Abstract
Phosphate amendments, especially phosphate rock (PR), are one of the most commonly used materials to stabilize heavy metals in contaminated soils. However, most of PR reserve consists of low-grade ore, which limits the efficiency of PR for stabilizing heavy metals. This study was to enhance the stabilization of heavy metals through improving the available phosphorous (P) release of PR by oxalic acid activation. Raw PR and activated PR (APR) were characterized by scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDS), X-ray powder diffraction (XRD), Brunauer–Emmett–Teller (BET) surface analysis, and laser diffraction to determine the changes of structure and composition of APR. The stabilization effectiveness of lead (Pb), zinc (Zn), and cadmium (Cd) in soils by APR was investigated through toxicity leaching test and speciation analysis. The results indicated that after treatment by oxalic acid, (1) the crystallinity of the fluorapatite phase of PR transformed into the weddellite phase; (2) the surface area of PR increased by 37%; (3) the particle size of PR became homogenized (20–70 μm); and (4) the available P content in PR increased by 22 times. These changes of physicochemical characteristics of PR induced that APR was more effective to transform soil heavy metals from the non-residual fraction to the residual fraction and enhance the stabilization efficiency of Pb, Zn, and Cd than PR. These results are significant for the future use of low-grade PR to stabilize heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil contamination with heavy metals is of great concern because of their toxicity and threat to human health and the environment (Li et al. 2014a, b). Nonferrous metal production is one of the most significant sources for heavy metals to enter various environmental mediums (e.g., atmosphere, surface water, and soils) as a result of worldwide human activities (Li et al. 2015). China is the largest producer of lead (Pb) and zinc (Zn) in the world, with an annual output of 4.16 and 5.21 million tons, respectively, in 2010 (Li et al. 2014a, b). The combined contamination of Pb, Zn, and cadmium (Cd) in soils near Pb/Zn mine has been shown to pose a potential risk to the surrounding environment (Guo et al. 2006; Park et al. 2011; Wu et al. 2013).
There has been an increasing effort to develop cost-effective technologies to minimize the mobility, solubility, and availability of heavy metals in soils around Pb/Zn mines (Mignardi et al. 2012a). Phosphate compounds and related materials have demonstrated to be effective amendments for stabilizing heavy metals in soils during the past 1 or 2 years (Li et al. 2015; Du et al. 2016). Phosphate amendments are one of the most commonly used materials for stabilizing Pb in contaminated soils (Fang et al. 2012; Cao et al. 2013; Du et al. 2014; Wei et al. 2015). Mignardi et al. reported that phosphate rock (PR) can reduce the solubility and availability of Pb, copper (Cu), Cd, and Zn in mine waste soil (Mignardi et al. 2012a). The formation of metal-phosphate complexes through the application of phosphate materials is considered a major immobilization approach for Pb, Zn, and Cd contaminated soils (Jiang et al. 2012; Mignardi et al. b). Certain Pb-P, Zn-P, and Cd-P complexes are highly stable, with limited solubility and mobility in soils (Austruy et al. 2014; Waterlot et al. 2011). Phosphate material-induced stabilization of heavy metals in soil is recommended by the United States Environmental Protection Agency (US EPA) as one of the best management practices for heavy metals in soils (Chrysochoou et al. 2007).
China has the second largest PR reserve in the world, but most of it consists of low-grade (P2O5 < 23%) and refractory ore (Zhang et al. 2008). The reactivity of the phosphate in PR is a crucial factor that directly affects the immobilization efficiency of heavy metals by PR. PR, with the low quality, is a raw material used to produce phosphate fertilizer (Scheckel et al. 2013). Low-grade ores have poor effectiveness when applied directly and need to be activated by converting the phosphate into available forms. Some studies have indicated that the release of phosphate from low reactivity PRs and the phytoavailability of P in PR-treated soils can be promoted after activated by organic acids, especially oxalic acid (Elouear et al. 2008; Jiang et al. 2012; Liu et al. 2012; Zhu et al. 2015). Thus, it can be assumed that PR activation by organic acids could induce the release of P from PR and improve stabilization efficiency of heavy metals through precipitation in contaminated soil. However, there have been few studies of the effects of activation of PR by organic acids on the immobilization of heavy metals (e.g., Pb, Zn, and Cd) in contaminated soils.
