018) Remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci Total Environ 633:206–219
Marin AR, Masscheleyn PH, Patrick WH (1993) Soil redox-pH stability of arsenic species and its influence on arsenic uptake by rice. Plant Soil 152:245–253
McBride MB, Simon T, Tam G, Wharton S (2013) Lead and arsenic uptake by leafy vegetables grown on contaminated soils: effects of mineral and organic amendments. Water Air Soil Pollut 224. https://doi.org/10.1007/s11270-012-1378-z
MDI (2005) Jade version 7.1. Material’s Data Inc., Livermore
Miller WP, Miller DM (1987) A micro-pipette method for soil mechanical analysis. Commun Soil Sci Plant Anal 18:1–15
MOE (2002) The Korean Standard Test (KST) methods for soils. Republic of Korea
MOE (2018) The Korean Standard Test (KST) methods for soils. Republic of Korea
Moon DH, Dermatas D, Menounou N (2004) Arsenic immobilization by calcium-arsenic precipitates in lime treated soils. Sci Total Environ 330:171–185. https://doi.org/10.1016/j.scitotenv.2004.03.016
Moon DH, Cheong K-H, Kim T-S et al (2010) Stabilization of Pb contaminated army firing range soil using calcined waste oyster shells. J Korean Soc Environ Eng 32:185–192
Moon DH, Kim KW, Yoon IH et al (2011) Stabilization of arsenic-contaminated mine tailings using natural and calcined oyster shells. Environ Earth Sci 64:597–605. https://doi.org/10.1007/s12665-010-0890-y
Moon DH, Park JW, Cheong KH et al (2013) Stabilization of lead and copper contaminated firing range soil using calcined oyster shells and fly ash. Environ Geochem Health 35:705–714. https://doi.org/10.1007/s10653-013-9528-9
Moon DH, Wazne M, Cheong KH et al (2015) Stabilization of As-, Pb-, and Cu-contaminated soil using calcined oyster shells and steel slag. Environ Sci Pollut Res 22:11162–11169. https://doi.org/10.1007/s11356-015-4612-6
Moon DH, Hwang I, Koutsospyros A et al (2018) Stabilization of lead (Pb) and zinc (Zn) in contaminated rice paddy soil using starfish: a preliminary study. Chemosphere 199:459–467. https://doi.org/10.1016/j.chemosphere.2018.01.090
Moon DH, Chang YY, Lee M et al (2021) Assessment of soil washing for heavy metal contaminated paddy soil using FeCl3 washing solutions. Environ Geochem Health 43:3343–3350. https://doi.org/10.1007/s10653-021-00815-3
Ok YS, Lim JE, Moon DH (2011) Stabilization of Pb and Cd contaminated soils and soil quality improvements using waste oyster shells. Environ Geochem Health 33:83–91. https://doi.org/10.1007/s10653-010-9329-3
Park SH, An J, Koutsospyros A, Moon DH (2023) Assessment of the stabilization of Cu-, Pb-, and Zn-contaminated fine soil using cockle shells, scallop shells, and starfish. Agriculture 13:1414. https://doi.org/10.3390/agriculture13071414
Pérez-Sirvent C, Martínez-Sánchez MJ, Veiga JM et al (2019) In situ chemical immobilisation by limestone filler of potentially harmful metal(loid) in contaminated soils: monitoring by Raman spectroscopy. Appl Geochem 111. https://doi.org/10.1016/j.apgeochem.2019.104441
Raffa CM, Chiampo F, Shanthakumar S (2021) Remediation of metal/metalloid-polluted soils: a short review. Appl Sci 11. https://doi.org/10.3390/app11094134
RDA (2013) Methods of soil chemical analysis. Republic of Korea
Rose J, Moulin I, Hazemann JL et al (2000) X-ray absorption spectroscopy study of immobilization processes for heavy metals in calcium silicate hydrates: 1. Case of lead. Langmuir 16:9900–9906. https://doi.org/10.1021/la0005208
Ruby MV, Davis A, Schoof R et al (1996) Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ Sci Technol 30:422–430
Satyro S, Race M, Marotta R et al (2017) Photocatalytic processes assisted by artificial solar light for soil washing effluent treatment. Environ Sci Pollut Res 24:6353–6360. https://doi.org/10.1007/s11356-016-6431-9
Schulte EE, Hopkins BG (1996) Estimation of organic matter by weight loss-on-ignition. In: Magdoff FR, Tabatabai MA, Hanlon Jr. EA (eds) Soil organic matter: analysis and interpretation, SSSA Special Publication, Madison, pp 21–31
Taylor HFW (1953) 33. Hydrated calcium silicates. Part V.* The water content of calcium silicate hydrate (I). J Chem Soc 163–171
Tun TZ, Bonnet S, Gheewala SH (2020) Life cycle assessment of Portland cement production in Myanmar. Int J Life Cycle Assess 25:2106–2121. https://doi.org/10.1007/s11367-020-01818-5
USEPA (2023) Superfund remedy report 17th edition. USA
Yoon IH, Moon DH, Kim KW et al (2010) Mechanism for the stabilization/solidification of arsenic-contaminated soils with Portland cement and cement kiln dust. J Environ Manage 91:2322–2328. https://doi.org/10.1016/j.jenvman.2010.06.018
Funding
This study was supported by the research fund from Chosun University, 2022.
Author information
Authors and Affiliations
Contributions
HGJ involved in conceptualization, data collection, data management, statistical analyses, and writing—original draft; AK involved in writing—review and editing; and DHM involved in conceptualization, funding acquisition, supervision, data management, and writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Manyun Zhang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jeon, H.G., Koutsospyros, A. & Moon, D.H. Stabilization of lead (Pb)- and arsenic (As)-contaminated soil using pen shells (Atrina pectinata). Environ Sci Pollut Res 31, 48663–48673 (2024). https://doi.org/10.1007/s11356-024-34362-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34362-y