Under certain conditions, some plant roots can secrete the low-molecular-weight organic acids, and some of them could promote dissolution of phosphate (Kohler et al. 2007). In addition, low-molecular-weight organic acids were found in the secretion of phosphorus-solubilizing bacteria (Ouahmane et al. 2009). The main low-molecular-weight organic acids that have been detected in soil are oxalic, citric, and matric acids (Kpomblekou and Tabatabai 2003). Most of them play a vital role in soil formation, rhizosphere changes, and nutrient cycling (Ström et al. 2005). Considering that oxalic acid has highly effectiveness in releasing P from PR (Liu et al. 2012,) and is a natural and biodegradable low molecular weight organic acids in soil, organic acid (oxalic acid) as an environmental acceptability material was used to activate PR. In this study, the stabilization of Pb, Zn, and Cd in polluted soils with raw PR and oxalic acid-activated PR (APR) was investigated. The objectives of the study were to investigate the effects of APR on the stabilization of Pb, Zn, and Cd in contaminated soils and provide further information on the appropriate utilization of PR resources.
Materials and methods
Materials
Uncontaminated soil was collected from the upper 20 cm of land in Zhuzhou city, Hunan province (soil-Z), and contaminated soil was collected from the upper 20 cm of land near Zhuzhou Smelter, Hunan province in China (soil-ZY). After being air-dried, the soil samples were crushed and then passed through a 2-mm sieve. A preliminary analysis showed that the concentration of Pb, Zn, and Cd in soil-Z were 66, 122, and 0.5 mg/kg, respectively. The concentration of metals in soil-Z was far below the Level II China Environmental Quality Standard for Soils (GB15618–1995). Thus, soil-Z was uncontaminated according to the CEQSS. To meet the study objectives, a further soil (soil-ZZ) was artificially prepared by spiking Pb(NO3)2, Zn(NO3)2, and Cd(NO3)2 into soil-Z and equilibrated for 3 weeks at room temperature and 50–70% field moisture capacity (Cao et al. 2013). All reagents were of analytical grade, and deionized water was used for all the experiments. The soils were spiked with Pb, Zn, and Cd nitrates because metal nitrate was easily soluble and nitrate ions have less influence on reaction system including the main chemical compositions of Ca, Mg, and phosphate ions, compared to carbonate, sulfate, and acetate ions (Du et al. 2016). The concentration of Pb, Zn, and Cd in soil-ZZ was 503, 10,010, and 99 mg/kg, respectively. Selected physical and chemical properties of soil-ZZ and soil-ZY are presented in Table 1.
The PR used in this study is originated from Zhongxiang county of Hubei province. The PR was crushed to pass through a #100 sieve (0.149 mm) and its basic properties are presented in Table 2. Oxalic acid was chosen as the activating agent. The PR was mixed with 0.5 mol/L oxalic acid at a liquid to solid ratio of 10:1 and equilibrated at 28 (±1) °C for 6 days (Liu et al. 2012). Then, the sample was dried in an oven at 60 °C and crushed to pass through a #100 sieve to obtain the APR. The structure and mineral composition of PR and APR were identified by scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDS) and X-ray powder diffraction (XRD).
Stabilization treatment
A 200-g soil sample was placed in a stainless steel mixing bowl and amended with pre-weighed PR/APR with ratios of 2, 5, 7, and 10% by soil dry weight, after which deionized water was added to give a water to solid ratio (W/S) of 30% and was thoroughly mixed 10 min (62 ± 5 rpm) by electronic cement paste mixer to achieve homogeneous mixture. The experiments were run as three replicates. After materials were mixed well, the mixture was placed in a polypropylene box with a cover and stored in a curing chamber at room temperature, 95% relative humidity for 7 days, after which they were dried and the mobility of Pb, Zn, and Cd were assessed using the toxicity characteristic leaching procedure (TCLP) (Agency 1986). All treatments were conducted in triplicate.
Toxicity characteristic leachability procedure
The leachability characteristics of heavy metals are fundamental in the regulatory characterization of the heavy metal contaminated soils. The leachability of heavy metals was analyzed by TCLP in this study (Agency 1986). Before TCLP treatment, the air-dried stabilized soil was crushed to pass through a 9.5-mm sieve. Briefly, a 20-g representative soil sample was extracted with leachant (acetic acid solution pH 4.93 ± 0.05) at a solid to liquid weight ratio of 1:20 and agitated in a 500-mL polypropylene bottle on a reciprocal shaker at 30 ± 2 rpm for 18 ± 2 h. At the end of agitation period, the fluid was filtered through a Millipore fiber filter of 0.45 μm pore size. The soluble heavy metal concentration in the filtrate was analyzed by inductively coupled plasma mass spectrometry (ICP-MS). The stabilization effectiveness of heavy metals was assessed by comparing the soluble heavy metal concentrations in the treated soils with that of the original soils.
Speciation analysis
The European Community Bureau of Reference (BCR) sequential extraction procedure (SEP) provides useful information to assess potential heavy metals mobility and bioavailability (Hu et al. 2006). The SEP has been shown to be reproducible and give better recoveries than other sequential extraction procedures (Rao et al. 2008). The SEP is made up of four steps to obtain the chemical speciation of heavy metals: the acid soluble/exchangeable fraction (AS), the reducible fraction (RD), the oxidizable fraction (OD), and the residual fraction (RS). The main reagents used in this experimental process included acetic acid, hydroxylamine hydrochloride, hydrogen peroxide, and nitric acid-perchloric acid-hydrofluoric acid, respectively.
Analytical methods
Metal concentrations of Pb, Zn, and Cd were analyzed by ICP-MS (model 7500, Agilent, Santa Clara, CA, USA). The total and available P content was detected by vanadium-molybdate-yellow colorimetry (Lambda 25 spectrophotometer, Perkin Elmer, Waltham, MA, USA). The pH of the soil samples was measured by a Delta320 pH/conductivity meter (Mettler Toledo, Colombus, OH, USA) (Zhang et al. 2016). An X-ray diffraction meter (BRUKER-AXS, D8 Advance, Bruker, Billerica, MA, USA) was used to determine the crystalline forms of PR and APR. The diffraction meter was operated at 40 kV and 40 mA with CuKa radiation. Scans were in the range of 10–85° in 2θ at a scan rate of 1°/min. Data analyses were conducted by Jade 6.5 software. The morphology of PR and APR was performed by SEM (S-4800, Hitachi, Tokyo, Japan). Prior to the examination, the samples were sputter-coated with Au by an Auto Fine Coater under a nitrogen atmosphere to render them electrically conductive, after which SEM was conducted at a low acceleration voltage of 15 kV. The particle size distributions of PR samples before and after activation were analyzed by laser diffraction techniques using a laser-scattering particle size distribution analyzer (LA-950, Horiba, Kyoto, Japan). And the samples were separated by ultrasonic dispersion in water and adding sodium hexametaphosphate as dispersion reagents before the measurement. The specific surface area of PR and APR samples was determined by standard N2-adsorption techniques using a surface area analyzer (Nova 4200e, Quantachrome, Boynton Beach, FL, USA).
Results and discussion
Characterization of PRs
X-ray diffraction analysis of PR/APR
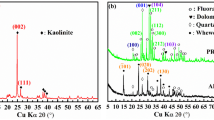
The XRD patterns of PR and APR are shown in Fig. 1. Clearly defined fluorapatite peaks could be observed in both PR and APR. After treatment with oxalic acid, the crystallinity of the fluorapatite phase gradually decreased as manifested by a broadening peak width and decreasing peak intensity. The gradual decrease of crystallinity was an indication of the increasing P release from fluorapatite. Dolomite was also observed in the test PR. The formation of weddellite was observed in the APR, with C2O4 2− in solution combining with Ca2+ released by dissolving the raw PR.
SEM with EDS analysis of PR/APR
The SEM micrographs and elemental spectra of selected particles obtained by the EDS analysis of PR and APR are shown in Fig. 2. The tested sample of raw PR powder contained different sized agglomerate crystals with micro size containing a significant amount of calcium (Ca), P, oxygen (O), magnesium (Mg), and carbon (C), with a low fluorine (F) content, which indicated the existence of fluorapatite (Fig. 1). The raw PR with high crystallinity was compact structure. After PR was treated by oxalic acid, the original agglomerate crystal structures became the poor crystallinity aggregation with porous structure (Fig. 2d–f). The amount of O and C in APR increased for the oxalic acid activation. The APR with porous structure and rough surface would probably increase the surface area, which would facilitate the adsorption and stabilization of heavy metals.
BET and particle size distribution analysis of PR/APR
The surface area of the APR obtained by the Brunauer–Emmett–Teller (BET) method was 3.64 m2/g. This was larger than the surface area of PR, which was only 2.67 m2/g. The activation of PR contributed to an increase in its surface area. The particle size distribution of PR and APR by the laser diffraction method is shown in Fig. 3. The percentage of PR particles with a size < 30 μm after activation was lower than for raw PR, and the percentage of particles with a size > 30 μm of APR was higher than for raw PR. APR had a more concentrated particle size distribution (20–70 μm) than PR, and the results of the particle size distribution and SEM micrographs were highly consistent. APR powder with uniform particle size distribution has larger surface area than PR.
Effectiveness of heavy metal stabilization by PR and APR
The leached concentrations of Pb, Zn, and Cd in two soils treated by PR and APR are shown in Fig. 4. Compared to the raw PR, it was clear that APR was more effective at stabilizing Pb, Zn, and Cd in contaminated soils. It can also be seen that the stabilization efficiency of the heavy metals was in the order Pb > Zn ≈ Cd. With the addition of an amendment ranging from 2 to 10%, the Pb leaching concentration decreased by 53–89% and 36–88% in soil-ZZ and soil-ZY treated with APR, respectively. In contrast, the Pb leaching concentration in soil-ZZ and soil-ZY treated by PR decreased by 36–67% and 16–54%, respectively. The leachable Zn in 10% APR- and PR-treated soils (soil-ZZ and soil-ZY) reduced by < 52 and < 22%, respectively. The leachable Cd in 10% ARR and PR stabilized soils reduced by < 61 and 26%, respectively.
The pH of APR was 5.10. The pH of APR-treated soils decreased slightly, while the pH of soil-ZZ and soil-ZY decreased from 7.9 and 5.4 to 7.2–7.9 and 3.9–4.9 (Fig. 4). In PR stabilized soils, the pH increased slightly. APR was a compound of PR and oxalic acid and released H2PO4 − and HPO4 2− in soils, which caused the relatively low soil pH values.
APR was created by the activation of PR with oxalic acid. Oxalic acid is a low-molecular-weight organic acid. The coordination ability of the oxalate anion was stronger than either of the other ligands, and the active group Ox2− could form steady coordination compounds or insoluble materials with the cations in PR (Liu et al. 2012). Oxalic acid was therefore effective in promoting the dissolution of PR and releasing P. The available P content of APR (600.47 mg/kg) was 23 times higher than that of PR (25.26 mg/kg). The dominant mechanism of heavy metal ions stabilization by PR is controlled by its dissolution in the acidic environment, followed by the subsequent precipitation formation of P in the form of H2PO4 − (Saxena and D’Souza 2006). The P in the form of H2PO4 − helped in precipitating heavy metal ions in polluted soils. Therefore, the greater available P content of APR was beneficial for the stabilization of heavy metals in soils, and especially for Pb, because Pb stabilization was mainly attributed to phosphate—induced the formation of fluoropyomorphite, and to a lesser extent to complexation or surface adsorption (Cao et al. 2004). In comparison, Zn and Cd were less chemisorbed via surface adsorption or complexation. The stabilization of Zn by PR can be mainly attributed to complexation or surface adsorption (accounting for up to 95.7% of the total stabilized) (Basta et al. 2001). The higher the solubility of PR, the more effective it is in stabilizing heavy metal ions.
Isomorphic substitution of calcium with divalent metal ions occurs and is related to their electronegativity and ionic radius (Perrone et al. 2001). The hydrated ionic radius is a function of charge and ionic radius, which affects stabilization effect of heavy metal ions. The stronger retention of Pb2+, with a large ionic radius (1.20 Å) and higher levels of electronegativity (2.33), is due to that the ionic radius of Pb2+ is very close to that of Ca2+ (0.99 Å). Because Zn2+ (ionic radius 0.75 Å, electronegativity 1.65) and Cd2+ (ionic radius 0.94 Å, electronegativity 1.69) have a smaller ionic radius than Ca2+ (ionic radius 0.99 Å, electronegativity 1.01) and higher electronegativity, they display intermediate behavior. Hence, the higher retention of Pb may be due to that the ionic radius of Cd2+ and Zn2+ is smaller than the ionic radius of Pb. To a certain degree, this reveals the observations of earlier researchers, where cations with a larger ionic radius than Ca2+ may be incorporated in the apatite lattice to a much more extent than cations with a small ionic radius (Xu et al. 1994). This may be the reason for the lower stabilization effectiveness of Cd2+ and Zn2+ in comparison to Pb2+.
The possible mechanisms for heavy metal stabilization by phosphate minerals include the following: (1) the formation of amorphous or poorly crystalline metal-phosphate precipitation; (2) ion-exchange interaction and surface complexation at the surface of PR; (3) isomorphic substitution of Ca in PR by other heavy metals during recrystallization or coprecipitation process (Cao et al. 2004; Saxena and D’Souza 2006).
Changes of the chemical form of heavy metals stabilized by PR and APR
As shown in Fig. 5, different chemical fractions of Pb, Zn, and Cd were detected in the three test soils before and after the stabilization treatment. Lead in original soils was mainly associated with the acid soluble (AS) fraction and reducible (RD) fraction (up to 65–76%); Zn and Cd were primarily associated with the AS fraction (45–75% and 42–59%, respectively). Therefore, Pb, Zn, and Cd have high mobility and bioavailability in contaminated soils.
The results demonstrated that APR was more effective than PR at transforming soil heavy metals from the non-residual fraction (the sum of AS, RD, and oxidizable (OD) fractions) to the residual (RS) fraction than PR. AS-Pb in soils treated by APR and PR decreased by 24–38% and 23–25%, respectively, and RS-Pb increased by 28–42% and 18–40%, respectively. In comparison with PR, the available P in APR increased and more easily converted soil Pb to highly insoluble Pb-phosphate mineral. Oxalic acid effectively increased the binding sites on soil organic matter and raised the proportion of organic combined-Pb. The RS-Zn in soils treated by PR and APR increased by 9–13% and 11–15%, respectively. The RS-Cd in soils treated by PR and APR increased by 14–19% and 21–25%, respectively. Generally, non-residual heavy metal is considered more potentially mobile and bioavailable than residual fraction. The stabilization effectiveness of heavy metals in polluted soils can be assessed by converting amounts of heavy metals from non-residual to the residual fraction, and the higher the conversion rates, the better stabilization effectiveness of heavy metals in soils (Zhang et al. 2015). The results of speciation demonstrated that APR could be used to reduce the mobility and bioavailability of heavy metals by changing the chemical forms of heavy metals in polluted soils.
Conclusions
The objective of this study was to activate PR by oxalic acid and form APR, and then evaluate the effectiveness of the APR in stabilizing Pb, Zn, and Cd in soils. The results indicated that after PR was treated by oxalic acid, the original agglomerate crystal structures became the poor crystallinity aggregation with porous structure; the particle size of PR became homogenized (20–70 μm); the surface area increased by 37%; and the available P content of APR increased by 22 times. Based on the changes of physicochemical properties of PR, APR could more effectively stabilize Pb, Zn, and Cd in soils than raw PR. With a 2–10% addition of APR, the concentration of Pb, Zn, and Cd leached from the soils decreased by 36–89%, 15–52%, and12–62%, respectively. AS-Pb in soils treated by APR decreased by 24–38% and RS-Pb increased by 28–42%, while RS-Zn and RS-Cd increased by 11–15% and 21–25%, respectively. The stabilization efficiency of the heavy metals by APR was in the order of Pb > Zn ≈ Cd. This suggests that APR can be used as a green amendment to stabilize Pb, Zn, and Cd contaminated soils, especially Pb contaminated soils.
References
Austruy A, Shahid M, Xiong T, Castrec M, Payre V, Niazi NK (2014) Mechanisms of metal-phosphates formation in the rhizosphere soils of pea and tomato: environmental and sanitary consequences. J Soil Sediment 14:666–678
Basta NT, Gradwohl R, Snethen KL, Schroder JL (2001) Chemical immobilization of lead, zinc, and cadmium in smelter-contaminated soils using biosolids and rock phosphate. J Environ Qual 30:1222–1230
Cao X, Ma LQ, Rhue DR, Appel CS (2004) Mechanisms of lead, copper, and zinc retention by phosphate rock. Environ Pollut 131:435–444
Cao X, Liang Y, Zhao L, Le H (2013) Mobility of Pb, Cu, and Zn in the phosphorus-amended contaminated soils under simulated landfill and rainfall conditions. Environ Sci Pollut Res 20:5913–5921
Chrysochoou M, Dermatas D, Grubb DG (2007) Phosphate application to firing range soils for Pb immobilization: the unclear role of phosphate. J Hazard Mater 144:1–14
Du Y, Wei M, Reddy KR, Jin F, Wu H, Liu Z (2014) New phosphate-based binder for stabilization of soils contaminated with heavy metals: leaching, strength and microstructure characterization. J Environ Manag 146:179–188
Du Y, Wei M, Reddy KR, Wu H (2016) Effect of carbonation on leachability, strength and microstructural characteristics of KMP binder stabilized Zn and Pb contaminated soils. Chemosphere 144:1033–1042
Elouear Z, Bouzid J, Boujelben N, Feki M, Jamoussi F, Montiel A (2008) Heavy metal removal from aqueous solutions by activated phosphate rock. J Hazard Mater 156:412–420
Fang Y, Cao X, Zhao L (2012) Effects of phosphorus amendments and plant growth on the mobility of Pb, Cu, and Zn in a multi-metal-contaminated soil. Environ Sci Pollut R 19:1659–1667
Guo G, Zhou Q, Ma Q (2006) Availability and assessment of fixing additives for the in situ remediation of heavy metal contaminated soils: a review. Environ Monit Assess 116:513–528
Hu N, Li Z, Huang P, Tao C (2006) Distribution and mobility of metals in agricultural soils near a copper smelter in South China. Environ Geochem Hlth 28:19–26
Jiang G, Liu Y, Huang L, Fu Q, Deng Y, Hu H (2012) Mechanism of lead immobilization by oxalic acid-activated phosphate rocks. J Environ Sci 24:919–925
Kohler J, Caravaca F, Carrasco L, Roldán A (2007) Interactions between a plant growth-promoting rhizobacterium, an AM fungus and a phosphate-solubilising fungus in the rhizosphere of Lactuca sativa. Appl Soil Ecol 35:480–487
Kpomblekou AK, Tabatabai MA (2003) Effect of low-molecular weight organic acids on phosphorus release and phytoavailabilty of phosphorus in phosphate rocks added to soils. Agric Ecosyst Environ 100:275–284
Li L, Holm PE, Marcussen H, Bruun HC (2014a) Release of cadmium, copper and lead from urban soils of Copenhagen. Environ Pollut 187:90–97
Li Z, Feng X, Bi X, Li G, Lin Y, Sun G (2014b) Probing the distribution and contamination levels of 10 trace metal/metalloids in soils near a Pb/Zn smelter in Middle China. Environ Sci Pollut Res 21:4149–4162
Li P, Lin C, Cheng H, Duan X, Lei K (2015) Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotox Environ Safe 113:391–399
Liu Y, Feng L, Hu H, Jiang G, Cai Z (2012) Phosphorus release from low-grade rock phosphates by low molecular weight organic acids. J Food Agric Environ 10:1001–1007
Mignardi S, Corami A, Ferrini V (2012) Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn. Chemosphere 86:354–360
Ouahmane L, Revel JC, Hafidi M, Thioulouse J, Prin Y, Galiana A (2009) Responses of Pinus halepensis growth, soil microbial catabolic functions and phosphate-solubilizing bacteria after rock phosphate amendment and ectomycorrhizal inoculation. Plant Soil 320:169–179
Park JH, Bolan N, Megharaj M, Naidu R (2011) Comparative value of phosphate sources on the immobilization of lead, and leaching of lead and phosphorus in lead contaminated soils. Sci Total Environ 409:853–860
Perrone J, Fourest B, Giffaut E (2001) Sorption of nickel on carbonate fluoroapatites. J Colloid Interf 239:303–313
Rao CRM, Sahuquillo A, Sanchez JFL (2008) A review of the different methods applied in environmental geochemistry for single and sequential extraction of trace elements in soils and related materials. Water Air Soil Poll 189:291–333
Saxena S, D’Souza SF (2006) Heavy metal pollution abatement using rock phosphate mineral. Environ Int 32:199–202
Scheckel KG, Diamond GL, Burgess MF, Klotzbach JM, Maddaloni M, Miller BW (2013) Amending soils with phosphate as means to mitigate soil lead hazard: a critical review of the state of the science. J Toxicol Env Heal B 16:337–380
Ström L, Owen AG, Godbold DL, Jones DL (2005) Organic acid behaviour in a calcareous soil implications for rhizosphere nutrient cycling. Soil Biol Biochem 37:2046–2054
US EPA (1986) Method 1311 toxicity characteristic leaching procedure (TCLP). United States Environmental Protection Agency
Waterlot C, Pruvot C, Ciesielski H, Douay F (2011) Effects of a phosphorus amendment and the pH of water used for watering on the mobility and phytoavailability of Cd, Pb and Zn in highly contaminated kitchen garden soils. Ecol Eng 37:1081–1093
Wei M, Du Y, Reddy KR, Wu H (2015) Effects of freeze-thaw on characteristics of new KMP binder stabilized Zn- and Pb-contaminated soils. Environ Sci Pollut R 22:19473–19484
Wu W, Xie Z, Xu J, Wang F, Shi J, Zhou R (2013) Immobilization of trace metals by phosphates in contaminated soil near lead/zinc mine tailings evaluated by sequential extraction and TCLP. J Soil Sediment 13:1386–1395
Xu Y, Schwartz FW, Traina SJ (1994) Sorption of Zn2+ and Cd2+ on hydroxyapatite surfaces. Environ Sci Technol 28:1472–1480
Zhang W, Ma W, Ji Y, Fan M, Oenema O, Zhang F (2008) Efficiency, economics, and environmental implications of phosphorus resource use and the fertilizer industry in China. Nutr Cycl Agroecosys 80:131–144
Zhang Z, Guo G, Teng Y, Wang J, Rhee JS, Wang S (2015) Screening and assessment of solidification/stabilization amendments suitable for soils of lead-acid battery contaminated site. J Hazard Mater 288:140–146
Zhang Z, Ren J, Wang M, Song X, Zhang C, Chen J (2016) Competitive immobilization of Pb in an aqueous ternary-metals system by soluble phosphates with varying pH. Chemosphere 159:58–65
Zhu J, Cai Z, Su X, Fu Q, Liu Y, Huang Q (2015) Immobilization and phytotoxicity of Pb in contaminated soil amended with γ-polyglutamic acid, phosphate rock, and γ-polyglutamic acid-activated phosphate rock. Environ Sci Pollut R 22:2661–2667
Funding
We are grateful to the National High Technology Research and Development Program of China (“863” program) (2012AA101402) and the National Natural Science Foundation of China (Grant No. 41071165) for their financial support for this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Zhang, Z., Guo, G., Wang, M. et al. Enhanced stabilization of Pb, Zn, and Cd in contaminated soils using oxalic acid-activated phosphate rocks. Environ Sci Pollut Res 25, 2861–2868 (2018). https://doi.org/10.1007/s11356-017-0664-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0664-0